Identification of Protective Amino Acid Metabolism Events in Nursery Pigs Fed Thermally Oxidized Corn Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals, Experimental Design, and Sample Collection
2.3. LC-MS-Based Metabolomic Analysis
2.3.1. Sample Preparation
2.3.2. Chemical Derivatization
2.3.3. Conditions of LC-MS Analysis
2.3.4. Targeted Quantitative Analysis
2.3.5. Untargeted Multivariate Data Analysis and Marker Characterization
2.4. Gene Expression Analysis
2.5. In Vitro Analysis of Threonine Dehydrogenase (TDG) and Threonine Dehydrotase (TDH) Enzymatic Activities
2.6. Statistical Analysis
3. Results
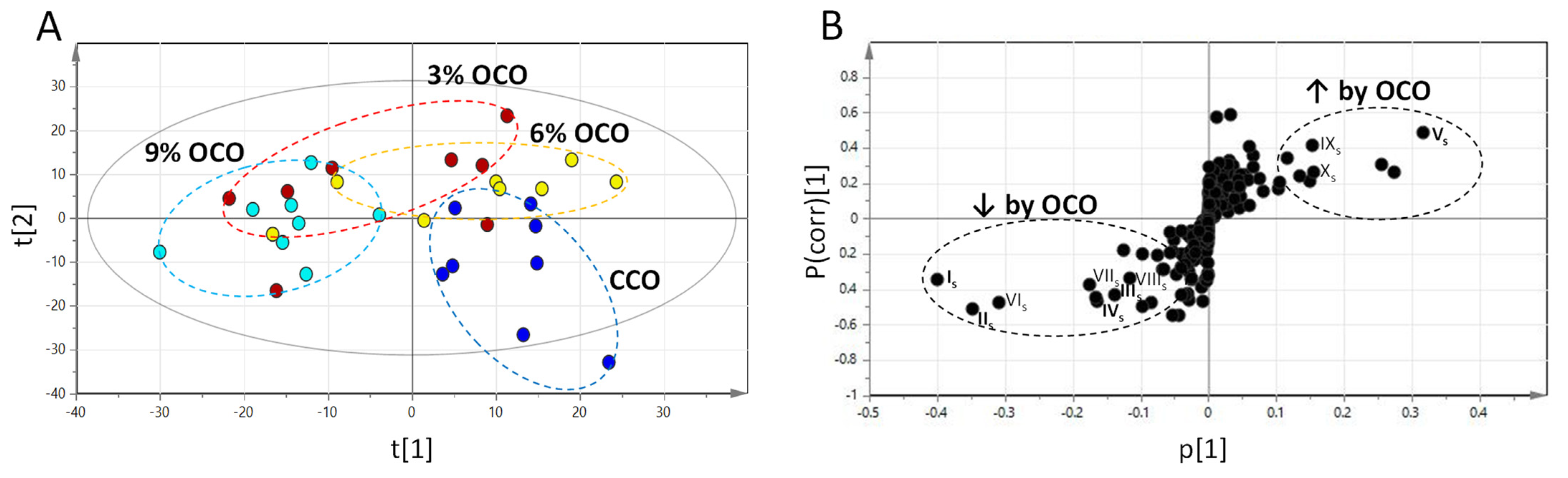
3.1. Influences of OCO on Serum-Free Amino Acids
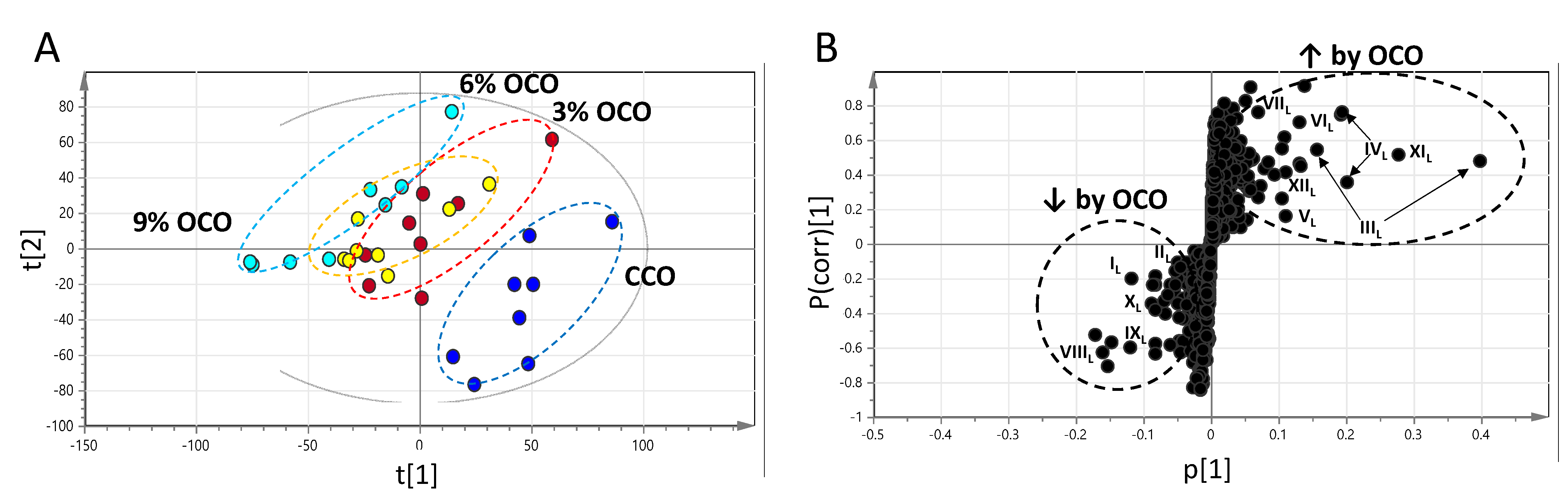
3.2. Influences of OCO on the Hepatic Metabolome
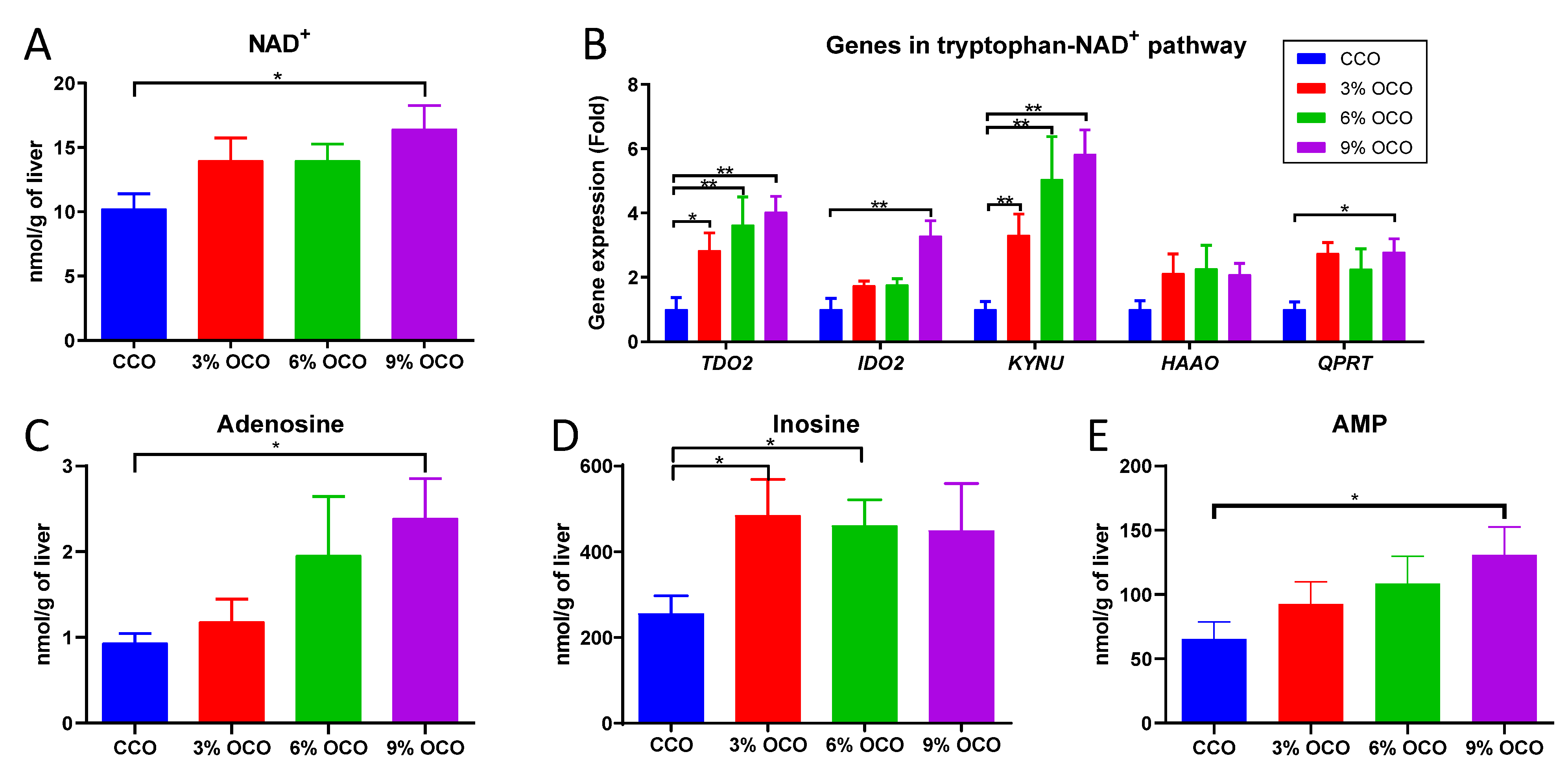
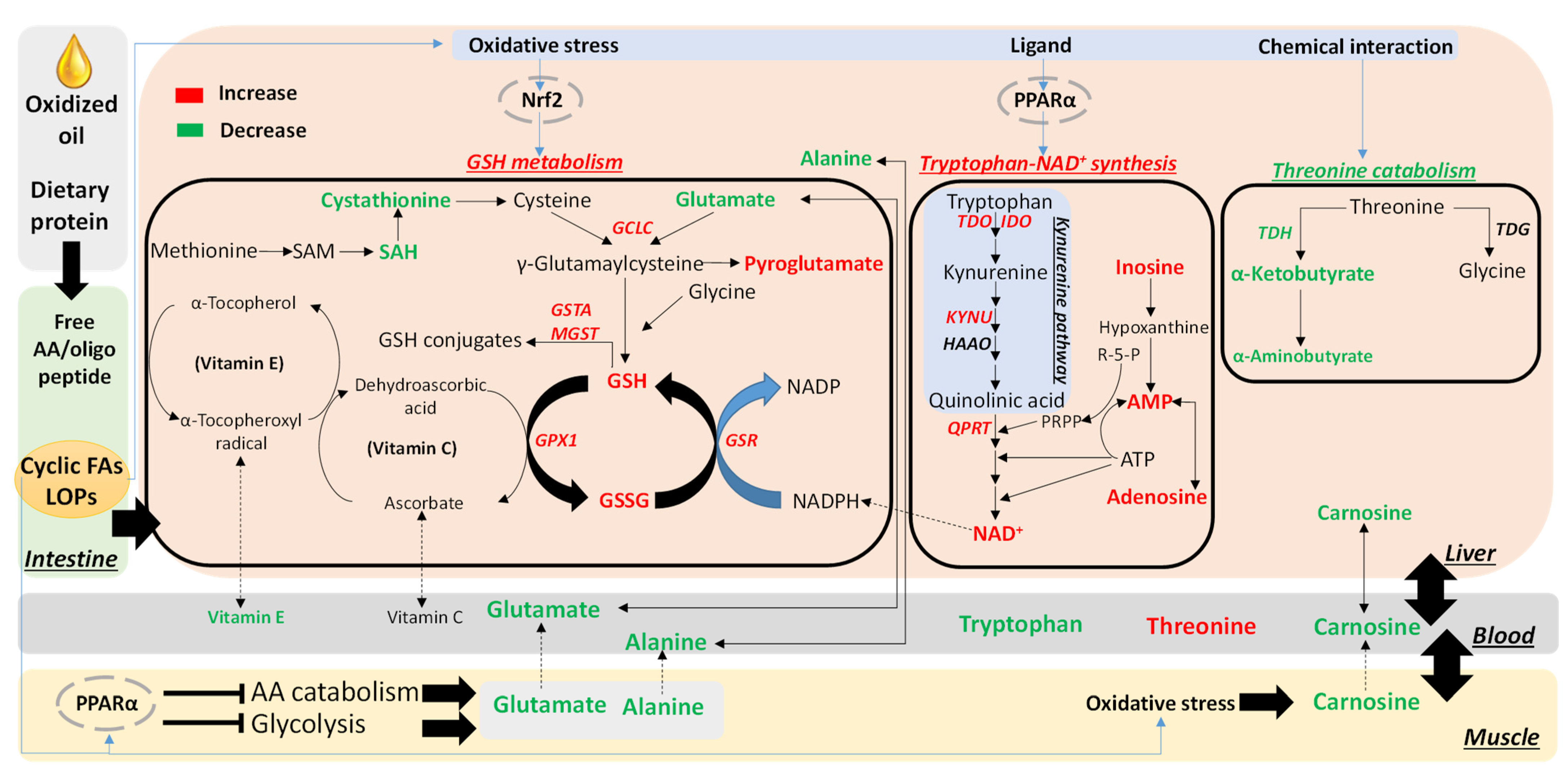
3.3. Effects of OCO on the Hepatic Tryptophan-NAD+ Pathway
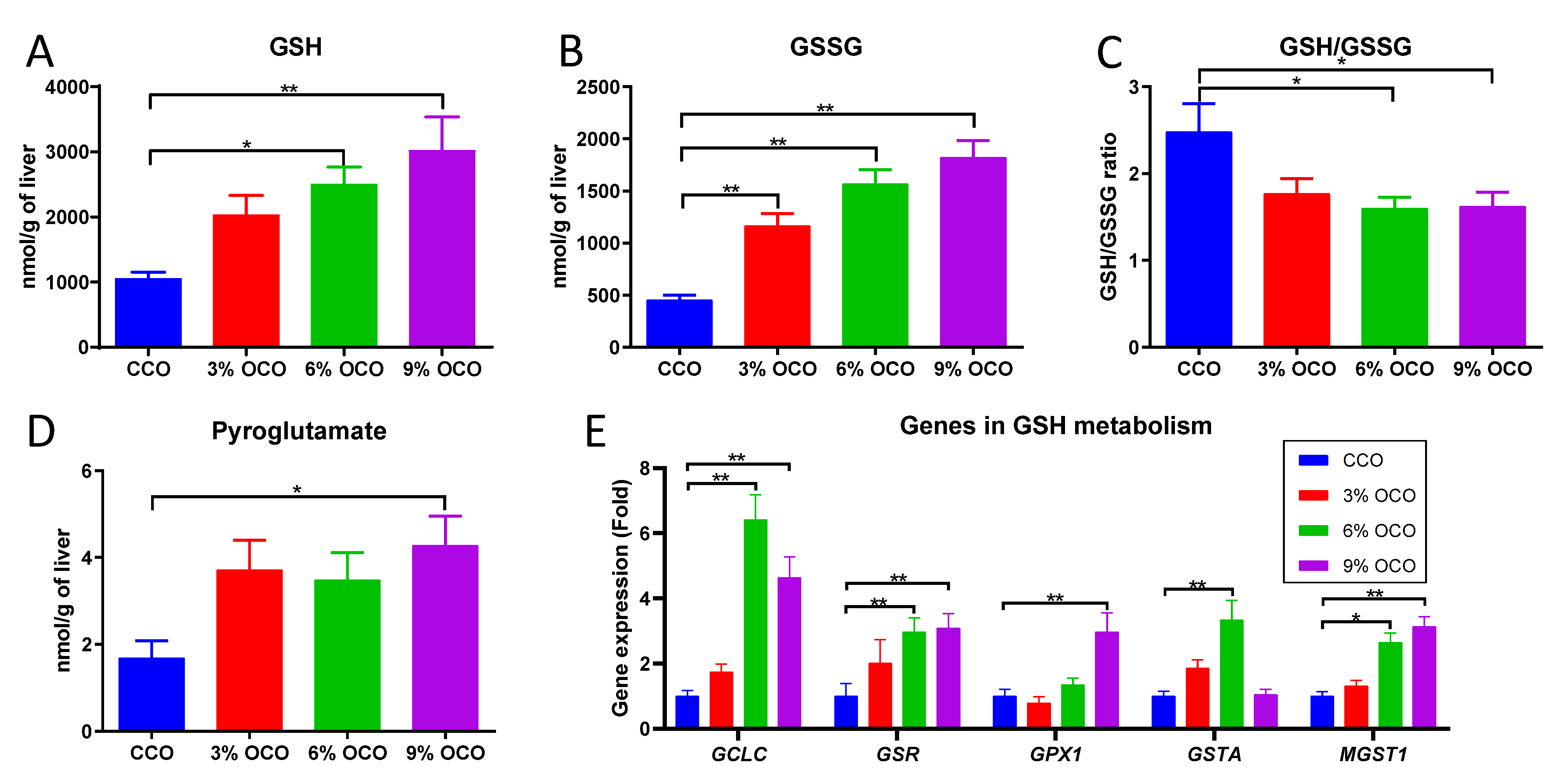
3.4. Effects of OCO on GSH Metabolism
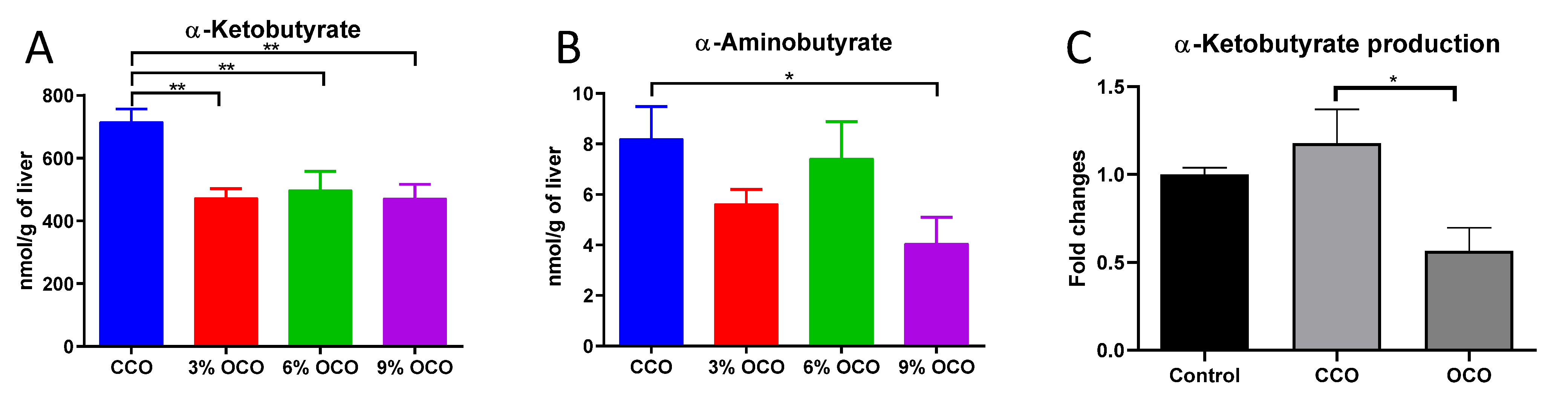
3.5. Effects of OCO on Threonine Catabolism
4. Discussion
4.1. Activation of Tryptophan-NAD+ Biosynthesis as a Protective Metabolic Event against Dietary Oxidized Lipids
4.2. Expansion of GSH Metabolism as a Protective Metabolic Event against Dietary Oxidized Lipids
4.3. Potential Protective Effects of Decreased Threonine Catabolism against Dietary Oxidized Lipids
4.4. Correlations between Serum and the Liver on Decreased Alanine and Glutamate
4.5. Tolerance to Oxidized Lipid Feeding and Protective Amino acid Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canakci, M. The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour. Technol. 2007, 98, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Kerr, B.J.; Kellner, T.A.; Shurson, G.C. Characteristics of lipids and their feeding value in swine diets. J. Anim. Sci. Biotechnol. 2015, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Saleh, A.S.M.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, C.; Kerr, B.J.; Weber, T.E.; Johnston, L.J.; Shurson, G.C. Influence of thermally oxidized vegetable oils and animal fats on growth performance, liver gene expression, and liver and serum cholesterol and triglycerides in young pigs1. J. Anim. Sci. 2014, 92, 2960–2970. [Google Scholar] [CrossRef]
