Serum Metabolites Characterization Produced by Cats CKD Affected, at the 1 and 2 Stages, before and after Renal Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Diet and Feeding Protocol
2.3. Sample Collection and Preparation
2.4. GC-MS, Acquisition, Processing Parameters, and Identification of Serum Metabolites
2.5. Statistical Analysis
3. Results
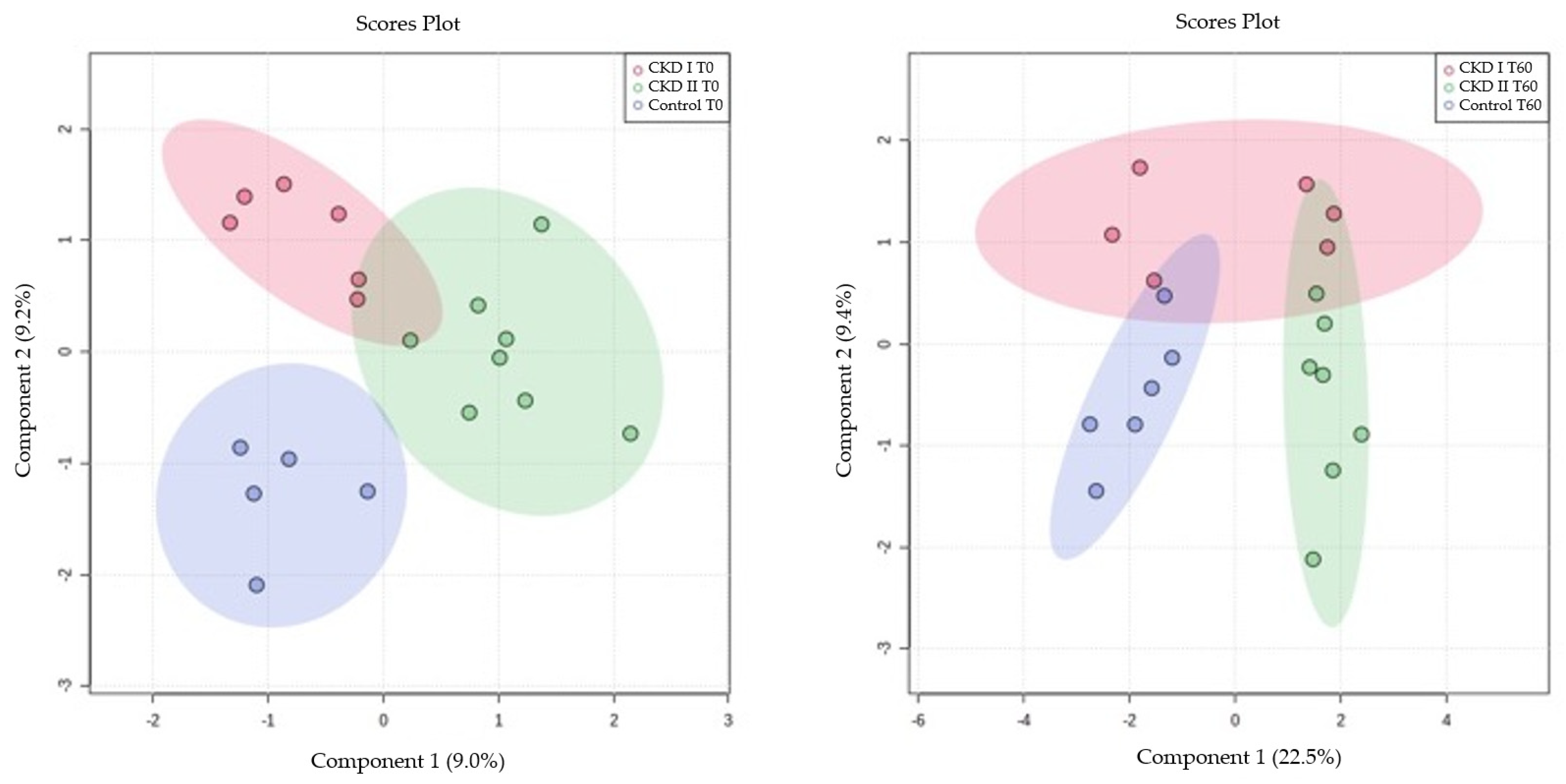
3.1. Partial Least Squares (PLS) for Serum Metabolomics Data at Baseline (T0) and after Sixty Days of Renal Diet (T60) between CKD Cats (Stages 1 and 2) and Control Group
3.2. Univariate Analysis for Serum Metabolomics Data at Baseline (T0) and after Sixty Days of Renal Diet (T60) between CKD Cats (Stages 1 and 2) and Control Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, C.A.; Elliott, J.; Schmiedt, C.W.; Brown, S.A. Chronic Kidney Disease in Aged Cats: Clinical Features, Morphology, and Proposed Pathogeneses. Vet. Pathol. 2016, 53, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Polzin, D.J. Chronic Kidney Disease in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Polzin, D.J. Chronic Kidney Disease. In Nephrology and Urology of Small Animals; Bartges, J., Polzin, D., Eds.; Wiley-Blackwell: West Sussex, UK, 2011; pp. 433–471. [Google Scholar]
- Bartges, J.W. Chronic Kidney Disease in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 669–692. [Google Scholar] [CrossRef]
- Reynolds, B.S.; Lefebvre, H.P. Feline CKD: Pathophysiology and risk factors-what do we know? J. Feline Med. Surg. 2013, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Finch, N.; Heiene, R. Early Detection of Chronic Kidney Disease. In BSAVA Manual of Canine and Feline Nephrology and Urology; Elliott, J., Grauer, G.F., Westropp, J.L., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2017; pp. 130–142. [Google Scholar]
- Polzin, D.J. Chronic Kidney Disease. In Textbook of Veterinary Internal Medicine; Ettinger, S.J., Feldman, E.C., Côté, E., Eds.; Elsevier: St Louis, MO, USA, 2017; pp. 4693–4734. [Google Scholar]
- Chew, D.J.; Dibartola, S.P.; Schenck, P. Canine and Feline Nephrology and Urology, 2nd ed.; Elsevier Saunders: St. Louis, Mo, USA, 2011; ISBN 978-0-7216-8178-8. [Google Scholar]
- IRIS, 2019 International Renal Interest Society. Available online: http://www.iris-kidney.com/pdf/IRIS_Staging_of_CKD_modified_2019.pdf (accessed on 17 January 2022).
- Weiss, R.H.; Kim, K. Metabolomics in the study of kidney diseases. Nat. Rev. Nephrol. 2012, 8, 22–33. [Google Scholar] [CrossRef]
