Hair-Based Assessment of Sex Steroid Hormones in Patients with Anorexia Nervosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Clinical Measures
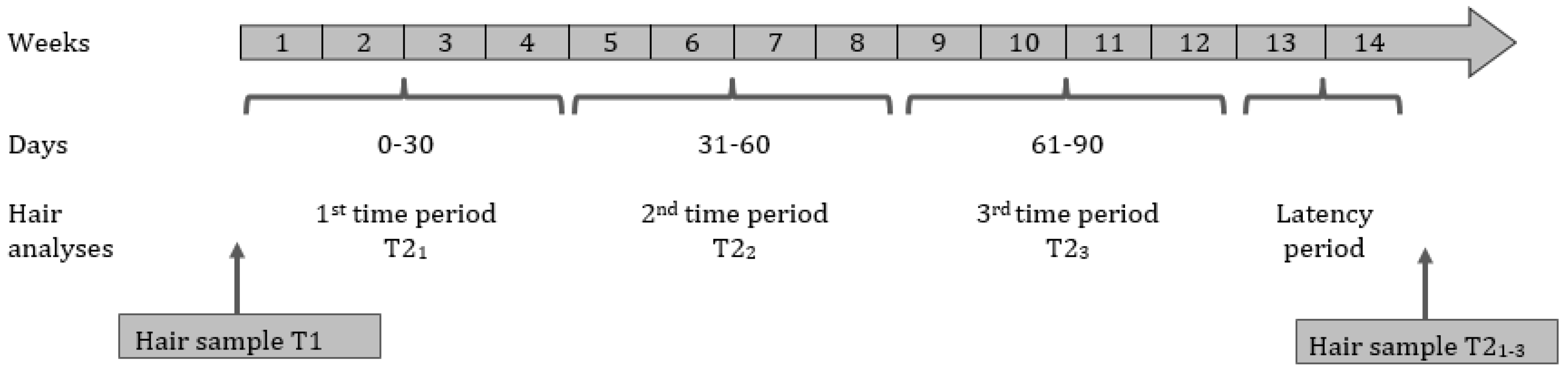
2.3. Hair Sampling Procedure and Biochemical Analysis
2.4. Plasma Hormone Measures
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schorr, M.; Miller, K.K. The Endocrine Manifestations of Anorexia Nervosa: Mechanisms and Management. Nat. Rev. Endocrinol. 2017, 13, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Milos, G.; Hebebrand, J. Endocrine Consequences of Anorexia Nervosa. Acta Acad. Med. Sin. 2019, 108, 899–904. [Google Scholar] [CrossRef]
- Berner, L.A.; Brown, T.A.; Lavender, J.M.; Lopez, E.; Wierenga, C.E.; Kaye, W.H. Neuroendocrinology of Reward in Anorexia Nervosa and Bulimia Nervosa: Beyond Leptin and Ghrelin. Mol. Cell. Endocrinol. 2019, 497, 110320. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.P. Endocrine Manifestations of Eating Disorders. J. Clin. Endocrinol. Metab. 2011, 96, 333–343. [Google Scholar] [CrossRef]
- Miller, K.K. Endocrine Effects of Anorexia Nervosa. Endocrinol. Metab. Clin. N. Am. 2013, 42, 515–528. [Google Scholar] [CrossRef]
- Baker, J.H.; Girdler, S.S.; Bulik, C.M. The Role of Reproductive Hormones in the Development and Maintenance of Eating Disorders. Expert Rev. Obstet. Gynecol. 2012, 7, 573–583. [Google Scholar] [CrossRef]
- Allaway, H.C.M.; Southmayd, E.A.; De Souza, M.J. The Physiology of Functional Hypothalamic Amenorrhea Associated with Energy Deficiency in Exercising Women and in Women with Anorexia Nervosa. Horm. Mol. Biol. Clin. Investig. 2016, 25, 91–119. [Google Scholar] [CrossRef]
- Prough, R.A.; Clark, B.J.; Klinge, C.M. Novel Mechanisms for DHEA Action. J. Mol. Endocrinol. 2016, 56, R139–R155. [Google Scholar] [CrossRef]
- Zumoff, B.; Walsh, B.T.; Katz, J.L.; Levin, J.; Rosenfeld, R.S.; Kream, J.; Weiner, H. Subnormal Plasma Dehydroisoandrosterone to Cortisol Ratio in Anorexia Nervosa: A Second Hormonal Parameter of Ontogenic Regression*. J. Clin. Endocrinol. Metab. 1983, 56, 668–672. [Google Scholar] [CrossRef]
- WINTERER, J.; GWIRTSMAN, H.E.; GEORGE, D.T.; KAYE, W.H.; LORIAUX, D.L.; CUTLER, G.B. Adrenocorticotropin-Stimulated Adrenal Androgen Secretion in Anorexia Nervosa: Impaired Secretion at Low Weight with Normalization after Long-Term Weight Recovery. J. Clin. Endocrinol. Metab. 1985, 61, 693–697. [Google Scholar] [CrossRef]
