Simultaneous Quantification of Serum Lipids and Their Association with Type 2 Diabetes Mellitus-Positive Hepatocellular Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Study Population
2.3. Measurement of Clinical Parameters
2.4. Sample Preparation
2.5. LC-MS/MS Analysis
2.6. Method Validation
2.7. Statistical Analysis
3. Results
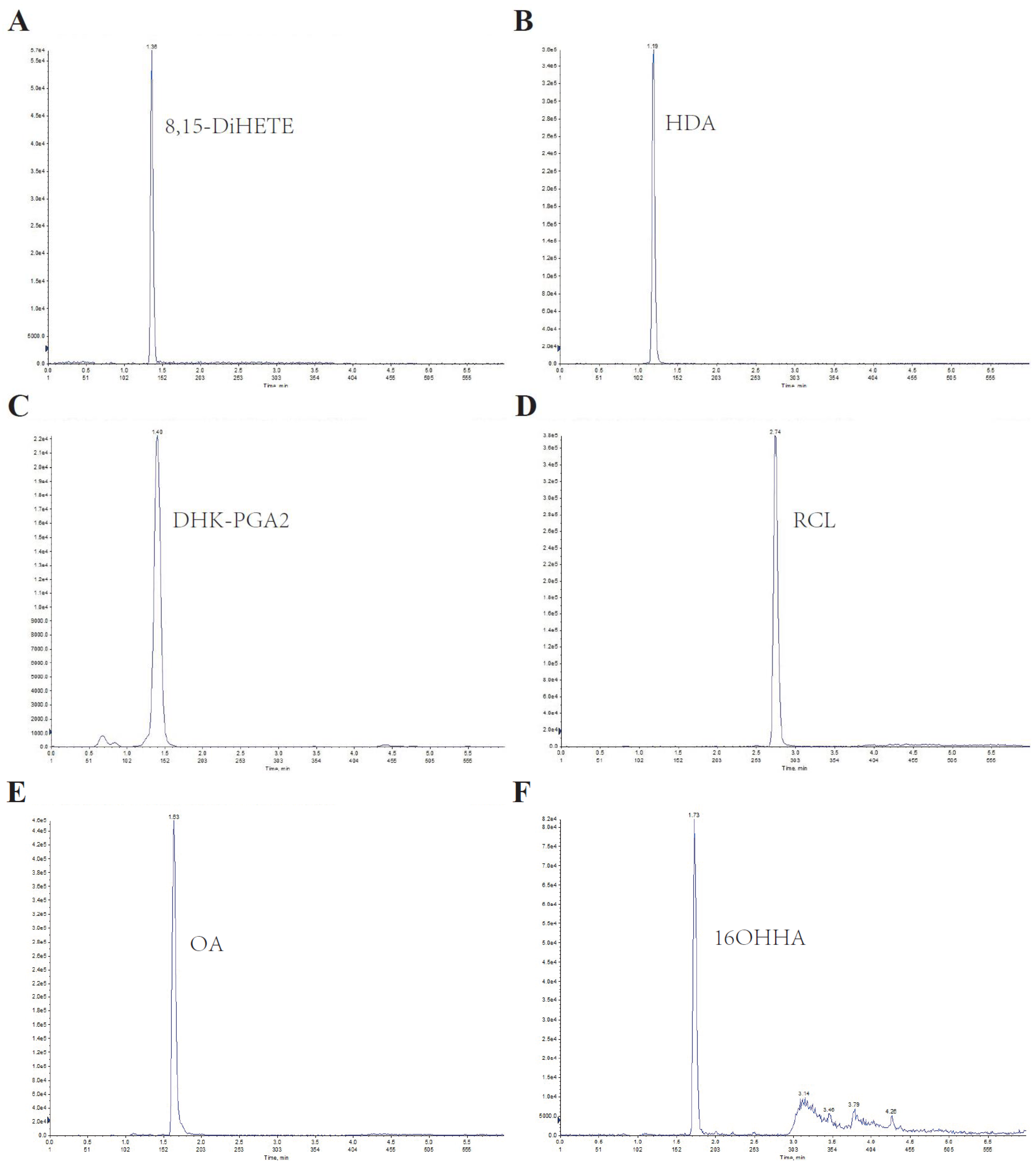
3.1. Method Development and Validation
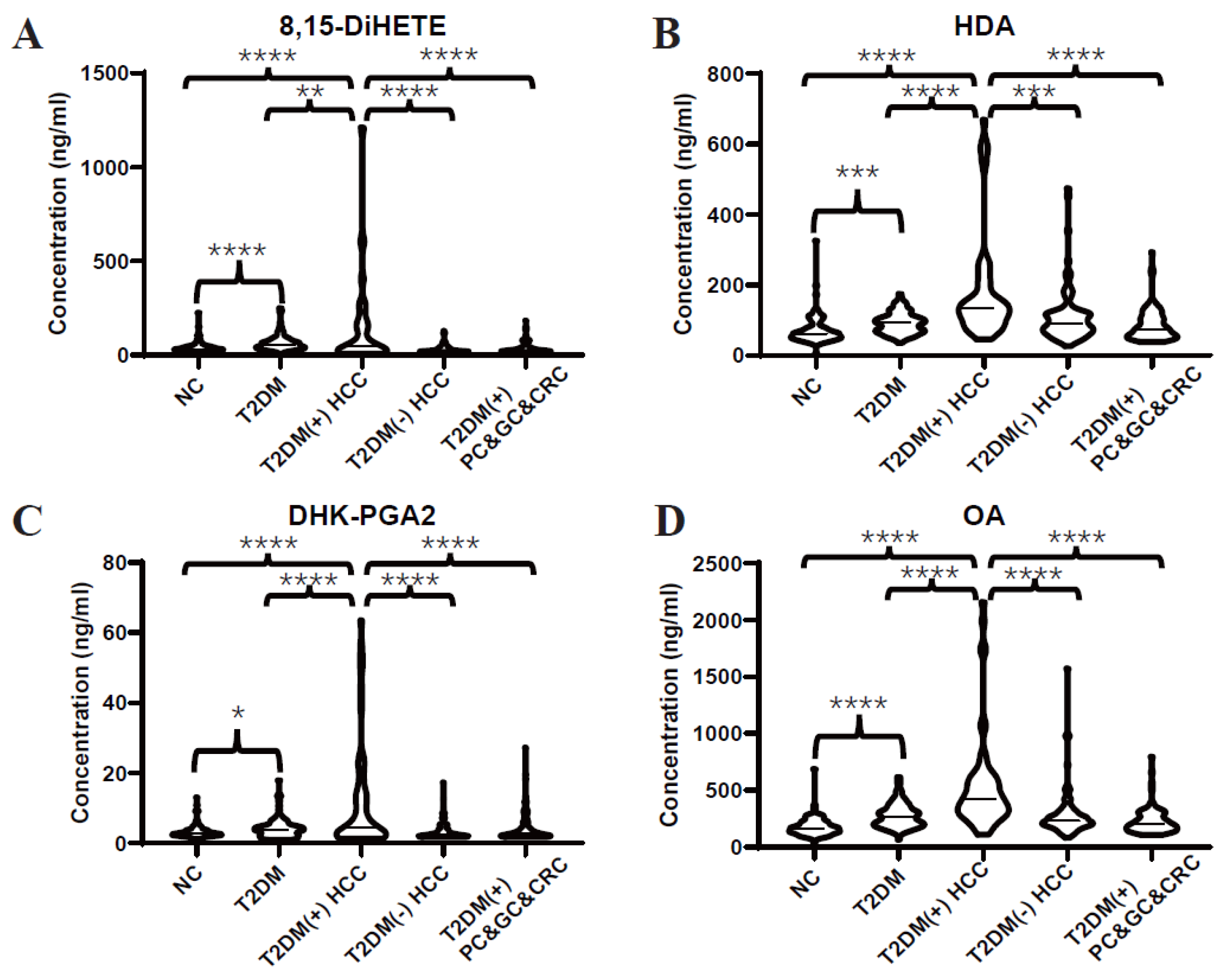
3.2. Validation of Dysregulated Lipids by Targeted LC-MS/MS Analyses
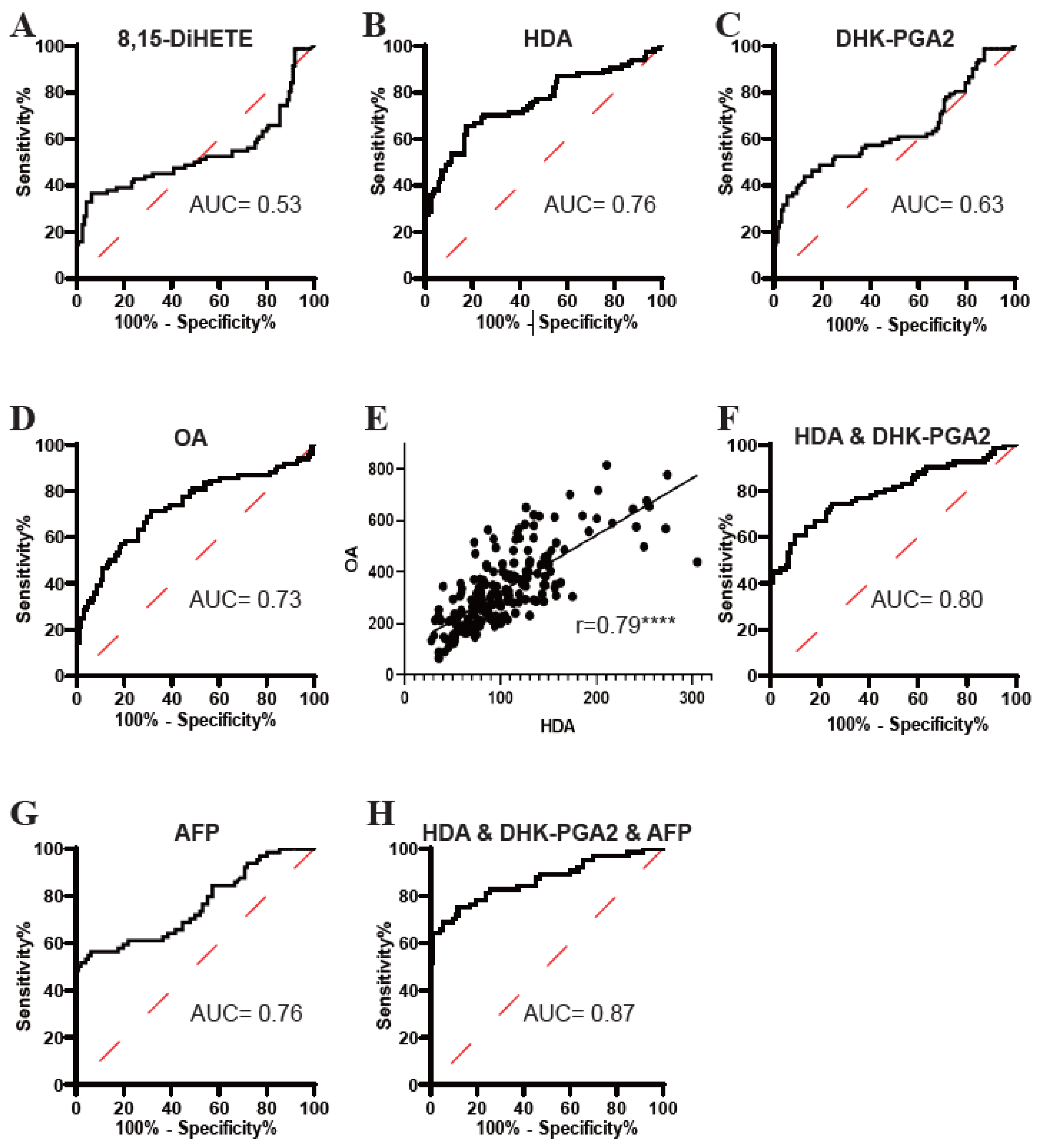
3.3. Evaluation of the Diagnostic Efficacy of the Targeted Lipids
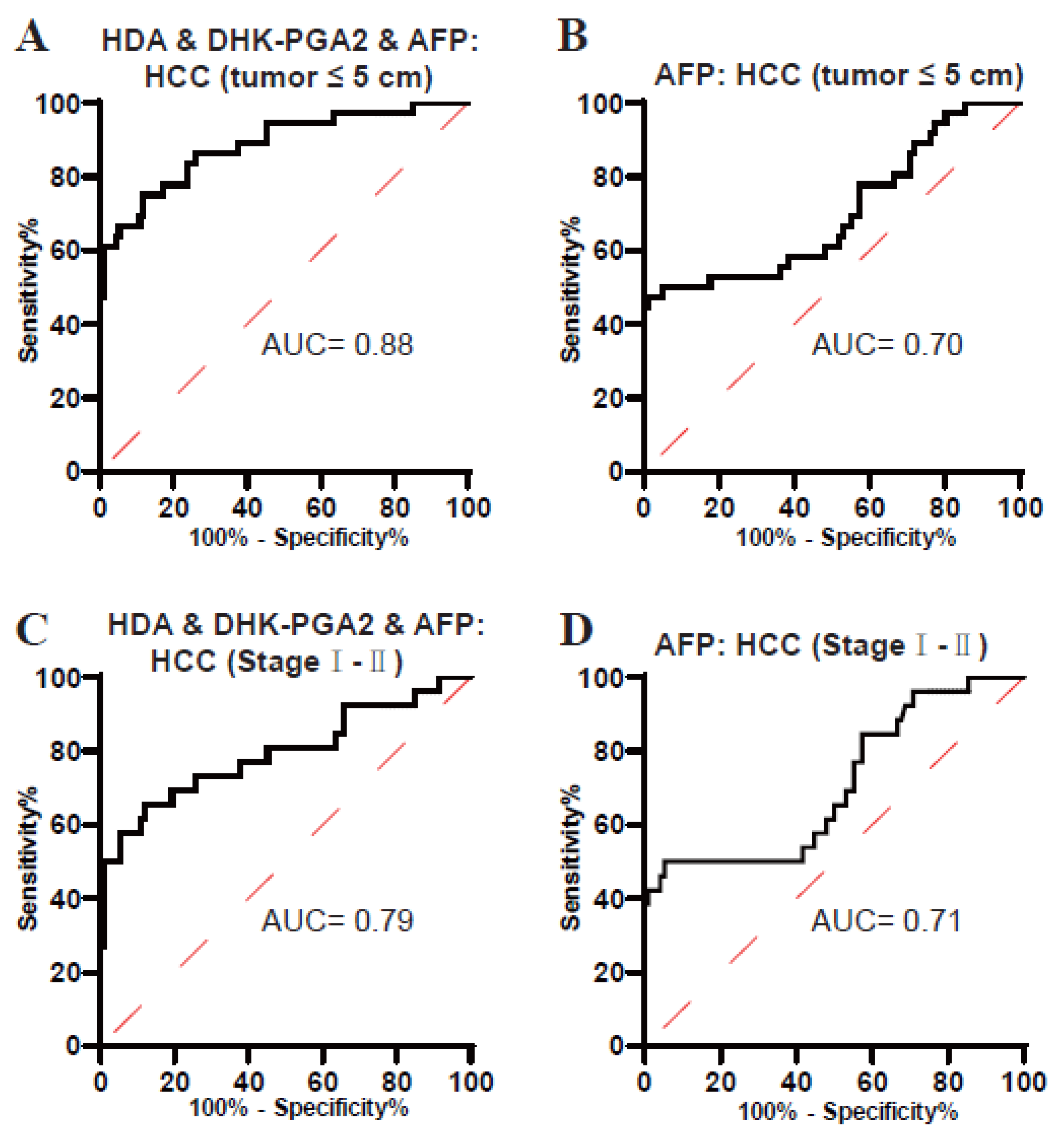
3.4. Clinical Value of the Biomarker Signature for Small-Size and Early-Stage T2DM(+) HCC
3.5. The Diagnostic Specificity of the Biomarker Signature
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Giannini, E.G.; Farinati, F.; Ciccarese, F.; Pecorelli, A.; Rapaccini, G.L.; Di Marco, M.; Benvegnu, L.; Caturelli, E.; Zoli, M.; Borzio, F.; et al. Prognosis of untreated hepatocellular carcinoma. Hepatology 2015, 61, 184–190. [Google Scholar] [CrossRef]
- Koh, W.P.; Wang, R.; Jin, A.; Yu, M.C.; Yuan, J.M. Diabetes mellitus and risk of hepatocellular carcinoma: Findings from the Singapore Chinese Health Study. Br. J. Cancer 2013, 108, 1182–1188. [Google Scholar] [CrossRef]
- Kasmari, A.J.; Welch, A.; Liu, G.; Leslie, D.; McGarrity, T.; Riley, T. Independent of Cirrhosis, Hepatocellular Carcinoma Risk Is Increased with Diabetes and Metabolic Syndrome. Am. J. Med. 2017, 130, 746.e1–746.e7. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef]