- Rivera-Vélez, S.-M.; Villarino, N.F. Feline urine metabolomic signature: Characterization of low-molecular-weight substances in urine from domestic cats. J. Feline Med. Surg. 2018, 20, 155–163. [Google Scholar] [CrossRef]
- Yu, S.; Gross, K.; Allen, T. A renal food supplemented with vitamins E, C and beta-carotene reduces oxidative stress and improves kidney function in client-owned dogs with stages 2 or 3 kidney disease. J. Vet. Intern. Med. 2006, 20, 1537. [Google Scholar]
- Yu, S.; Paetau-Robinson, I. Dietary supplements of vitamins E and C and β-carotene reduce oxidative stress in cats with renal insufficiency. Vet. Res. Commun. 2006, 30, 403–413. [Google Scholar] [CrossRef]
- Brown, S.A. Oxidative Stress and Chronic Kidney Disease. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 157–166. [Google Scholar] [CrossRef]
- Halfen, D.P.; Caragelasco, D.S.; De Souza Nogueira, J.P.; Jeremias, J.T.; Pedrinelli, V.; Oba, P.M.; Ruberti, B.; Pontieri, C.F.F.; Kogika, M.M.; Brunetto, M.A. Evaluation of electrolyte concentration and pro-inflammatory and oxidative status in dogs with advanced chronic kidney disease under dietary treatment. Toxins 2019, 12, 3. [Google Scholar] [CrossRef]
- Brown, S.A.; Brown, C.A.; Crowell, W.A.; Barsanti, J.A.; Allen, T.; Cowell, C.; Finco, D.R. Beneficial effects of chronic administration of dietary ω-3 polyunsaturated fatty acids in dogs with renal insufficiency. J. Lab. Clin. Med. 1998, 131, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Polzin, D.J. Evidence-based step-wise approach to managing chronic kidney disease in dogs and cats. J. Vet. Emerg. Crit. Care 2013, 23, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, E.; Everts, H.; Kastelein, A.; Beynen, A. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets. Vet. Rec. 2005, 157, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Scherk, M.A.; Laflamme, D.P. Controversies in Veterinary Nephrology: Renal Diets Are Indicated for Cats with International Renal Interest Society Chronic Kidney Disease Stages 2 to 4: The Con View. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 1067–1094. [Google Scholar] [CrossRef]
- Polzin, D.; Osborne, C.; Hayden, D.; Stevens, J. Influence of reduced protein diets on morbidity, mortality, and renal function in dogs with induced chronic renal failure. Am. J. Vet. Res. 1984, 45, 506–517. [Google Scholar]
- Jacob, F.; Polzin, D.J.; Osborne, C.A.; Allen, T.A.; Kirk, C.A.; Neaton, J.D.; Lekcharoensuk, C.; Swanson, L.L. Clinical evaluation of dietary modification for treatment of spontaneous chronic renal failure in dogs. J. Am. Vet. Med. Assoc. 2002, 220, 1163–1170. [Google Scholar] [CrossRef]
