Adenosine Triphosphate (ATP) and Protein Aggregation in Age-Related Vision-Threatening Ocular Diseases
Abstract
1. Introduction
2. Materials and Methods
3. Results
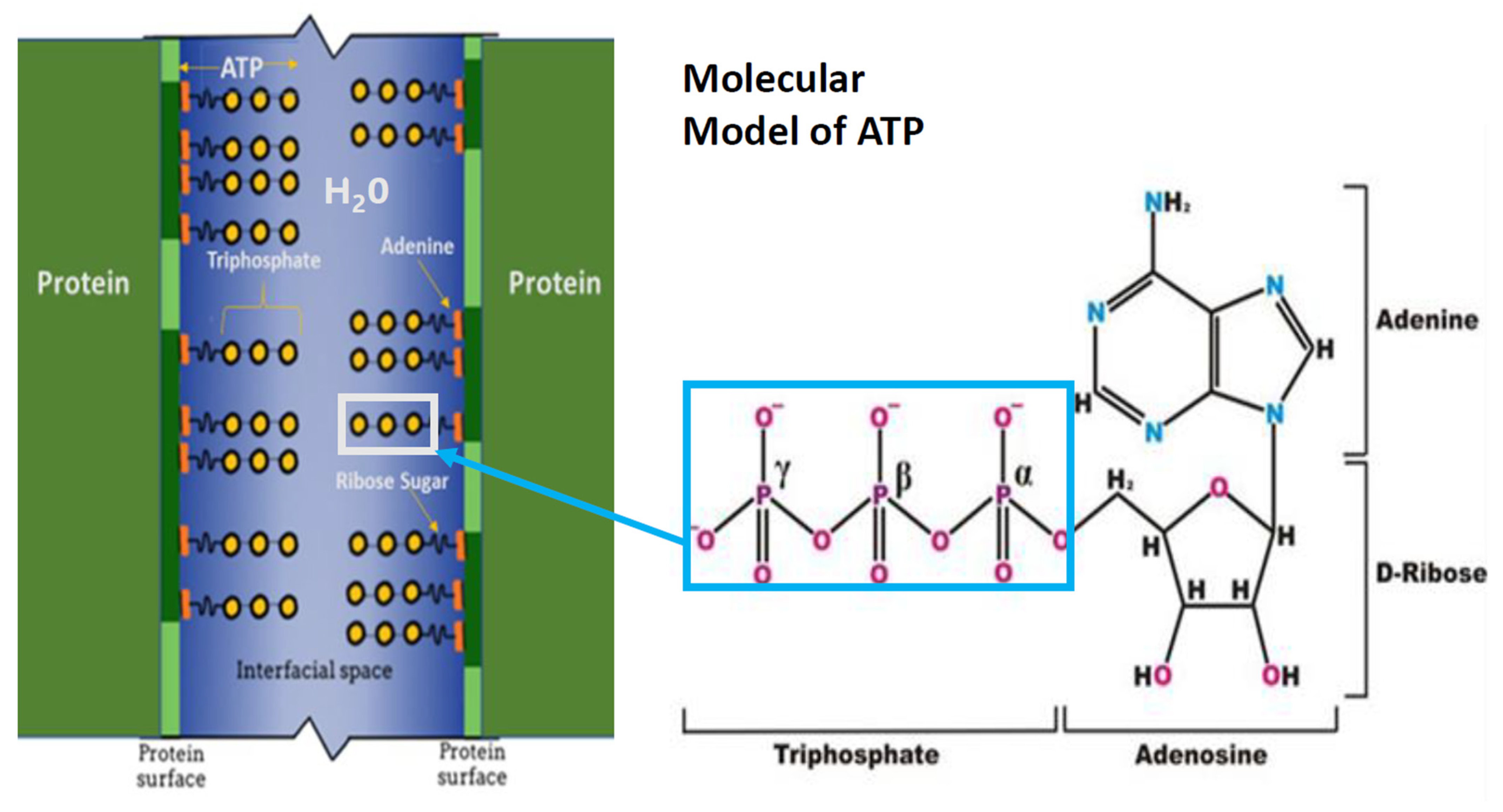
3.1. ATP as A Hydrotrope
3.2. The Millimolar Concentration of ATP
3.3. ATP and Protein Aggregation
3.4. The ATP Hydrotropic Layer
3.5. The Hydrotropic Nature of ATP
3.6. Functions of ATP
4. Discussion
4.1. Strengths of the Hypothesis
4.2. Limitations of the Hypothesis
4.3. Future Directions
4.3.1. Crystalline Lens and Age-Related Cataract
4.3.2. Presbyopia
4.3.3. Age-Related Macular Degeneration
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Merchant, T.E.; Lass, J.H.; Meneses, P.; Greiner, J.V.; Glonek, T. The effects of age on phosphatic metabolites of the human crystalline lens. Exp. Eye Res. 1991, 52, 641–646. [Google Scholar] [CrossRef]
- Moreau, K.L.; King, J.A. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol. Med. 2012, 18, 273–282. [Google Scholar] [CrossRef]
- Budnar, P.; Tangirala, R.; Bakthisaran, R.; Rao, C.M. Protein Aggregation and Cataract: Role of Age-Related Modifications and Mutations in α-Crystallins. Biochemistry 2022, 87, 225–241. [Google Scholar] [CrossRef]
- Bloemendal, H.; de Jong, W.; Jaenicke, R.; Lubsen, N.H.; Slingsby, C.; Tardieu, A. Ageing and vision: Structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 2004, 86, 407–485. [Google Scholar] [CrossRef]
- Serebryany, E.; Chowdhury, S.; Woods, C.N.; Thorn, D.C.; Watson, N.E.; McClelland, A.A.; Klevit, R.E.; Shakhnovich, E.I. A native chemical chaperone in the human eye lens. Elife 2022, 11, e76923. [Google Scholar] [CrossRef]
- Truscott, R.J.; Zhu, X. Presbyopia and cataract: A question of heat and time. Prog. Retin. Eye Res. 2010, 29, 487–499. [Google Scholar] [CrossRef]
- Nandi, S.K.; Nahomi, R.B.; Rankenberg, J.; Glomb, M.A.; Nagaraj, R.H. Glycation-mediated inter-protein cross-linking is promoted by chaperone-client complexes of α-crystallin: Implications for lens aging and presbyopia. J. Biol. Chem. 2020, 295, 5701–5716. [Google Scholar] [CrossRef]
- Paraoan, L.; Sharif, U.; Carlsson, E.; Supharattanasitthi, W.; Mahmud, N.M.; Kamalden, T.A.; Hiscott, P.; Jackson, M.; Grierson, I. Secretory proteostasis of the retinal pigmented epithelium: Impairment links to age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100859. [Google Scholar] [CrossRef]
- Leger, F.; Fernagut, P.O.; Canron, M.H.; Léoni, S.; Vital, C.; Tison, F.; Bezard, E.; Vital, A. Protein aggregation in the aging retina. J. Neuropathol. Exp. Neurol. 2011, 70, 63–68. [Google Scholar] [CrossRef]
- Surgucheva, I.; Ninkina, N.; Buchman, V.L.; Grasing, K.; Surguchov, A. Protein aggregation in retinal cells and approaches to cell protection. Cell. Mol. Neurobiol. 2005, 25, 1051–1066. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Veréb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 2013, 9, 973–984. [Google Scholar] [CrossRef]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef]