- Jia, W.; Weng, J.; Zhu, D.; Ji, L.; Lu, J.; Zhou, Z.; Zou, D.; Guo, L.; Ji, Q.; Chen, L.; et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab. Res. Rev. 2019, 35, e3158. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 2015, 350, g7607. [Google Scholar] [CrossRef]
- Suh, S.; Kim, K.W. Diabetes and Cancer: Cancer Should Be Screened in Routine Diabetes Assessment. Diabetes Metab. J. 2019, 43, 733–743. [Google Scholar] [CrossRef]
- Kanwal, F.; Singal, A.G. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology 2019, 157, 54–64. [Google Scholar] [CrossRef]
- Satriano, L.; Lewinska, M.; Rodrigues, P.M.; Banales, J.M.; Andersen, J.B. Metabolic rearrangements in primary liver cancers: Cause and consequences. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 748–766. [Google Scholar] [CrossRef]
- Sangineto, M.; Villani, R.; Cavallone, F.; Romano, A.; Loizzi, D.; Serviddio, G. Lipid Metabolism in Development and Progression of Hepatocellular Carcinoma. Cancers 2020, 12, 1419. [Google Scholar] [CrossRef]
- Fitian, A.I.; Nelson, D.R.; Liu, C.; Xu, Y.; Ararat, M.; Cabrera, R. Integrated metabolomic profiling of hepatocellular carcinoma in hepatitis C cirrhosis through GC/MS and UPLC/MS-MS. Liver Int. 2014, 34, 1428–1444. [Google Scholar] [CrossRef]
- Gao, R.; Cheng, J.; Fan, C.; Shi, X.; Cao, Y.; Sun, B.; Ding, H.; Hu, C.; Dong, F.; Yan, X. Serum Metabolomics to Identify the Liver Disease-Specific Biomarkers for the Progression of Hepatitis to Hepatocellular Carcinoma. Sci. Rep. 2015, 5, 18175. [Google Scholar] [CrossRef]
- Luo, P.; Yin, P.; Hua, R.; Tan, Y.; Li, Z.; Qiu, G.; Yin, Z.; Xie, X.; Wang, X.; Chen, W.; et al. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology 2018, 67, 662–675. [Google Scholar] [CrossRef]