- Ephraim, E.; Jewell, D.E. High Protein Consumption with Controlled Phosphorus Level Increases Plasma Concentrations of Uremic Toxins in Cats with Early Chronic Kidney Disease. Food Sci. Nutr. 2021, 7, 096. [Google Scholar] [CrossRef]
- Laflamme, D.P. Development and validation of a body condition score system for cats: A clinical tool. Feline Pract. 1997, 25, 13–18. [Google Scholar]
- Michel, K.E.; Anderson, W.; Cupp, C.; Laflamme, D.P. Correlation of a feline muscle mass score with body composition determined by dual-energy X-ray absorptiometry. Br. J. Nutr. 2011, 106, S57–S59. [Google Scholar] [CrossRef][Green Version]
- Vallejo, M.; García, A.; Tuñón, J.; García-Martínez, D.; Angulo, S.; Martin-Ventura, J.L.; Blanco-Colio, L.M.; Almeida, P.; Egido, J.; Barbas, C. Plasma fingerprinting with GC-MS in acute coronary syndrome. Anal. Bioanal. Chem. 2009, 394, 1517–1524. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass Spectral and Retention Index Libraries for Metabolomics Based on Quadrupole and Time-of-Flight Gas Chromatography/Mass Spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, A.; Ferrarini, A.; Rey-Stolle, F.; García, A.; Barbas, C. From sample treatment to biomarker discovery: A tutorial for untargeted metabolomics based on GC-(EI)-Q-MS. Anal. Chim. Acta 2015, 900, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Uribe, A.P.; Hernández-Cruz, E.Y.; Ramírez-Magaña, K.J.; Pedraza-Chaverri, J. Involvement of Tricarboxylic Acid Cycle Metabolites in Kidney Diseases. Biomolecules 2021, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.L.; Billings, F.T.; Bojanowski, M.T.; Gilliam, L.A.; Yu, C.; Roshanravan, B.; Roberts, L.J.; Himmelfarb, J.; Ikizler, T.A.; Brown, N.J. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol. Rep. 2016, 4, e12780. [Google Scholar] [CrossRef]
- Granata, S.; Zaza, G.; Simone, S.; Villani, G.; Latorre, D.; Pontrelli, P.; Carella, M.; Schena, F.P.; Grandaliano, G.; Pertosa, G. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genom. 2009, 10, 388. [Google Scholar] [CrossRef]
- Hallan, S.; Afkarian, M.; Zelnick, L.R.; Kestenbaum, B.; Sharma, S.; Saito, R.; Darshi, M.; Barding, G.; Raftery, D.; Ju, W.; et al. Metabolomics and Gene Expression Analysis Reveal Down-regulation of the Citric Acid (TCA) Cycle in Non-diabetic CKD Patients. EBioMedicine 2017, 26, 68–77. [Google Scholar] [CrossRef]
- Huang, H.; van Dullemen, L.F.A.; Akhtar, M.Z.; Faro, M.-L.L.; Yu, Z.; Valli, A.; Dona, A.; Thézénas, M.-L.; Charles, P.D.; Fischer, R.; et al. Proteo-metabolomics reveals compensation between ischemic and non-injured contralateral kidneys after reperfusion. Sci. Rep. 2018, 8, 8539. [Google Scholar] [CrossRef]