- Westermark, P.; Andersson, A.; Westermark, G.T. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol. Rev. 2011, 91, 795–826. [Google Scholar] [CrossRef]
- Neuberg, C. Hydrotropische erscheinngern. Biochem. Z. 1916, 76, 107–176. [Google Scholar]
- Patel, A.; Malinovska, L.; Saha, S.; Wang, J.; Alberti, S.; Krishnan, Y.; Hyman, A.A. ATP as a biological hydrotrope. Science 2017, 356, 753–756. [Google Scholar] [CrossRef]
- Hayes, M.H.; Peuchen, E.H.; Dovichi, N.J.; Weeks, D.L. Dual roles for ATP in the regulation of phase separated protein aggregates in Xenopus oocyte nucleoli. Elife 2018, 7, e35224. [Google Scholar] [CrossRef]
- Pu, Y.; Li, Y.; Jin, X.; Tian, T.; Ma, Q.; Zhao, Z.; McVey, A.F.; Bai, F. ATP-Dependent Dynamic Protein Aggregation Regulates Bacterial Dormancy Depth Critical for Antibiotic Tolerance. Mol. Cell 2019, 73, 143–156.e4. [Google Scholar] [CrossRef]
- Sridharan, S.; Kurzawa, N.; Werner, T.; Günthner, I.; Helm, D.; Huber, W.; Bantscheff, M.; Savitski, M.M. Proteome-wide solubility and thermal stability profiling reveals distinct regulatory roles for ATP. Nat. Commun. 2019, 10, 1155. [Google Scholar] [CrossRef]
- Greiner, J.V.; Glonek, T. Hydrotropic function of ATP in the crystalline lens. Exp. Eye Res. 2020, 190, 107862. [Google Scholar] [CrossRef]
- Takaine, M.; Imamura, H.; Yoshida, S. High and stable ATP levels prevent aberrant intracellular protein aggregation in yeast. Elife 2022, 11, e67659. [Google Scholar] [CrossRef]
- Greiner, J.V.; Glonek, T. Intracellular ATP Concentration and Implication for Cellular Evolution. Biology 2021, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.V.; Glonek, T. Implications of the hydrotropic function of intralenticular ATP in catractogenesis and presbyopiogenesis. New Front. Ophthalmol. 2020, 6, 1–2. [Google Scholar] [CrossRef]
- Hoult, D.I.; Busby, S.J.; Gadian, D.G.; Radda, G.K.; Richards, R.E.; Seeley, P.J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature 1974, 252, 285–287. [Google Scholar] [CrossRef]
- Bárány, M.; Bárány, K.; Burt, C.T.; Glonek, T.; Myers, T.C. Structural changes in myosin during contraction and the state of ATP in the intact frog muscle. J. Supramol. Struct. 1975, 3, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Burt, C.T.; Glonek, T.; Bárány, M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J. Biol. Chem. 1976, 251, 2584–2591. [Google Scholar] [CrossRef]
- Greiner, J.V.; Kopp, S.J.; Sanders, D.R.; Glonek, T. Organophosphates of the crystalline lens: A nuclear magnetic resonance spectroscopic study. Investig. Ophthalmol. Vis. Sci. 1981, 21, 700–713. [Google Scholar]
- Kuwabara, T. The maturation of the lens cell: A morphologic study. Exp. Eye Res. 1975, 20, 427–443. [Google Scholar] [CrossRef]
- Glonek, T.; Greiner, J.V. Intralenticular water interactions with phosphates in the intact crystalline lens. Ophthalmic Res. 1990, 22, 302–309. [Google Scholar] [CrossRef]