- Han, J.; Han, M.L.; Xing, H.; Li, Z.L.; Yuan, D.Y.; Wu, H.; Zhang, H.; Wang, M.D.; Li, C.; Liang, L.; et al. Tissue and serum metabolomic phenotyping for diagnosis and prognosis of hepatocellular carcinoma. Int. J. Cancer 2020, 146, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.L.; Han, Y.; Pei, L.; Yue, Z.H.; Liu, B.Y.; Cui, J.W.; Jia, M.; Wang, H. A Serum Metabolite Classifier for the Early Detection of Type 2 Diabetes Mellitus-Positive Hepatocellular Cancer. Metabolites 2022, 12, 610. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, H.; Wang, Z.; Cong, W.; Wang, J.; Zeng, M.; Zhou, W.; Bie, P.; Liu, L.; Wen, T.; et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer 2020, 9, 682–720. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Singh, M.K.; Das, B.K.; Choudhary, S.; Gupta, D.; Patil, U.K. Diabetes and hepatocellular carcinoma: A pathophysiological link and pharmacological management. Biomed Pharm. 2018, 106, 991–1002. [Google Scholar] [CrossRef]
- Wainwright, P.; Scorletti, E.; Byrne, C.D. Type 2 Diabetes and Hepatocellular Carcinoma: Risk Factors and Pathogenesis. Curr. Diab. Rep. 2017, 17, 20. [Google Scholar] [CrossRef]
- Xia, H.; Chen, J.; Sekar, K.; Shi, M.; Xie, T.; Hui, K.M. Clinical and metabolomics analysis of hepatocellular carcinoma patients with diabetes mellitus. Metabolomics 2019, 15, 156. [Google Scholar] [CrossRef]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2021, 218, e20201606. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.M. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef]
- Calvisi, D.F.; Wang, C.; Ho, C.; Ladu, S.; Lee, S.A.; Mattu, S.; Destefanis, G.; Delogu, S.; Zimmermann, A.; Ericsson, J.; et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology 2011, 140, 1071–1083. [Google Scholar] [CrossRef]