- Cao, D.-S.; Wang, B.; Zeng, M.-M.; Liang, Y.-Z.; Xu, Q.-S.; Zhang, L.-X.; Li, H.-D.; Hu, Q.-N. A new strategy of exploring metabolomics data using Monte Carlo tree. Analyst 2011, 136, 947–954. [Google Scholar] [CrossRef]
- Wei, Q.; Xiao, X.; Fogle, P.; Dong, Z. Changes in Metabolic Profiles during Acute Kidney Injury and Recovery following Ischemia/Reperfusion. PLoS ONE 2014, 9, e106647. [Google Scholar] [CrossRef]
- Vaziri, N.D. Dyslipidemia of chronic renal failure: The nature, mechanisms, and potential consequences. Am. J. Physiol. Ren. Physiol. 2006, 290, 262–272. [Google Scholar] [CrossRef]
- Behling-Kelly, E. Serum Lipoprotein Changes in Dogs with Renal Disease. J. Vet. Intern. Med. 2014, 28, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Fürst, P. Amino acid metabolism in uremia. J. Am. Coll. Nutr. 1989, 8, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, M.H.; Yamaguchi, N.; Takahashi, H.; Hase, R.; Yamamoto, H.; Kikuchi, S.; Tanabe, T. Relationship of reduced glomerular filtration rate with alterations in plasma free amino acids and uric acid evaluated in healthy control and hypertensive subjects. Sci. Rep. 2019, 9, 10252. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.C.; Quimby, J.; Blake, A.; Keys, D.; Steiner, J.M.; Suchodolski, J. Serum and Fecal Amino Acid Profiles in Cats with Chronic Kidney Disease. Vet. Sci. 2022, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, M.A.; Halfen, D.P.; Risolia, L.W.; Pedrinelli, V.; Caragelasco, D.S.; Vendramini, T.H.A.; de Carvalho Balieiro, J.C.; Pontieri, C.F.F.; Jeremias, J.T.; Ruberti, B.; et al. Evaluation of Serum and Urine Amino Acids in Dogs with Chronic Kidney Disease and Healthy Dogs Fed a Renal Diet. Metabolites 2021, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Sanchez-Lozada, L.G.; Andres-Hernando, A.; Kojima, H.; Kasahara, M.; Rodriguez-Iturbe, B.; Bjornstad, P.; Lanaspa, M.A.; Johnson, R.J. Endogenous Fructose Metabolism could Explain the Warburg Effect and the Protection of SGLT2 Inhibitors in Chronic Kidney Disease. Front. Immunol. 2021, 12, 694457. [Google Scholar] [CrossRef]
- Roshanravan, B.; Zelnick, L.R.; Djucovic, D.; Gu, H.; Alvarez, J.A.; Ziegler, T.R.; Gamboa, J.L.; Utzschneider, K.; Kestenbaum, B.; Himmelfarb, J.; et al. Chronic kidney disease attenuates the plasma metabolome response to insulin. JCI Insight 2018, 3, e122219. [Google Scholar] [CrossRef]
- Prescott, B.A.; Waelsch, H. Free and Combined Glutamic Acid in Human Blood Plasma and Serum. J. Biol. Chem. 1947, 167, 855–860. [Google Scholar] [CrossRef]