- Glonek, T.; Snogren, T.; Schmidt, S.Y.; Hearn, S.L.; Isreb, M.A.; Greiner, J.V. Phosphatic metabolism in dark- and light-adapted rat retinas. Exp. Eye Res. 2022, 221, 109141. [Google Scholar] [CrossRef]
- Dang, M.; Li, Y.; Song, J. Tethering-induced destabilization and ATP-binding for tandem RRM domains of ALS-causing TDP-43 and hnRNPA1. Sci. Rep. 2021, 11, 1034. [Google Scholar] [CrossRef]
- Nelson, D.L.; Hoskins, A. Lehninger Principles of Biochemistry, 8th ed.; WH Freeman & Company: New York, NY, USA, 2021. [Google Scholar]
- Ratajczak, K.; Tobiecka, M. DNA Aptamer beacon probe (ABP) for monitoring of adneosine triphophate level in SW480 cancer cells treted with glycolysis inhibitor 2-doxyglucose. Int. J. Mol. Sci. 2023, 24, 9295. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.V.; Kopp, S.J.; Glonek, T. Distribution of phosphatic metabolites in the crystalline lens. Investig. Ophthalmol. Vis. Sci. 1985, 26, 537–544. [Google Scholar]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, G.M.; Greig, N.H.; Khan, T.A.; Hassan, I.; Tabrez, S.; Shakil, S.; Sheikh, I.A.; Zaidi, S.K.; Akram, M.; Jabir, N.R.; et al. Protein misfolding and aggregation in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Morales-Scheihing, D.; Butler, P.C.; Soto, C. Type 2 diabetes as a protein misfolding disease. Trends Mol. Med. 2015, 21, 439–449. [Google Scholar] [CrossRef]
- Kennard, O.I.N.; Motherwell, W.D.S.; Coppola, J.C.; Wampler, D.L.; Larson, A.C.; Watson, D.G. The crystal and molecular structure of adenosine triphosphate. Proc. R. Soc Lond. Ser. A Math. Phys. Sci. 1971, 325, 401–436. [Google Scholar]
- Mogami, G.; Wazawa, T.; Morimoto, N.; Kodama, T.; Suzuki, M. Hydration properties of adenosine phosphate series as studied by microwave dielectric spectroscopy. Biophys. Chem. 2011, 154, 1–7. [Google Scholar] [CrossRef]
- Friedrich, T.; Steinmüller, K.; Weiss, H. The proton-pumping respiratory complex I of bacteria and mitochondria and its homologue in chloroplasts. FEBS Lett. 1995, 367, 107–111. [Google Scholar] [CrossRef]
- Rice, A.M.; Rosen, M.K. ATP controls the crowd. Science 2017, 356, 701–702. [Google Scholar] [CrossRef]
- Parry, B.R.; Surovtsev, I.V.; Cabeen, M.T.; O’Hern, C.S.; Dufresne, E.R.; Jacobs-Wagner, C. The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell 2014, 156, 183–194. [Google Scholar] [CrossRef]
- Cyr, D.M. Cooperation of the molecular chaperone Ydj1 with specific Hsp70 homologs to suppress protein aggregation. FEBS Lett. 1995, 359, 129–132. [Google Scholar] [PubMed]
- Biswas, A.; Das, K.P. Role of ATP on the interaction of alpha-crystallin with its substrates and its implications for the molecular chaperone function. J. Biol. Chem. 2004, 279, 42648–42657. [Google Scholar] [CrossRef]
- Sharma, K.K.; Santhoshkumar, P. Lens aging: Effects of crystallins. Biochim. Biophys. Acta 2009, 1790, 1095–1108. [Google Scholar] [CrossRef]