- Monteleone, P.; Luisi, M.; Colurcio, B.; Casarosa, E.; Monteleone, P.; Ioime, R.; Genazzani, A.R.; Maj, M. Plasma Levels of Neuroactive Steroids Are Increased in Untreated Women With Anorexia Nervosa or Bulimia Nervosa. Psychosom. Med. 2001, 63, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Luisi, M.; Martiadis, V.; Serritella, C.; Longobardi, N.; Casarosa, E.; Genazzani, A.R.; Maj, M. Impaired Reduction of Enhanced Levels of Dehydroepiandrosterone by Oral Dexamethasone in Anorexia Nervosa. Psychoneuroendocrinology 2006, 31, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Misra, M.; Meenaghan, E.; Rosenblum, L.; Donoho, D.A.; Herzog, D.; Klibanski, A.; Miller, K.K. Adrenal Glucocorticoid and Androgen Precursor Dissociation in Anorexia Nervosa. J. Clin. Endocrinol. Metab. 2009, 94, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.; Maayan, R.; Ram, A.; Loewenthal, R.; Achiron, A.; Modan-Moses, D.; Feigin, M.; Weizman, A.; Valevski, A. Circulatory Neurosteroid Levels in Underweight Female Adolescent Anorexia Nervosa Inpatients and Following Weight Restoration. Eur. Neuropsychopharmacol. 2005, 15, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Kao, T.; Cheng, Y.; Fan, K.; Huang, Y.; Liu, C. Dehydroepiandrosterone Status and Efficacy of Dehydroepiandrosterone Supplementation for Bone Health in Anorexia Nervosa: A Systematic Review and Meta-analysis. Int. J. Eat. Disord. 2022, 55, 733–746. [Google Scholar] [CrossRef]
- Hammer, F.; Subtil, S.; Lux, P.; Maser-Gluth, C.; Stewart, P.M.; Allolio, B.; Arlt, W. No Evidence for Hepatic Conversion of Dehydroepiandrosterone (DHEA) Sulfate to DHEA: In Vivo and in Vitro Studies. J. Clin. Endocrinol. Metab. 2005, 90, 3600–3605. [Google Scholar] [CrossRef]
- Stern, J.; Arslan, R.C.; Penke, L. Stability and Validity of Steroid Hormones in Hair and Saliva across Two Ovulatory Cycles. Compr. Psychoneuroendocrinology 2022, 9, 100114. [Google Scholar] [CrossRef]
- Murray, S.B.; Quintana, D.S.; Loeb, K.L.; Griffiths, S.; Le Grange, D. Treatment Outcomes for Anorexia Nervosa: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Psychol. Med. 2019, 49, 535–544. [Google Scholar] [CrossRef]
- Misra, M.; Prabhakaran, R.; Miller, K.K.; Tsai, P.; Lin, A.; Lee, N.; Herzog, D.B.; Klibanski, A. Role of Cortisol in Menstrual Recovery in Adolescent Girls with Anorexia Nervosa. Pediatr. Res. 2006, 59, 598–603. [Google Scholar] [CrossRef]
- Dempfle, A.; Herpertz-Dahlmann, B.; Timmesfeld, N.; Schwarte, R.; Egberts, K.M.; Pfeiffer, E.; Fleischhaker, C.; Wewetzer, C.; Bühren, K. Predictors of the Resumption of Menses in Adolescent Anorexia Nervosa. BMC Psychiatry 2013, 13, 308. [Google Scholar] [CrossRef]
- Traboulsi, S.; Itani, L.; Tannir, H.; Kreidieh, D.; El Masri, D.; El Ghoch, M. IS BODY FAT PERCENTAGE A GOOD PREDICTOR OF MENSTRUAL RECOVERY IN FEMALES WITH ANOREXIA NERVOSA AFTER WEIGHT RESTORATION? A SYSTEMATIC REVIEW AND EXPLORATORY AND SELECTIVE META-ANALYSIS. J. Popul. Ther. Clin. Pharmacol. 2019, 26, e25–e37. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.E.; Farahani, L.; Webber, L.; Jayasena, C. Current Understanding of Hypothalamic Amenorrhoea. Ther. Adv. Endocrinol. Metab. 2020, 11, 204201882094585. [Google Scholar] [CrossRef] [PubMed]