- Boler, D.D.; Fernández-Dueñas, D.M.; Kutzler, L.W.; Zhao, J.; Harrell, R.J.; Campion, D.R.; McKeith, F.K.; Killefer, J.; Dilger, A.C. Effects of oxidized corn oil and a synthetic antioxidant blend on performance, oxidative status of tissues, and fresh meat quality in finishing barrows. J. Anim. Sci. 2012, 90, 5159–5169. [Google Scholar] [CrossRef]
- Rosero, D.S.; Odle, J.; Moeser, A.J.; Boyd, R.D.; van Heugten, E. Peroxidised dietary lipids impair intestinal function and morphology of the small intestine villi of nursery pigs in a dose-dependent manner. Br. J. Nutr. 2015, 114, 1985–1992. [Google Scholar] [CrossRef]
- Yuan, J.; Kerr, B.J.; Curry, S.M.; Chen, C. Identification of C9-C11 unsaturated aldehydes as prediction markers of growth and feed intake for non-ruminant animals fed oxidized soybean oil. J. Anim. Sci. Biotechnol. 2020, 11, 49. [Google Scholar] [CrossRef]
- Eder, K.; Stangl, G.I. Plasma Thyroxine and Cholesterol Concentrations of Miniature Pigs Are Influenced by Thermally Oxidized Dietary Lipids. J. Nutr. 2000, 130, 116–121. [Google Scholar] [CrossRef]
- Silva-Guillen, Y.V.; Arellano, C.; Boyd, R.D.; Martinez, G.; Van Heugten, E. Growth performance, oxidative stress and immune status of newly weaned pigs fed peroxidized lipids with or without supplemental vitamin E or polyphenols. J. Anim. Sci. Biotechnol. 2020, 11, 22. [Google Scholar] [CrossRef]
- Chao, P.-M.; Yang, M.-F.; Tseng, Y.-N.; Chang, K.-M.; Lu, K.-S.; Huang, C.-J. Peroxisome Proliferation in Liver of Rats Fed Oxidized Frying Oil. J. Nutr. Sci. Vitaminol. 2005, 51, 361–368. [Google Scholar] [CrossRef]
- Tavárez, M.; Boler, D.; Bess, K.; Zhao, J.; Yan, F.; Dilger, A.; McKeith, F.; Killefer, J. Effect of antioxidant inclusion and oil quality on broiler performance, meat quality, and lipid oxidation. Poult. Sci. 2011, 90, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.K.; Else, P.L.; Hulbert, A.J. Membrane fatty acid composition of rat skeletal muscle is most responsive to the balance of dietary n-3 and n-6 PUFA. Br. J. Nutr. 2010, 103, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Staprans, I.; Rapp, J.H.; Pan, X.M.; Feingold, K.R. Oxidized lipids in the diet are incorporated by the liver into very low density lipoprotein in rats. J. Lipid Res. 1996, 37, 420–430. [Google Scholar] [CrossRef]
- Fruehwirth, S.; Zehentner, S.; Salim, M.; Sterneder, S.; Tiroch, J.; Lieder, B.; Zehl, M.; Somoza, V.; Pignitter, M. In Vitro Digestion of Grape Seed Oil Inhibits Phospholipid-Regulating Effects of Oxidized Lipids. Biomolecules 2020, 10, 708. [Google Scholar] [CrossRef]