- Greiner, J.V.; Chylack, L.T., Jr. Posterior subcapsular cataracts: Histopathologic study of steroid-associated cataracts. Arch. Ophthalmol. 1979, 97, 135–144. [Google Scholar] [CrossRef]
- Heier, J.S.; Topping, T.M.; Baumann, W.; Dirks, M.S.; Chern, S. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology 2000, 107, 2034–2038; discussion 2039. [Google Scholar] [CrossRef]
- Mulhern, M.L.; Madson, C.J.; Danford, A.; Ikesugi, K.; Kador, P.F.; Shinohara, T. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3951–3959. [Google Scholar] [CrossRef]
- Berthoud, V.M.; Beyer, E.C. Oxidative stress, lens gap junctions, and cataracts. Antioxid. Redox Signal. 2009, 11, 339–353. [Google Scholar] [CrossRef]
- Prokofyeva, E.; Wegener, A.; Zrenner, E. Cataract prevalence and prevention in Europe: A literature review. Acta Ophthalmol. 2013, 91, 395–405. [Google Scholar] [CrossRef]
- Lee, C.M.; Afshari, N.A. The global state of cataract blindness. Curr. Opin. Ophthalmol. 2017, 28, 98–103. [Google Scholar] [CrossRef]
- Congdon, N.G.; Friedman, D.S.; Lietman, T. Important causes of visual impairment in the world today. JAMA 2003, 290, 2057–2060. [Google Scholar] [CrossRef]
- Asbell, P.A.; Dualan, I.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-related cataract. Lancet 2005, 365, 599–609. [Google Scholar] [CrossRef]
- Goodman, D.F.; Stark, W.J.; Gottsch, J.D. Complications of cataract extraction with intraocular lens implantation. Ophthalmic Surg. 1989, 20, 132–140. [Google Scholar] [CrossRef]
- Chen, X.J.; Hu, L.D.; Yao, K.; Yan, Y.B. Lanosterol and 25-hydroxycholesterol dissociate crystallin aggregates isolated from cataractous human lens via different mechanisms. Biochem. Biophys. Res. Commun. 2018, 506, 868–873. [Google Scholar] [CrossRef]
- Xi, Y.B.; Chen, X.J.; Zhao, W.J.; Yan, Y.B. Congenital Cataract-Causing Mutation G129C in γC-Crystallin Promotes the Accumulation of Two Distinct Unfolding Intermediates That Form Highly Toxic Aggregates. J. Mol. Biol. 2015, 427, 2765–2781. [Google Scholar] [CrossRef]
- Evans, P.; Wyatt, K.; Wistow, G.J.; Bateman, O.A.; Wallace, B.A.; Slingsby, C. The P23T cataract mutation causes loss of solubility of folded gammaD-crystallin. J. Mol. Biol. 2004, 343, 435–444. [Google Scholar] [CrossRef]
- Muranov, K.O.; Maloletkina, O.I.; Poliansky, N.B.; Markossian, K.A.; Kleymenov, S.Y.; Rozhkov, S.P.; Goryunov, A.S.; Ostrovsky, M.A.; Kurganov, B.I. Mechanism of aggregation of UV-irradiated β(L)-crystallin. Exp. Eye Res. 2011, 92, 76–86. [Google Scholar] [CrossRef]
- Zorić, L.M.D.; Kisic, B. Basic Review of the Oxidative Stress Role in Age-Related Cataractogensis. In Studies on the Cornea and Lens; Babizhayev, M.A., Li, D.W.C., Kasus-Jacobi, A., Zorić, L., Aliό, J.L., Eds.; Springer Science + Business Media: New York, NY, USA, 2015. [Google Scholar]
- Dragoi, V. Chapter 14: Visual Processing: Eye and Retina. In Neuroscience Online Section 2: Sensory Systems; The University of Texas Health Science Center at Houston: Houston, TX, USA, 2020. [Google Scholar]