- El-Gayar, A.; Sobh, M.; El-Kholy, A.; Sallam, S.; Wafa, E. Alterations of Plasma Free Amino Acids in Nephrotic Syndrome. Int. Urol. Nephrol. 1994, 26, 707–712. [Google Scholar] [CrossRef]
- Suliman, M.E.; Qureshi, A.R.; Stenvinkel, P.; Pecoits-Filho, R.; Bárány, P.; Heimbürger, O.; Anderstam, B.; Ayala, E.R.; Divino Filho, J.C.; Alvestrand, A.; et al. Inflammation contributes to low plasma amino acid concentrations in patients with chronic kidney disease. Am. J. Clin. Nutr. 2005, 82, 342–349. [Google Scholar] [CrossRef]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef] [PubMed]
- Currie, M.G.; Needleman, P. Renal Arachidonic Acid Metabolism. Ann. Rev. Physiol. 1984, 46, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Han, L.-D.; Xia, J.-F.; Liang, Q.-L.; Wang, Y.; Wang, Y.-M.; Hu, P.; Li, P.; Luo, G.-A. Plasma esterified and non-esterified fatty acids metabolic profiling using gas chromatography–mass spectrometry and its application in the study of diabetic mellitus and diabetic nephropathy. Anal. Chim. Acta 2011, 689, 85–91. [Google Scholar] [CrossRef]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.-A.; Han, S.H.; Chinga, F.; Park, A.S.D.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function—Measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef]
- O’Connell, J.M.B.; Romeo, J.A.; Mudge, G.H. Renal tubular secretion of creatinine in the dog. Am. J. Physiol. Content 1962, 203, 985–990. [Google Scholar] [CrossRef]
- Stevens, L.A.; Levey, A.S. Measurement of kidney function. Med. Clin. N. Am. 2005, 89, 457–473. [Google Scholar] [CrossRef]
- Preiss, D.J.; Godber, I.M.; Lamb, E.J.; Dalton, R.N.; Gunn, I.R. The influence of a cooked-meat meal on estimated glomerular filtration rate. Ann. Clin. Biochem. 2007, 44, 35–42. [Google Scholar] [CrossRef]
- Schutte, J.E.; Longhurst, J.C.; Gaffney, F.A.; Bastian, B.C.; Blomqvist, C.G. Total plasma creatinine: An accurate measure of total striated muscle mass. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 762–766. [Google Scholar] [CrossRef]
- Levey, A.S. Measurement of renal function in chronic renal disease. Kidney Int. 1990, 38, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Jackson, M.I.; Jewell, D.E.; Ephraim, E. Chronic kidney disease in cats alters response of the plasma metabolome and fecal microbiome to dietary fiber. PLoS ONE 2020, 15, e0235480. [Google Scholar] [CrossRef] [PubMed]
- Younes, H.; Garleb, K.; Behr, S.; Rémésy, C.; Demigné, C. Fermentable Fibers or Oligosaccharides Reduce Urinary Nitrogen Excretion by Increasing Urea Disposal in the Rat Cecum. J. Nutr. 1995, 125, 1010–1016. [Google Scholar] [PubMed]