- Seidenfeld, M.E.; Rickert, V.I. Impact of Anorexia, Bulimia and Obesity on the Gynecologic Health of Adolescents. Am. Fam. Physician 2001, 64, 445–450. [Google Scholar] [PubMed]
- Grotzinger, A.D.; Briley, D.A.; Engelhardt, L.E.; Mann, F.D.; Patterson, M.W.; Tackett, J.L.; Tucker-Drob, E.M.; Harden, K.P. Genetic and Environmental Influences on Pubertal Hormones in Human Hair across Development. Psychoneuroendocrinology 2018, 90, 76–84. [Google Scholar] [CrossRef]
- Walther, A.; Tsao, C.; Pande, R.; Kirschbaum, C.; Field, E.; Berkman, L. Do Dehydroepiandrosterone, Progesterone, and Testosterone Influence Women’s Depression and Anxiety Levels? Evidence from Hair-Based Hormonal Measures of 2105 Rural Indian Women. Psychoneuroendocrinology 2019, 109, 104382. [Google Scholar] [CrossRef]
- Hansen, B.B.; Klopfer, S.O. Optimal Full Matching and Related Designs via Network Flows. J. Comput. Graph. Stat. 2006, 15, 609–627. [Google Scholar] [CrossRef]
- Fichter, M.; Quadflieg, N. Strukturiertes Inventar Für Anorektische Und Bulimische Essstörungen (SIAB); Fragebogen (SIAB-S) Und Interview (SIAB-EX) Nach DSM-IV Und ICD-10; Handanweisung; Hogrefe: Göttingen, Germany, 1999. [Google Scholar]
- Thiel, A.; Jacobi, C.; Horstmann, S.; Paul, T.; Nutzinger, D.O.; Schüssler, G. A German Version of the Eating Disorder Inventory EDI-2. Psychother. Psychosom. Med. Psychol. 1997, 47, 365–376. [Google Scholar]
- Hautzinger, M.; Keller, F.; Kühner, C. BDI-II. Beck-Depressions-Inventar; 2. Auflage; Pearson Assessment: Frankfurt, Germany, 2009. [Google Scholar]
- Franke, G.; Derogatis, L. Symptom-Checkliste von LR Derogatis: SCL-90-R; Deutsche Version; Beltz Test: Göttingen, Germany, 2002. [Google Scholar]
- Hemmelmann, C.; Brose, S.; Vens, M.; Hebebrand, J.; Ziegler, A. Perzentilen Des Body-Mass-Index Auch Für 18- Bis 80-Jährige? Daten Der Nationalen Verzehrsstudie II. Dtsch. Med. Wochenschr. (DMW) 2010, 135, 848–852. [Google Scholar] [CrossRef]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile Für Den Body-Mass-Index Für Das Kindes-Und Jugendalter Unter Heranziehung Verschiedener Deutscher Stichproben. Mon. Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Wennig, R. Potential Problems with the Interpretation of Hair Analysis Results. Forensic Sci. Int. 2000, 107, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Harkey, M.R. Anatomy and Physiology of Hair. Forensic Sci. Int. 1993, 63, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Stalder, T.; Foley, P.; Rauh, M.; Deng, H.; Kirschbaum, C. Quantitative Analysis of Steroid Hormones in Human Hair Using a Column-Switching LC–APCI–MS/MS Assay. J. Chromatogr. B 2013, 928, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Kirschbaum, C.; Grass, J.; Stalder, T. LC–MS Based Analysis of Endogenous Steroid Hormones in Human Hair. J. Steroid Biochem. Mol. Biol. 2016, 162, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Croghan, C.W.; Egeghy, P.P. Methods of Dealing with Values Below the Limit of Detection Using SAS; US-EPA: Las Vegas, NV, USA, 2003; p. 5. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Sominsky, L.; Hodgson, D.M.; McLaughlin, E.A.; Smith, R.; Wall, H.M.; Spencer, S.J. Linking Stress and Infertility: A Novel Role for Ghrelin. Endocr. Rev. 2017, 38, 432–467. [Google Scholar] [CrossRef]
- Klump, K.L. Puberty as a Critical Risk Period for Eating Disorders: A Review of Human and Animal Studies. Horm. Behav. 2013, 64, 399–410. [Google Scholar] [CrossRef]
- Gray, J.M.; Wade, G.N. Food Intake, Body Weight, and Adiposity in Female Rats: Actions and Interactions of Progestins and Antiestrogens. Am. J. Physiol. Metab. 1981, 240, E474–E481. [Google Scholar] [CrossRef]
- Kemnitz, J.W.; Gibber, J.R.; Lindsay, K.A.; Eisele, S.G. Effects of Ovarian Hormones on Eating Behaviors, Body Weight, and Glucoregulation in Rhesus Monkeys. Horm. Behav. 1989, 23, 235–250. [Google Scholar] [CrossRef]
- VARMA, M.; CHAI, J.; MEGUID, M.; LAVIANO, A.; GLEASON, J.; YANG, Z.; BLAHA, V. Effect of Estradiol and Progesterone on Daily Rhythm in Food Intake and Feeding Patterns in Fischer Rats. Physiol. Behav. 1999, 68, 99–107. [Google Scholar] [CrossRef]
- King, S.R. Neurosteroids and the Nervous System; SpringerBriefs in Neuroscience; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-5558-5. [Google Scholar]
- Dichtel, L.E.; Lawson, E.A.; Schorr, M.; Meenaghan, E.; Paskal, M.L.; Eddy, K.T.; Pinna, G.; Nelson, M.; Rasmusson, A.M.; Klibanski, A.; et al. Neuroactive Steroids and Affective Symptoms in Women Across the Weight Spectrum. Neuropsychopharmacology 2018, 43, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.; Wainwright, S.R.; Galea, L.A.M. Sex Hormones and Adult Hippocampal Neurogenesis: Regulation, Implications, and Potential Mechanisms. Front. Neuroendocrinol. 2016, 41, 129–152. [Google Scholar] [CrossRef]
- Støving, R.K. MECHANISMS IN ENDOCRINOLOGY: Anorexia Nervosa and Endocrinology: A Clinical Update. Eur. J. Endocrinol. 2019, 180, R9–R27. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, F.; Monteleone, P.; Bortolotti, F.; Dalle Grave, R.; Todisco, P.; Favaro, A.; Santonastaso, P.; Ramacciotti, C.; Paoli, R.; Maj, M. Persistent Amenorrhoea in Weight-Recovered Anorexics: Psychological and Biological Aspects. Psychiatry Res. 2003, 118, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Van Binsbergen, C.J.M.; Bennink, H.J.T.C.; Odink, J.; Haspels, A.A.; Koppeschaar, H.P.F. A Comparative and Longitudinal Study on Endocrine Changes Related to Ovarian Function in Patients with Anorexia Nervosa. J. Clin. Endocrinol. Metab. 