- Hejtmancik, J.F. The genetics of cataract: Our vision becomes clearer. Am. J. Hum. Genet. 1998, 62, 520–525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Markossian, K.A.; Yudin, I.K.; Kurganov, B.I. Mechanism of suppression of protein aggregation by α-crystallin. Int. J. Mol. Sci. 2009, 10, 1314–1345. [Google Scholar] [CrossRef] [PubMed]
- Kopylova, L.V.; Cherepanov, I.V.; Snytnikova, O.A.; Rumyantseva, Y.V.; Kolosova, N.G.; Tsentalovich, Y.P.; Sagdeev, R.Z. Age-related changes in the water-soluble lens protein composition of Wistar and accelerated-senescence OXYS rats. Mol. Vis. 2011, 17, 1457–1467. [Google Scholar] [PubMed]
- Kumari, P.S.R.; Thankappan, B. Kumarasamy Oxidative stress in applied basic research and clinical practice. In Studies on the Cornea and Lens; Babizhayev, M.A., Li, D.W.-C., Kasus-Jacobi, A., Zorić, L., Aliό, J.L., Eds.; Springer Science + Business Media: New York, NY, USA, 2015. [Google Scholar]
- Lam, D.; Rao, S.K.; Ratra, V.; Liu, Y.; Mitchell, P.; King, J.; Tassignon, M.-J.; Jonas, J.; Pang, C.P.; Chang, D.F. Cataract. Nat. Rev. Dis. Primers 2015, 1, 15014. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, R.A. Drug Discovery. A new dawn for cataracts. Science 2015, 350, 636–637. [Google Scholar] [PubMed]
- Skinner, C.; Miraldi Utz, V. Pharmacological approaches to restoring lens transparency: Real world applications. Ophthalmic Genet. 2017, 38, 201–205. [Google Scholar] [CrossRef]
- Makley, L.N.; McMenimen, K.A.; DeVree, B.T.; Goldman, J.W.; McGlasson, B.N.; Rajagopal, P.; Dunyak, B.M.; McQuade, T.J.; Thompson, A.D.; Sunahara, R.; et al. Pharmacological chaperone for α-crystallin partially restores transparency in cataract models. Science 2015, 350, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, X.J.; Zhu, J.; Xi, Y.B.; Yang, X.; Hu, L.D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol reverses protein aggregation in cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef]
- Xu, J.; Fu, Q.; Chen, X.; Yao, K. Advances in pharmacotherapy of cataracts. Ann. Transl. Med. 2020, 8, 1552. [Google Scholar] [CrossRef]
- Hu, L.D.; Wang, J.; Chen, X.J.; Yan, Y.B. Lanosterol modulates proteostasis via dissolving cytosolic sequestosomes/aggresome-like induced structures. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118617. [Google Scholar] [CrossRef] [PubMed]
- Fricke, T.R.; Tahhan, N.; Resnikoff, S.; Papas, E.; Burnett, A.; Ho, S.M.; Naduvilath, T.; Naidoo, K.S. Global Prevalence of Presbyopia and Vision Impairment from Uncorrected Presbyopia: Systematic Review, Meta-analysis, and Modelling. Ophthalmology 2018, 125, 1492–1499. [Google Scholar] [CrossRef]
- Hickenbotham, A.; Roorda, A.; Steinmaus, C.; Glasser, A. Meta-analysis of sex differences in presbyopia. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3215–3220. [Google Scholar] [CrossRef]