- Wernimont, S.M.; Radosevich, J.; Jackson, M.I.; Ephraim, E.; Badri, D.V.; MacLeay, J.M.; Jewell, D.E.; Suchodolski, J.S. The Effects of Nutrition on the Gastrointestinal Microbiome of Cats and Dogs: Impact on Health and Disease. Front. Microbiol. 2020, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.C.; Quimby, J.M.; Isaiah, A.; Suchodolski, J.S.; Lunghofer, P.J.; Gustafson, D.L. The fecal microbiome and serum concentrations of indoxyl sulfate and p-cresol sulfate in cats with chronic kidney disease. J. Vet. Intern. Med. 2019, 33, 662–669. [Google Scholar] [CrossRef] [PubMed]


| Nutrients (g/100 kcal DM) | Senior Diet | Renal Test Diet |
|---|---|---|
| Protein | 10.34 | 8.68 |
| Fat | 5.23 | 3.94 |
| Crude fiber | 0.62 | 0.41 |
| Ash | 1.87 | 1.08 |
| Calcium | 0.32 | 0.13 |
| Phosphorus | 0.29 | 0.12 |
| Ca/P ratio | 1.12 | 1.08 |
| Potassium | 0.17 | 0.22 |
| Sodium | 0.20 | 0.08 |
| Omega-3 | 0.10 | 0.28 |
| Metabolizable energy (kcal/kg) | 3.920 | 4.353 |
| Essential amino acids | ||
| Arginine | 0.70 | 0.48 |
| Phenylalanine | 0.49 | 0.36 |
| Histidine | 0.26 | 0.18 |
| Isoleucine | 0.42 | 0.37 |
| Leucine | 0.94 | 0.79 |
| Lysine | 0.59 | 0.49 |
| Methionine | 0.28 | 0.17 |
| Taurine | 0.07 | 0.05 |
| Threonine | 0.41 | 0.35 |
| Tryptophan | 0.08 | 0.09 |
| Valine | 0.52 | 0.46 |
| Variables | Control Group (n = 10) | CKD1 Group (n = 6) | CKD2 Group (n = 9) | |||
|---|---|---|---|---|---|---|
| T0 | T60 | T0 | T60 | T0 | T60 | |
| Age (years) | 5.30 ± 1.07 | - | 10.83 ± 1.05 | - | 10.22 ± 1.35 | - |
| Body weight (kg) | 4.52 ± 0.65 | 4.53 ± 0.58 | 5.29 ± 0.73 | 5.28 ± 0.67 | 4.72 ± 1.51 | 4.60 ± 1.58 |
| Total protein (g/dL) | 7.90 ± 0.57 | 7.68 ± 0.82 | 8.10 ± 0.60 | 7.87 ± 0.75 | 8.14 ± 0.42 | 7.92 ± 0.43 |
| Albumin (g/dL) | 3.30 ± 0.19 | 3.29 ± 0.41 | 3.60 ± 0.24 | 3.53 ± 0.14 | 3.50 ± 0.21 | 3.51 ± 0.28 |
| Glucose (mg/dL) | 79.40 ± 9.74 | 109 ± 34.98 | 81.67 ± 13.41 | 83 ± 6.87 | 79.67 ± 5.29 | 95.22 ± 37.65 |
| Creatinine (mg/dL) | 1.35 ± 0.21 | 1.16 ± 0.24 | 1.32 ± 0.12 | 1.29 ± 0.10 | 2.03 ± 0.32 | 1.94 ± 0.81 |
| BUN (mg/dL) | 24.67 ± 2.98 | 23.08 ± 2.43 | 23.42 ± 1.53 | 23.02 ± 2.08 | 35.06 ± 5.28 | 35.81 ± 11.81 |
| SDMA (µg/dL) | 9.56 ± 4.10 | 9.5 ± 3.37 | 10.60 ± 3.36 | 7.50 ± 1.05 | 14.44 ± 3.88 | 11.33 ± 5.94 |
| Phosphorus (mg/dL) | 5.58 ± 0.65 | 5.97 ± 0.84 | 4.83 ± 0.51 | 4.97 ± 0.57 | 5.08 ± 0.48 | 5.96 ± 1.01 |
| Total calcium (mg/dL) | 9.96 ± 0.51 | 9.43 ± 0.85 | 10.40 ± 0.32 | 10.22 ± 0.50 | 10.49 ± 0.31 | 10.02 ± 0.56 |
| Sodium (mEq/L) | 152 ± 2.11 | 156.40 ± 1.17 | 154 ± 2.53 | 156.17 ± 2.14 | 152.67 ± 2.00 | 157.33 ± 3.39 |