- Eder, K. The effects of a dietary oxidized oil on lipid metabolism in rats. Lipids 1999, 34, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Varady, J.; Gessner, D.K.; Most, E.; Eder, K.; Ringseis, R. Dietary moderately oxidized oil activates the Nrf2 signaling pathway in the liver of pigs. Lipids Health Dis. 2012, 11, 31. [Google Scholar] [CrossRef]
- Koch, A.; König, B.; Spielmann, J.; Leitner, A.; Stangl, G.I.; Eder, K. Thermally Oxidized Oil Increases the Expression of Insulin-Induced Genes and Inhibits Activation of Sterol Regulatory Element-Binding Protein-2 in Rat Liver. J. Nutr. 2007, 137, 2018–2023. [Google Scholar] [CrossRef]
- Stadelmann-Ingrand, S.; Pontcharraud, R.; Fauconneau, B. Evidence for the reactivity of fatty aldehydes released from oxidized plasmalogens with phosphatidylethanolamine to form Schiff base adducts in rat brain homogenates. Chem. Phys. Lipids 2004, 131, 93–105. [Google Scholar] [CrossRef]
- Hashiba, H.; Koshiyama, I.; Fukushima, D. Oxidative Browning of Amadori Compounds from Amino Acids and Peptides. Adv. Exp. Med. Biol. 1977, 86b, 419–448. [Google Scholar]
- Ansari, N.A.; Dash, D. Amadori glycated proteins: Role in production of autoantibodies in diabetes mellitus and effect of inhibitors on non-enzymatic glycation. Aging Dis. 2012, 4, 50–56. [Google Scholar]
- Zhang, Q.; Ames, J.M.; Smith, R.; Baynes, J.W.; Metz, T.O. A Perspective on the Maillard Reaction and the Analysis of Protein Glycation by Mass Spectrometry: Probing the Pathogenesis of Chronic Disease. J. Proteome Res. 2008, 8, 754–769. [Google Scholar] [CrossRef]
- Mol, M.; Regazzoni, L.; Altomare, A.; Degani, G.; Carini, M.; Vistoli, G.; Aldini, G. Enzymatic and non-enzymatic detoxification of 4-hydroxynonenal: Methodological aspects and biological consequences. Free. Radic. Biol. Med. 2017, 111, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Haberzettl, P.; Ahmed, Y.; Srivastava, S.; Bhatnagar, A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem. J. 2008, 410, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yao, D.; Urriola, P.E.; Hanson, A.R.; Saqui-Salces, M.; Kerr, B.J.; Shurson, G.C.; Chen, C. Identification of activation of tryptophan-NAD+ pathway as a prominent metabolic response to thermally oxidized oil through metabolomics-guided biochemical analysis. J. Nutr. Biochem. 