- Weale, R.A. Epidemiology of refractive errors and presbyopia. Surv. Ophthalmol. 2003, 48, 515–543. [Google Scholar] [CrossRef]
- Frick, K.D.; Joy, S.M.; Wilson, D.A.; Naidoo, K.S.; Holden, B.A. The Global Burden of Potential Productivity Loss from Uncorrected Presbyopia. Ophthalmology 2015, 122, 1706–1710. [Google Scholar] [CrossRef]
- Katz, J.A.; Karpecki, P.M.; Dorca, A.; Chiva-Razavi, S.; Floyd, H.; Barnes, E.; Wuttke, M.; Donnenfeld, E. Presbyopia–A Review of Current Treatment Options and Emerging Therapies. Clin. Ophthalmol. 2021, 15, 2167–2178. [Google Scholar] [CrossRef]
- Heys, K.R.; Friedrich, M.G.; Truscott, R.J. Presbyopia and heat: Changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging Cell. 2007, 6, 807–815. [Google Scholar] [CrossRef]
- Drazic, A.; Myklebust, L.M.; Ree, R.; Arnesen, T. The world of protein acetylation. Biochim. Biophys. Acta 2016, 1864, 1372–1401. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, R.H.; Linetsky, M.; Stitt, A.W. The pathogenic role of Maillard reaction in the aging eye. Amino Acids 2012, 42, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Wilmarth, P.A.; Tanner, S.; Dasari, S.; Nagalla, S.R.; Riviere, M.A.; Bafna, V.; Pevzner, P.A.; David, L.L. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: Does deamidation contribute to crystallin insolubility? J. Proteome Res. 2006, 5, 2554–2566. [Google Scholar] [CrossRef] [PubMed]
- Boscia, F.; Grattagliano, I.; Vendemiale, G.; Micelli-Ferrari, T.; Altomare, E. Protein oxidation and lens opacity in humans. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2461–2465. [Google Scholar]
- Hooi, M.Y.; Raftery, M.J.; Truscott, R.J. Racemization of two proteins over our lifespan: Deamidation of asparagine 76 in γS crystallin is greater in cataract than in normal lenses across the age range. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3554–3561. [Google Scholar] [CrossRef] [PubMed]
- Korlimbinis, A.; Berry, Y.; Thibault, D.; Schey, K.L.; Truscott, R.J. Protein aging: Truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Exp. Eye Res. 2009, 88, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Garner, W.H.; Garner, M.H. Protein Disulfide Levels and Lens Elasticity Modulation: Applications for Presbyopia. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2851–2863. [Google Scholar] [CrossRef]
- Korenfeld, M.S.; Robertson, S.M.; Stein, J.M.; Evans, D.G.; Rauchman, S.H.; Sall, K.N.; Venkataraman, S.; Chen, B.-L.; Wuttke, M.; Burns, W. Topical lipoic acid choline ester eye drop for improvement of near visual acuity in subjects with presbyopia: A safety and preliminary efficacy trial. Eye 2021, 35, 3292–3301. [Google Scholar] [CrossRef]
- Zhang, H.; Singh, M.; Nair, A.; Larin, K.V.; Aglyamov, S.R. Elasticity Changes in the Crystalline Lens during Oxidative Damage and the Antioxidant Effect of Alpha-Lipoic Acid Measured by Optical Coherence Elastography. Photonics 2021, 8, 207. [Google Scholar] [CrossRef]