| Potassium (mEq/L) | 4.84 ± 0.31 | 5.01 ± 0.48 | 5.15 ± 0.57 | 4.68 ± 0.49 | 4.88 ± 0.50 | 5.13 ± 0.57 |
| Chloride (mEq/L) | 115.30 ± 3.74 | 120.20 ± 2.90 | 118 ± 2.28 | 121.33 ± 1.37 | 117.67 ± 1.32 | 122.67 ± 3.67 |
| Cholesterol (mg/dL) | 153.60 ± 44.82 | 129.20 ± 45.16 | 221.83 ± 40.48 | 200 ± 46.38 | 206.33 ± 58.19 | 163.56 ± 51.10 |
| Tryglicerides (mg/dL) | 46.50 ± 22.42 | 47 ± 15.96 | 61.50 ± 31.16 | 60.83 ± 12.12 | 52.78 ± 13.98 | 68.67 ± 36.53 |
| ALP (mg/dL) | 29.90 ± 7.00 | 26.50 ± 5.10 | 33.67 ± 8.04 | 32 ± 8.76 | 38.33 ± 24.35 | 37.33 ± 27.80 |
| ALT (mg/dL) | 65.60 ± 20.32 | 56.80 ± 18.96 | 65.17 ± 19.34 | 57 ± 14.39 | 82.67 ± 61.80 | 76.56 ± 50.94 |
| Biochemical Class | Compound Names | Code | Identification Level | Identification in the Human Metabolome Database (HMDB) | Relative Standard Deviation (%) |
|---|---|---|---|---|---|
| Amino acids and derivatives | L-Alanine | ALA | 2 | 0000161 | 7.3 |
| L-Valine | VAL | 2 | 0000883 | 7.1 | |
| L-Isoleucine | ILE | 2 | 0000172 | 7.0 | |
| L-Proline | PRO | 2 | 0000162 | 11.0 | |
| L-Glycine | GLY | 2 | 0000123 | 10.1 | |
| L-Serine | SER | 2 | 0000187 | 14.2 | |
| L-Threonine | THR | 2 | 0000167 | 4.8 | |
| L-Homoserine | hSER | 2 | 0000719 | 23.3 | |
| 5-Oxoproline | PYR | 2 | 0000267 | 19.7 | |
| Creatinine | CRE | 2 | 0000562 | 23.2 | |
| L-Glutamic acid | GLU | 2 | 0000148 | 6.5 | |
| L-Phenylalanine | PHE | 2 | 0000159 | 10.1 | |
| L-Arginine | ARG | 2 | 0000517 | 9.8 | |
| L-Tyrosine | TYR | 2 | 0000158 | 22.7 | |
| L-Tryptophan | TRP | 2 | 0000929 | 25.3 | |
| Carbohydrates and conjugates | D-Fructose | FRU | 2 | 0000660 | 25.3 |
| D-Ribose | RIB | 2 | 0000283 | 16.2 | |
| D-Mannose | MAN | 2 | 000169 | 10.5 | |
| D-Allose | ALLO | 2 | 0001151 | 10.5 | |
| Inositol-Phosphate | INO | 2 | 0000213 | 23.4 | |
| Lactose | LAC | 2 | 0000186 | 27.3 | |
| Fatty acids | Palmitoleic acid | C16:1 | 2 | 0003229 | 24.5 |
| Palmitic acid | C16:0 | 2 | 0000220 | 11.9 | |
| Heptadecanoic acid | HEP | 2 | 0002259 | 18.3 | |
| Linoleic acid | LNL | 2 | 0000673 | 25.3 | |
| Oleic acid | OLC | 2 | 0000207 | 19.4 | |
| Stearic acic | STE | 2 | 0000827 | 13.5 | |
| Arachidonic acid | ARA | 2 | 0001043 | 15.0 | |
| Docosahexaenoic acid | DHA | 2 | 0002183 | 7.0 | |
| Arachidic acid | C20:0 | 2 | 0002212 | 22.1 | |
| 1-Monopalmitin | MG16:0 | 2 | 0011564 | 13.6 | |
| Monostearin | MG18:0 | 2 | 0011131 | 15.3 | |
| Prenol Lipids | Alpha-Tocopherol | αTOC | 2 | 0001893 | 14.7 |
| Steroids | Cholesterol | CHO | 2 | 0000067 | 13.5 |
| Carboxilic acid | Citric acid | CIT | 2 | 0000094 | 29.4 |
| Sarcosine | SAR | 2 | 0000271 | 21.3 | |