1990, 71, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.H. Resumption of Menses in Anorexia Nervosa. Arch. Pediatr. Adolesc. Med. 1997, 151, 16. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.H.; Jacobson, M.S.; Sterling, W.M.; Hertz, S. Treatment Goal Weight in Adolescents with Anorexia Nervosa: Use of BMI Percentiles. Int. J. Eat. Disord. 2008, 41, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Guo, E.L.; Katta, R. Diet and Hair Loss: Effects of Nutrient Deficiency and Supplement Use. Dermatol. Pract. Concept. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef]
- Xiang, L.; Sunesara, I.; Rehm, K.E.; Marshall Jr., G.D. Hair Cortisol Concentrations Are Associated with Hair Growth Rate. Neuroimmunomodulation 2016, 23, 287–294. [Google Scholar] [CrossRef]
- Tyler, I.; Wiseman, M.C.; Crawford, R.I.; Birmingham, C.L. Cutaneous Manifestations of Eating Disorders. J. Cutan. Med. Surg. 2002, 6, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Strumia, R. Dermatologic Signs in Patients with Eating Disorders. Am. J. Clin. Dermatol. 2005, 6, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Herpertz-Dahlmann, B. Intensive Treatments in Adolescent Anorexia Nervosa. Nutrients 2021, 13, 1265. [Google Scholar] [CrossRef] [PubMed]
- Schury, K.; Koenig, A.M.; Isele, D.; Hulbert, A.L.; Krause, S.; Umlauft, M.; Kolassa, S.; Ziegenhain, U.; Karabatsiakis, A.; Reister, F.; et al. Alterations of Hair Cortisol and Dehydroepiandrosterone in Mother-Infant-Dyads with Maternal Childhood Maltreatment. BMC Psychiatry 2017, 17, 213. [Google Scholar] [CrossRef] [PubMed]
- Talmon, A.; Widom, C.S. Childhood Maltreatment and Eating Disorders: A Prospective Investigation. Child Maltreat. 2022, 27, 88–99. [Google Scholar] [CrossRef]
- Toufexis, D.; Rivarola, M.A.; Lara, H.; Viau, V. Stress and the Reproductive Axis. J. Neuroendocrinol. 2014, 26, 573–586. [Google Scholar] [CrossRef]
| AN-T1 N = 33 | HC N = 33 | Test Statistic T/X2/U | p | |
|---|---|---|---|---|
| Demographics and clinical variables | ||||
| Age (years) | 15.0 ± 2.9 | 15.9 ± 2.8 | −0.69 | 0.496 |
| Age of onset (years) | 14.4 ± 2.6 | - | - | - |
| EDI-2 (total score) a | 185.4 ± 43.1 | 142.0 ± 34.4 | 4.4 | <0.001 *** |
| BDI-II (total score) | 15.4 ± 9.6 | 5.1 ± 5.1 | 5.5 | <0.001 *** |
| SCL-90-R (global severity index) | 33.9 ± 20.0 | 20.4 ± 19.7 | 2.8 | <0.001 *** |
| Clinical variables and plasma hormone measures | ||||
| BMI (kg/m2) | 14.8 ± 1.1 | 20.2 ± 2.1 | −13.10 | <0.001 *** |
| BMI-SDS | −2.7 ± 1.0 | −0.05 ± 0.6 | −12.50 | <0.001 *** |
| Menarche (# of participants) | 28 | 29 | 0.13 (X2) | 0.720 |