2018, 57, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.R.; Urriola, P.E.; Wang, L.; Johnston, L.J.; Chen, C.; Shurson, G.C. Dietary peroxidized maize oil affects the growth performance and antioxidant status of nursery pigs. Anim. Feed. Sci. Technol. 2016, 216, 251–261. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, D.; Chen, C. 2-Hydrazinoquinoline as a Derivatization Agent for LC-MS-Based Metabolomic Investigation of Diabetic Ketoacidosis. Metabolites 2013, 3, 993–1010. [Google Scholar] [CrossRef]
- Bird, M.I.; Nunn, P.B. Metabolic homoeostasis of l-threonine in the normally-fed rat. Importance of liver threonine dehydrogenase activity. Biochem. J. 1983, 214, 687–694. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic and Medical Aspects in Humans. J. Amino Acids 2016, 2016, 8952520. [Google Scholar] [CrossRef]
- Jansman, A.J.; van Diepen, J.T.; Melchior, D. The effect of diet composition on tryptophan requirement of young piglets1. J. Anim. Sci. 2010, 88, 1017–1027. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 10th ed.; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Michael, A.F.; Drummond, K.N.; Doeden, D.; Anderson, J.A.; Good, R.A. Tryptophan metabolism in man. J. Clin. Investig. 1964, 43, 1730–1746. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Ohta, Y.; Nagamura, Y.; Sasaki, E.; Ishiguro, I. Relationship between L-tryptophan uptake and L-tryptophan 2,3-dioxygenase activity in rat hepatocytes. Biochem. Int. 1990, 20, 71–80. [Google Scholar] [PubMed]
- Belenky, P.; Bogan, K.L.; Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 2007, 32, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Pinson, B.; Ceschin, J.; Saint-Marc, C.; Daignan-Fornier, B. Dual control of NAD+ synthesis by purine metabolites in yeast. Elife 2019, 8, e43808. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Ohnishi, M.; Iguchi, S.; Sano, K.; Umezawa, C. Peroxisome-Proliferator Regulates Key Enzymes of the Tryptophan-NAD+ Pathway. Toxicol. Appl. Pharmacol. 1999, 158, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Joffre, F.; Siess, M.-H.; Vernevaut, M.-F.; Collenot, P.; Genty, M.; Sébédio, J.-L. Cyclic fatty acid monomers from heated oil modify the activities of lipid synthesizing and oxidizing enzymes in rat liver. J. Nutr. 2000, 130, 1524–1530. [Google Scholar] [CrossRef]
- Contreras, A.V.; Torres, N.; Tovar, A.R. PPAR-α as a key nutritional and environmental sensor for metabolic adaptation. Adv. Nutr. 2013, 4, 439–452. [Google Scholar] [CrossRef]