- Schug, Z.T.; Vande Voorde, J.; Gottlieb, E. The metabolic fate of acetate in cancer. Nat. Rev. Cancer 2016, 16, 708–717. [Google Scholar] [CrossRef]
- Bi, J.; Ichu, T.A.; Zanca, C.; Yang, H.; Zhang, W.; Gu, Y.; Chowdhry, S.; Reed, A.; Ikegami, S.; Turner, K.M.; et al. Oncogene Amplification in Growth Factor Signaling Pathways Renders Cancers Dependent on Membrane Lipid Remodeling. Cell Metab. 2019, 30, 525–538.e8. [Google Scholar] [CrossRef]
- Gimple, R.C.; Kidwell, R.L.; Kim, L.J.Y.; Sun, T.; Gromovsky, A.D.; Wu, Q.; Wolf, M.; Lv, D.; Bhargava, S.; Jiang, L.; et al. Glioma Stem Cell-Specific Superenhancer Promotes Polyunsaturated Fatty-Acid Synthesis to Support EGFR Signaling. Cancer Discov. 2019, 9, 1248–1267. [Google Scholar] [CrossRef]
- Banales, J.M.; Inarrairaegui, M.; Arbelaiz, A.; Milkiewicz, P.; Muntane, J.; Munoz-Bellvis, L.; La Casta, A.; Gonzalez, L.M.; Arretxe, E.; Alonso, C.; et al. Serum Metabolites as Diagnostic Biomarkers for Cholangiocarcinoma, Hepatocellular Carcinoma, and Primary Sclerosing Cholangitis. Hepatology 2019, 70, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.B.; Kim, Y.B.; Lee, J.C.; Moon, M.H. Optimisation of high-speed lipidome analysis by nanoflow ultrahigh-performance liquid chromatography-tandem mass spectrometry: Application to identify candidate biomarkers for four different cancers. J. Chromatogr. B Anal. Technol. Biomed Life Sci. 2021, 1175, 122739. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, G.B.; Lee, J.C.; Moon, M.H. High-Speed Screening of Lipoprotein Components Using Online Miniaturized Asymmetrical Flow Field-Flow Fractionation and Electrospray Ionization Tandem Mass Spectrometry: Application to Hepatocellular Carcinoma Plasma Samples. Anal. Chem. 2021, 93, 4867–4875. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.B.; Lee, J.C.; Moon, M.H. Plasma lipid profile comparison of five different cancers by nanoflow ultrahigh performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2019, 1063, 117–126. [Google Scholar] [CrossRef]
- Li, Y.; Lin, N.; Xu, J.; Lu, Y.; Chen, S.; Pan, C.; Wang, C.; Xu, M.; Zhou, B.; Xu, R.; et al. Measurement of Serum and Hepatic Eicosanoids by Liquid Chromatography Tandem-Mass Spectrometry (LC-MS/MS) in a Mouse Model of Hepatocellular Carcinoma (HCC) with Delivery of c-Met and Activated beta-Catenin by Hepatocyte Hydrodynamic Injection. Med. Sci. Monit. 2018, 24, 1670–1679. [Google Scholar] [CrossRef]
- Santockyte, R.; Kandoussi, H.; Chen, W.; Zheng, N.; Venkatarangan, L.; Gan, J.; Shen, H.; Bonacorsi, S.J.; Easter, J.; Burrell, R.; et al. LC-MS/MS bioanalysis of plasma 1, 14-tetradecanedioic acid and 1, 16-hexadecanedioic acid as candidate biomarkers for organic anion-transporting polypeptide mediated drug-drug interactions. Bioanalysis 2018, 10, 1473–1485. [Google Scholar] [CrossRef]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef]


| Discovery Set | Test Set | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | T2DM | T2DM(+) HCC | NC | T2DM | T2DM(+) HCC | T2DM(−) HCC | T2DM(+) CRC | T2DM(+) PC | T2DM(+) GC |
| N = 32 | N = 19 | N = 94 | N = 96 | N = 64 | N = 90 | N = 44 | N = 22 | N = 21 | |
| Age | 56.47 ± 11.37 | 64.32 ± 9.21 | 43.56 ± 15.02 | 55.15 ± 12.92 | 61.67 ± 9.15 | 56.88 ± 12.68 | 68.55 ± 9.99 | 68.86 ± 8.13 | 67.90 ± 11.61 |
| Gender Male/Female | 20/12 | 17/2 | 32/62 | 65/31 | 54/10 | 70/20 | 27/17 | 15/7 | 14/7 |
| FBG (mmol/L) | 7.43 ± 1.43 | 9.75 ± 4.94 | 4.99 ± 0.42 | 8.17 ± 2.37 | 8.30 ± 3.45 | 5.07 ± 0.56 | 7.65 ± 2.01 | 7.93 ± 2.45 | 8.85 ± 4.34 |