| Age at menarchea | 12.1 ± 0.9 | 12.6 ± 0.9 | −1.9 | 0.068 |
| Menses during the last three months (# of participants) b | 0 | 29 | 57.0 (X2) | <0.001 *** |
| Leptin (ng/mL) c | 0.77 (1.83) | 10.67 (9.11) | 770.0 (U) | <0.001 *** |
| Ghrelin (ng/mL) c | 417.33 (265.93) | 232.65 (150.90) | 80.0 (U) | <0.001 *** |
| Hair hormone measures | ||||
| Progesterone (pg/mg) | 0.07 (0.28) | 0.57 (1.22) | 814.0 (U) | <0.001 *** |
| DHEA (pg/mg) | 0.37 (0.43) | 0.40 (0.56) | 646.0 (U) | 0.193 |
| AN-T1 T1 Pre-Treatment Level | AN-T2 T21 Time Period 21 (Weeks 1–4 after Admission) | T22 Time Period 22 (Weeks 5–8 after Admission) | T23 Time Period 23 (Weeks 9–12 after Admission) | Test Statistic X2F/T | p | |
|---|---|---|---|---|---|---|
| Clinical variables and plasma hormone measures | ||||||
| Weight (kg)a | 41.35 (7.03) | 44.75 (7.3) | 49.60 (7.28) | 51.70 (7.28) | 77.7 (X2F) | <0.001 *** |
| BMI (kg/m2) a | 15.16 (1.52) | 16.30 (1.75) | 17.69 (2.10) | 18.96 (1.97) | 77.7 (X2F) | <0.001 *** |
| BMI-SDS a | −2.58 (1.19) | −1.74 (1.28) | −1.11 (1.11) | −0.54 (.64) | 77.7 (X2F) | <0.001 *** |
| Menses during the last three months (# of participants) b | 0 | - | - | 5 | - | - |
| Leptin (ng/mL) c | 1.04 (2.00) | - | - | 10.37 (11.86) | 9.0 (T) | <0.001 *** |
| Ghrelin (ng/mL) c | 441.50 (305.28) | - | - | 213.70 (150.06) | 210.0 (T) | <0.001 *** |
| Hair hormone measures | ||||||
| Progesterone b | 0.07 (0.25) | 0.07 (0.37) | 0.07 (0.22) | 0.07 (0.30) | 1.5 (X2F) | 0.682 |
| DHEA b | 0.37 (0.25) | 0.43 (0.25) | 0.43 (0.25) | 0.38 (0.29) | 2.3 (X2F) | 0.509 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batury, V.-L.; Tam, F.I.; Hellerhoff, I.; Wronski, M.-L.; Borucki, K.; Weidner, K.; Roessner, V.; Gao, W.; Ehrlich, S. Hair-Based Assessment of Sex Steroid Hormones in Patients with Anorexia Nervosa. Metabolites 2023, 13, 21. https://doi.org/10.3390/metabo13010021
Batury V-L, Tam FI, Hellerhoff I, Wronski M-L, Borucki K, Weidner K, Roessner V, Gao W, Ehrlich S. Hair-Based Assessment of Sex Steroid Hormones in Patients with Anorexia Nervosa. Metabolites. 2023; 13(1):21. https://doi.org/10.3390/metabo13010021
Chicago/Turabian StyleBatury, Victoria-Luise, Friederike I. Tam, Inger Hellerhoff, Marie-Louis Wronski, Katrin Borucki, Kerstin Weidner, Veit Roessner, Wei Gao, and Stefan Ehrlich. 2023. "Hair-Based Assessment of Sex Steroid Hormones in Patients with Anorexia Nervosa" Metabolites 13, no. 1: 21. https://doi.org/10.3390/metabo13010021
APA StyleBatury, V.-L., Tam, F. I., Hellerhoff, I., Wronski, M.-L., Borucki, K., Weidner, K., Roessner, V., Gao, W., & Ehrlich, S. (2023). Hair-Based Assessment of Sex Steroid Hormones in Patients with Anorexia Nervosa. Metabolites, 13(1), 21. https://doi.org/10.3390/metabo13010021