- Holden, P.R.; Tugwood, J.D. Peroxisome proliferator-activated receptor alpha: Role in rodent liver cancer and species differences. J. Mol. Endocrinol. 1999, 22, 1–8. [Google Scholar] [CrossRef]
- Cheon, Y.; Nara, T.Y.; Band, M.R.; Beever, J.E.; Wallig, M.A.; Nakamura, M.T. Induction of overlapping genes by fasting and a peroxisome proliferator in pigs: Evidence of functional PPARalpha in nonproliferating species. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, 1525–1535. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Tagnon, M.D.; Simeon, K.O. Aldehyde dehydrogenases may modulate signaling by lipid peroxidation-derived bioactive aldehydes. Plant Sig. Behav. 2017, 12, e1387707. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.; De Groot, H. NAD(P)H, a directly operating antioxidant? FASEB J. 2001, 15, 1569–1574. [Google Scholar] [CrossRef]
- Kamat, J.P.; Devasagayam, T.P. Methylene blue plus light-induced lipid peroxidation in rat liver microsomes: Inhibition by nicotinamide (vitamin B3) and other antioxidants. Chem. Interact. 1996, 99, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Arcaro, A.; Pizzimenti, S.; Daga, M.; Cetrangolo, G.P.; Dianzani, C.; Lepore, A.; Graf, M.; Ames, P.R.J.; Barrera, G. DNA damage by lipid peroxidation products: Implications in cancer, inflammation and autoimmunity. AIMS Genet. 2017, 4, 103–137. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.R.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Denno, R.; Takabayashi, A.; Sugano, M.; Awane, M.; Jin, M.-B.; Morimoto, T.; Tanaka, K.; Yamaoka, Y.; Kobayashi, N.; Ozawa, K. The ratio of reduced glutathione/oxidized glutathione is maintained in the liver during short-term hepatic hypoxia. J. Gastroenterol. 1995, 30, 338–346. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Gallant, S.C.; Sukhich, G.T. Carnosine, the protective, anti-aging peptide. Biosci. Rep. 1999, 19, 581–587. [Google Scholar] [CrossRef]
- Quinn, P.J.; Boldyrev, A.A.; Formazuyk, V.E. Carnosine: Its properties, functions and potential therapeutic applications. Mol. Asp. Med. 1992, 13, 379–444. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Yadav, S. The glutathione cycle: Glutathione metabolism beyond the γ-glutamyl cycle. IUBMB Life 2018, 70, 585–592. [Google Scholar] [CrossRef]