| AFP >7/≤7 ng/mL | 3/29 | 11/8 | 2/92 | 6/90 | 37/27 | 55/35 | 2/42 | 3/19 | 4/17 |
| Precisions | Linear Range (ng/mL) | Regression Coefficient (R2) | Recoveries | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | ||||||||
| Low | High | Low | High | Low | Median | High | |||
| 8,15-DiHETE | 2.05% | 1.91% | 1.72% | 7.05% | 3.125–1600 | ≥0.9989 | 96.83% | 96.45% | 92.08% |
| HDA | 2.45% | 3.05% | 1.84% | 2.52% | 6.25–800 | ≥0.9990 | 103.71% | 102.70% | 104.40% |
| DHK-PGA2 | 1.31% | 2.87% | 3.86% | 9.49% | 0.78125–200 | ≥0.9982 | 98.93% | 97.40% | 93.00% |
| RCL | 1.27% | 0.30% | 1.45% | 7.42% | 6.25–800 | ≥0.9992 | 97.04% | 95.42% | 91.87% |
| OA | 2.25% | 1.34% | 2.37% | 5.21% | 25–3200 | ≥0.9981 | 97.62% | 94.36% | 86.45% |
| 16OHHA | 1.87% | 2.25% | 2.02% | 5.31% | 3.125–400 | ≥0.9989 | 95.98% | 94.95% | 93.88% |
| Metabolite | MS2 Score | VIP | p Value | FC | Log_FC |
|---|---|---|---|---|---|
| Pregnanetriol | 0.7622 | 2.0363 | 0.0000 | 0.4133 | −1.2746 |
| PC(18:4(6Z,9Z,12Z,15Z)/18:1(11Z)) | 0.7082 | 1.4334 | 0.0136 | 0.6198 | −0.6901 |
| N-Cyclopropyl-trans-2-cis-6-nonadienamide | 0.9400 | 2.7859 | 0.0000 | 0.6872 | −0.5411 |
| 6beta-Hydroxytestosterone | 0.8066 | 1.2485 | 0.0389 | 0.7675 | −0.3817 |
| PC(22:5(7Z,10Z,13Z,16Z,19Z)/18:2(9Z,12Z)) | 0.7928 | 1.2568 | 0.0256 | 0.7891 | −0.3417 |
| PC(22:5(7Z,10Z,13Z,16Z,19Z)/16:0) | 0.6836 | 1.5313 | 0.0114 | 0.8529 | −0.2296 |
| Pelargonic acid | 0.9999 | 1.2747 | 0.0178 | 1.1232 | 0.1676 |
| PC(22:5(7Z,10Z,13Z,16Z,19Z)/P-16:0) | 0.8313 | 1.0545 | 0.0290 | 1.3449 | 0.4275 |
| (E)-2,6-Dimethyl-2,5-heptadienoic acid | 0.6134 | 1.5895 | 0.0005 | 1.3755 | 0.4600 |