| Hydroxy acids | 3-Hydroxybutyric acid | 3HBT | 2 | 0000011 | 11.0 |
| Organic Carbonic acids | Urea | URE | 2 | 0000294 | 15.8 |
| Imidazopyrimidines | Uric acid | URI | 2 | 0000289 | 24.3 |
| Purine nucleosides | Guanosine | GUA | 2 | 0000133 | 18.1 |
| Indoles | Serotonin | 5HTA | 2 | 0000259 | 12.6 |
| Glycerophospholipids | Glycerol-3-Phosphate | G3P | 2 | 0000126 | 22.5 |
| Benzene | 3-Hydroxyanthranilic acid | 3OHAA | 2 | 0001476 | 28.9 |
| Metabolites | p Value | FDR | Tukey’s HSD |
|---|---|---|---|
| Citric acid | 0.0010 | 0.0440 | CKD II T0-CKD I T0; Control T0-CKD II T0 |
| Monostearin | 0.0021 | 0.0444 | Control T0-CKD II T0 |
| Glycine | 0.0003 | 0.0055 | Control T60-CKD I T60; Control T60-CKD II T60 |
| Fructose | 0.0007 | 0.0107 | CKD II T60-CKD I T60; Control T60-CKD II T60 |
| Glutamic acid | 0.0013 | 0.0144 | Control T60-CKD II T60 |
| Arachidonic acid | 0.0042 | 0.0269 | Control T60-CKD II T60 |
| Stearic acid | 0.0044 | 0.0269 | Control T60-CKD II T60 |
| Creatinine | 0.0358 | 0.0002 | CKD II T60-CKD I T60; Control T60-CKD II T60 |
| Urea | 0.0031 | 0.0265 | Control T60-CKD II T60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruberti, B.; Machado, D.P.; Vendramini, T.H.A.; Pedrinelli, V.; Marchi, P.H.; Jeremias, J.T.; Pontieri, C.F.F.; Kogika, M.M.; Brunetto, M.A. Serum Metabolites Characterization Produced by Cats CKD Affected, at the 1 and 2 Stages, before and after Renal Diet. Metabolites 2023, 13, 43. https://doi.org/10.3390/metabo13010043
Ruberti B, Machado DP, Vendramini THA, Pedrinelli V, Marchi PH, Jeremias JT, Pontieri CFF, Kogika MM, Brunetto MA. Serum Metabolites Characterization Produced by Cats CKD Affected, at the 1 and 2 Stages, before and after Renal Diet. Metabolites. 2023; 13(1):43. https://doi.org/10.3390/metabo13010043
Chicago/Turabian StyleRuberti, Bruna, Daniela Pedrosa Machado, Thiago Henrique Annibale Vendramini, Vivian Pedrinelli, Pedro Henrique Marchi, Juliana Toloi Jeremias, Cristiana Fonseca Ferreira Pontieri, Marcia Mery Kogika, and Marcio Antonio Brunetto. 2023. "Serum Metabolites Characterization Produced by Cats CKD Affected, at the 1 and 2 Stages, before and after Renal Diet" Metabolites 13, no. 1: 43. https://doi.org/10.3390/metabo13010043
APA StyleRuberti, B., Machado, D. P., Vendramini, T. H. A., Pedrinelli, V., Marchi, P. H., Jeremias, J. T., Pontieri, C. F. F., Kogika, M. M., & Brunetto, M. A. (2023). Serum Metabolites Characterization Produced by Cats CKD Affected, at the 1 and 2 Stages, before and after Renal Diet. Metabolites, 13(1), 43. https://doi.org/10.3390/metabo13010043