- Metges, C.C.; Yu, Y.-M.; Cai, W.; Lu, X.-M.; Wong, S.; Regan, M.M.; Ajami, A.; Young, V.R. Oxoproline kinetics and oxoproline urinary excretion during glycine- or sulfur amino acid-free diets in humans. Am. J. Physiol. Metab. 2000, 278, E868–E876. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Glutathione, metabolism and function via the gamma-glutamyl cycle. Life Sci. 1974, 15, 177–190. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Tian, L.; Shi, M.M.; Forman, H.J. Increased transcription of the regulatory subunit of gamma-glutamylcysteine synthetase in rat lung epithelial L2 cells exposed to oxidative stress or glutathione depletion. Arch. Biochem. Biophys. 1997, 342, 126–133. [Google Scholar] [CrossRef] [PubMed]
- SShi, M.M.; Iwamoto, T.; Forman, H.J. Gamma-Glutamylcysteine synthetase and GSH increase in quinone-induced oxidative stress in BPAEC. Am. J. Physiol. Content 1994, 267 Pt 1, 414–421. [Google Scholar]
- Chen, C.; Kong, A.-N. Dietary chemopreventive compounds and ARE/EpRE signaling. Free. Radic. Biol. Med. 2004, 36, 1505–1516. [Google Scholar] [CrossRef]
- Jayaraman, B.; Htoo, J.; Nyachoti, C.M. Effects of dietary threonine:lysine ratioes and sanitary conditions on performance, plasma urea nitrogen, plasma-free threonine and lysine of weaned pigs. Anim. Nutr. 2015, 1, 283–288. [Google Scholar] [CrossRef]
- Tang, Q.; Tan, P.; Ma, N.; Ma, X. Physiological Functions of Threonine in Animals: Beyond Nutrition Metabolism. Nutrients 2021, 13, 2592. [Google Scholar] [CrossRef]
- Saldana, C.I.; Knabe, D.A.; Owen, K.Q.; Burgoon, K.G.; Gregg, E.J. Digestible threonine requirements of starter and finisher pigs. J. Anim. Sci. 1994, 72, 144–150. [Google Scholar] [CrossRef]
- Schaart, M.W.; Schierbeek, H.; Van Der Schoor, S.R.D.; Stoll, B.; Burrin, D.G.; Reeds, P.J.; Van Goudoever, J.B. Threonine Utilization Is High in the Intestine of Piglets. J. Nutr. 2005, 135, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Ballevre, O.; Cadenhead, A.; Calder, A.G.; Rees, W.D.; Lobley, G.E.; Fuller, M.F.; Garlick, P.J. Quantitative partition of threonine oxidation in pigs: Effect of dietary threonine. Am. J. Physiol. Metab. 1990, 259 Pt 1, E483–E491. [Google Scholar] [CrossRef] [PubMed]
- Eliot, A.C.; Kirsch, J.F. Pyridoxal Phosphate Enzymes: Mechanistic, Structural and Evolutionary Considerations. Annu. Rev. Biochem. 2004, 73, 383–415. [Google Scholar] [CrossRef] [PubMed]