- Panja, S.; Gaikwad, H.; Rankenberg, J.; Nam, M.H.; Nagaraj, R.H. Promotion of Protein Solubility and Reduction in Stiffness in Human Lenses by Aggrelyte-1: Implications for Reversing Presbyopia. Int. J. Mol. Sci. 2023, 24, 2196. [Google Scholar] [CrossRef] [PubMed]
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef] [PubMed]
- Gordois, A.; Cutler, H.; Pezzullo, L.; Gordon, K.; Cruess, A.; Winyard, S.; Hamilton, W.; Chua, K. An estimation of the worldwide economic and health burden of visual impairment. Glob. Public Health 2012, 7, 465–481. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Klaver, C.C.; Assink, J.J.; van Leeuwen, R.; Wolfs, R.C.; Vingerling, J.R.; Stijnen, T.; Hofman, A.; De Jong, P.T. Incidence and progression rates of age-related maculopathy: The Rotterdam Study. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2237–2241. [Google Scholar]
- Wong, T.Y.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The natural history and prognosis of neovascular age-related macular degeneration: A systematic review of the literature and meta-analysis. Ophthalmology 2008, 115, 116–126. [Google Scholar] [CrossRef]
- Stahl, A. The Diagnosis and Treatment of Age-Related Macular Degeneration. Dtsch. Arztebl. Int. 2020, 117, 513–520. [Google Scholar] [CrossRef]
- Fine, S.L.; Berger, J.W.; Maguire, M.G.; Ho, A.C. Age-related macular degeneration. N. Engl. J. Med. 2000, 342, 483–492. [Google Scholar] [CrossRef]
- Winkler, B.S.; Boulton, M.E.; Gottsch, J.D.; Sternberg, P. Oxidative damage and age-related macular degeneration. Mol. Vis. 1999, 5, 32. [Google Scholar]
- Handa, J.T. How does the macula protect itself from oxidative stress? Mol. Asp. Med. 2012, 33, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Feeney-Burns, L.; Eldred, G.E. The fate of the phagosome: Conversion to ‘age pigment’ and impact in human retinal pigment epithelium. Trans. Ophthalmol. Soc. UK 1983, 103 Pt 4, 416–421. [Google Scholar] [PubMed]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [PubMed]
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greiner, J.V.; Glonek, T. Adenosine Triphosphate (ATP) and Protein Aggregation in Age-Related Vision-Threatening Ocular Diseases. Metabolites 2023, 13, 1100. https://doi.org/10.3390/metabo13101100
Greiner JV, Glonek T. Adenosine Triphosphate (ATP) and Protein Aggregation in Age-Related Vision-Threatening Ocular Diseases. Metabolites. 2023; 13(10):1100. https://doi.org/10.3390/metabo13101100
Chicago/Turabian StyleGreiner, Jack V., and Thomas Glonek. 2023. "Adenosine Triphosphate (ATP) and Protein Aggregation in Age-Related Vision-Threatening Ocular Diseases" Metabolites 13, no. 10: 1100. https://doi.org/10.3390/metabo13101100
APA StyleGreiner, J. V., & Glonek, T. (2023). Adenosine Triphosphate (ATP) and Protein Aggregation in Age-Related Vision-Threatening Ocular Diseases. Metabolites, 13(10), 1100. https://doi.org/10.3390/metabo13101100