| PC(20:3(8Z,11Z,14Z)/20:1(11Z)) | 0.8495 | 1.0410 | 0.0146 | 1.3959 | 0.4812 |
| PC(P-18:1(11Z)/22:5(4Z,7Z,10Z,13Z,16Z)) | 0.8300 | 1.2593 | 0.0026 | 1.3976 | 0.4829 |
| PC(P-16:0/16:0) | 0.6759 | 1.6758 | 0.0073 | 1.4797 | 0.5653 |
| Azelaic acid | 0.6424 | 1.3805 | 0.0056 | 1.4960 | 0.5812 |
| PC(P-18:0/22:4(7Z,10Z,13Z,16Z)) | 0.8381 | 1.7603 | 0.0004 | 1.5029 | 0.5878 |
| 1-O-Hexadecyl-2-O-dihomogammalinolenoylglycero-3-phosphocholine | 0.8612 | 1.9964 | 0.0010 | 1.6454 | 0.7184 |
| PC(18:2(9Z,12Z)/P-18:1(11Z)) | 0.7329 | 1.7922 | 0.0003 | 1.6477 | 0.7204 |
| 3-Hydroxyisovaleric acid | 0.9370 | 1.7349 | 0.0001 | 1.6689 | 0.7389 |
| Ethyl oleate | 0.7968 | 1.1517 | 0.0072 | 1.6872 | 0.7546 |
| PC(18:0/P-16:0) | 0.6660 | 2.3506 | 0.0003 | 1.7399 | 0.7990 |
| 16-Hydroxy hexadecanoic acid | 0.9732 | 1.8678 | 0.0020 | 2.0424 | 1.0302 |
| Octadecanedioic acid | 0.6481 | 1.0454 | 0.0340 | 2.1652 | 1.1145 |
| Ricinoleic acid | 0.9862 | 1.4726 | 0.0454 | 2.6921 | 1.4288 |
| 15-Keto-13,14-dihydroprostaglandin A2 | 0.8173 | 1.6023 | 0.0457 | 2.7710 | 1.4704 |
| Hexadecanedioic acid | 0.9007 | 2.0187 | 0.0158 | 5.2489 | 2.3920 |
| 8,15-DiHETE | 0.9765 | 1.5252 | 0.0114 | 7.9630 | 2.9933 |
| AUC (95%CI) | Sensitivity (%) | Specificity (%) | p-Value | |
|---|---|---|---|---|
| 8,15-DiHETE | 0.53 (0.44–0.62) | 39.56 | 93.60 | 0.4534 |
| HDA | 0.76 (0.67–0.83) | 65.48 | 82.40 | <0.0001 |
| DHK-PGA2 | 0.63 (0.55–0.71) | 43.90 | 87.30 | 0.0014 |
| OA | 0.73 (0.65–0.80) | 71.43 | 68.75 | <0.0001 |
| HDA & DHK-PGA2 | 0.80 (0.73–0.86) | 60.98 | 90.24 | <0.0001 |
| AFP | 0.76 (0.68–0.84) | 56.25 | 93.75 | <0.0001 |
| HDA & DHK-PGA2 & AFP | 0.87 (0.80–0.93) | 68.75 | 94.62 | <0.0001 |
| Groups | AUC (95%CI) | Sensitivity (%) | Specificity (%) | p-Value | |