- Moundras, C.; Bercovici, D.; Rémésy, C.; Demigné, C. Influence of glucogenic amino acids on the hepatic metabolism of threonine. Biochim. Biophys. Acta 1992, 1115, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Polge, A.; Bancel, E.; Strubel, H.B.D.; Peray, S.P.P.; Carlet, C.; DE Bornier, B.M. Plasma amino acid concentrations in elderly patients with protein energy malnutrition. Age Ageing 1997, 26, 457–462. [Google Scholar] [CrossRef]
- Fujita, Y.; Yoshimura, Y.; Inoue, G. Effect of low-protein diets on free amino acids in plasma of young men: Effect of protein quality with maintenance or excess energy intake. J. Nutr. Sci. Vitaminol. 1978, 24, 297–309. [Google Scholar] [CrossRef]
- Noguchi, Y.; Zhang, Q.-W.; Sugimoto, T.; Furuhata, Y.; Sakai, R.; Mori, M.; Takahashi, M.; Kimura, T. Network analysis of plasma and tissue amino acids and the generation of an amino index for potential diagnostic use. Am. J. Clin. Nutr. 2006, 83, 513s–519s. [Google Scholar] [CrossRef] [PubMed]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; van de Graaf, S.F. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef] [PubMed]
- Felig, P. Amino Acid Metabolism in Man. Annu. Rev. Biochem. 1975, 44, 933–955. [Google Scholar] [CrossRef] [PubMed]
- Burri, L.; Thoresen, G.H.; Berge, R.K. The Role of PPARα Activation in Liver and Muscle. PPAR Res. 2010, 2010, 542359. [Google Scholar] [CrossRef]
- Contreras, A.V.; Rangel-Escareño, C.; Torres, N.; Alemán-Escondrillas, G.; Ortiz, V.; Noriega, L.G.; Torre-Villalvazo, I.; Granados, O.; Velázquez-Villegas, L.A.; Tobon-Cornejo, S.; et al. PPARα via HNF4α regulates the expression of genes encoding hepatic amino acid catabolizing enzymes to maintain metabolic homeostasis. Genes Nutr. 2015, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.; Hanson, A.; Shurson, G.; Urriola, P. Peroxidized lipids reduce growth performance of poultry and swine: A meta-analysis. Anim. Feed. Sci. Technol. 2017, 231, 47–58. [Google Scholar] [CrossRef]
- Kerr, B.J.; Lindblom, S.C.; Overholt, M.F. Influence of feeding thermally peroxidized soybean oil on growth performance, digestibility, gut integrity, and oxidative stress in nursery pigs. J. Anim. Sci. 2020, 98, skaa016. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.L.; Boyd, R.D.; Zier-Rush, C.; Rosero, D.S.; Van Heugten, E. Lipid peroxidation impairs growth and viability of nursery pigs reared under commercial conditions1. J. Anim. Sci. 2019, 97, 3379–3389. [Google Scholar] [CrossRef] [PubMed]

| Concentration (µM) | Percentage (%) # | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite | CCO | 3% OCO | 6% OCO | 9% OCO | p-Value | CCO | 3% OCO | 6% OCO | 9% OCO | p-Value |