|---|---|---|---|---|---|
| HCC (≤5 cm) vs. T2DM | |||||
| AFP | 0.70 (0.59–0.81) | 47.22 | 98.96 | 0.0004 | |
| The Biomarker Signature | 0.88 (0.81–0.95) | 75.00 | 88.17 | <0.0001 | |
| HCC (I-II) vs. T2DM | |||||
| AFP | 0.71 (0.58–0.83) | 50.00 | 94.79 | 0.0013 | |
| The Biomarker Signature | 0.79 (0.68–0.91) | 65.38 | 88.17 | <0.0001 | |
| AUC (95%CI) | Sensitivity (%) | Specificity (%) | p-Value | |
|---|---|---|---|---|
| AFP | 0.79(0.72–0.87) | 56.25 | 89.66 | <0.0001 |
| The Biomarker Signature | 0.88(0.83–0.94) | 68.75 | 94.12 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Z.; Pei, L.; Meng, G.; Zhang, A.; Li, M.; Jia, M.; Wang, H.; Cao, L. Simultaneous Quantification of Serum Lipids and Their Association with Type 2 Diabetes Mellitus-Positive Hepatocellular Cancer. Metabolites 2023, 13, 90. https://doi.org/10.3390/metabo13010090
Yue Z, Pei L, Meng G, Zhang A, Li M, Jia M, Wang H, Cao L. Simultaneous Quantification of Serum Lipids and Their Association with Type 2 Diabetes Mellitus-Positive Hepatocellular Cancer. Metabolites. 2023; 13(1):90. https://doi.org/10.3390/metabo13010090
Chicago/Turabian StyleYue, Zhihong, Lin Pei, Guangyan Meng, Aimin Zhang, Meng Li, Mei Jia, Hui Wang, and Linlin Cao. 2023. "Simultaneous Quantification of Serum Lipids and Their Association with Type 2 Diabetes Mellitus-Positive Hepatocellular Cancer" Metabolites 13, no. 1: 90. https://doi.org/10.3390/metabo13010090
APA StyleYue, Z., Pei, L., Meng, G., Zhang, A., Li, M., Jia, M., Wang, H., & Cao, L. (2023). Simultaneous Quantification of Serum Lipids and Their Association with Type 2 Diabetes Mellitus-Positive Hepatocellular Cancer. Metabolites, 13(1), 90. https://doi.org/10.3390/metabo13010090