| Ala | 318.28 a | 286.11 ab | 301.56 ab | 254.21 b | 0.04 | 7.37 | 6.87 | 7.35 | 6.50 | 0.16 |
| Arg * | 116.35 | 124.96 | 117.11 | 108.43 | 0.46 | 2.66 | 3.03 | 2.85 | 2.74 | 0.24 |
| Asn | 83.96 | 68.88 | 71.28 | 72.15 | 0.84 | 1.88 | 1.67 | 1.71 | 1.74 | 0.94 |
| Asp | 25.98 | 22.64 | 23.49 | 20.19 | 0.45 | 0.59 | 0.54 | 0.57 | 0.51 | 0.67 |
| Cit | 39.06 | 41.38 | 42.73 | 42.21 | 0.96 | 0.91 | 0.98 | 1.05 | 1.09 | 0.78 |
| Gln | 453.09 | 381.13 | 411.00 | 390.04 | 0.18 | 10.45 | 9.24 | 10.02 | 10.03 | 0.51 |
| Glu | 474.78 a | 348.01 ab | 397.11 ab | 310.29 b | 0.02 | 10.89 a | 8.36 ab | 9.65 ab | 7.89 b | 0.04 |
| Gly | 1073.68 | 1073.73 | 1036.56 | 1018.71 | 0.85 | 24.99 | 25.85 | 25.14 | 26.28 | 0.82 |
| His * | 51.63 | 55.21 | 49.85 | 48.08 | 0.47 | 1.18 | 1.34 | 1.22 | 1.20 | 0.67 |
| Iso/Leu * | 204.66 | 197.58 | 209.91 | 180.88 | 0.40 | 4.68 | 4.77 | 5.10 | 4.58 | 0.38 |
| Lys * | 149.08 | 132.75 | 124.84 | 154.35 | 0.62 | 3.40 | 3.23 | 2.97 | 3.85 | 0.33 |
| Met * | 22.84 | 19.34 | 22.80 | 23.94 | 0.66 | 0.52 | 0.47 | 0.55 | 0.59 | 0.58 |
| Orn | 60.10 | 63.74 | 61.31 | 67.11 | 0.80 | 1.36 | 1.53 | 1.48 | 1.71 | 0.15 |
| Phe * | 124.66 | 131.40 | 124.30 | 114.91 | 0.36 | 2.87 | 3.21 | 3.04 | 2.92 | 0.36 |
| Pro | 278.39 | 269.86 | 275.09 | 253.78 | 0.50 | 6.43 | 6.50 | 6.69 | 6.46 | 0.72 |
| Ser | 215.06 | 233.19 | 205.54 | 219.91 | 0.71 | 4.94 | 5.67 | 4.95 | 5.57 | 0.25 |
| Tau | 228.05 | 227.05 | 181.05 | 204.54 | 0.85 | 5.18 | 5.47 | 4.28 | 5.23 | 0.80 |
| Thr * | 118.64 | 150.78 | 147.48 | 146.60 | 0.44 | 2.69 | 3.63 | 3.55 | 3.67 | 0.06 |
| Trp * | 43.06 a | 34.34 ab | 35.80 ab | 30.98 b | 0.04 | 0.99 | 0.84 | 0.86 | 0.78 | 0.10 |
| Tyr | 46.75 | 47.14 | 56.25 | 60.60 | 0.33 | 1.05 | 1.13 | 1.38 | 1.46 | 0.11 |
| Val | 215.99 | 232.79 | 230.90 | 203.55 | 0.60 | 4.95 | 5.63 | 5.59 | 5.15 | 0.38 |
| Total | 4344.06 | 4141.98 | 4125.95 | 3925.44 | 0.40 | |||||
| Carnosine | 14.56 | 11.53 | 9.01 | 9.3 | 0.01 | |||||
| Ions | Modes of Ion Detection | m/z of Detection | Identity | Formula | ∆ppm | Database ID | Effects of OCO |
|---|---|---|---|---|---|---|---|
| Is | [M+DC]+ | 323.1053 | Alanine * | C3H7NO2 | 4.0 | HMDB0000161 | ↓ |
| IIs | [M+DC]+ | 381.1110 | Glutamate * | C5H9NO4 | 2.6 | HMDB0060475 | ↓ |
| IIIs | [M+DC]+ | 438.1478 | Tryptophan * | C11H12N2O2 | 2.3 | HMDB0030396 | ↓ |
| IVs | [M+DC]+ | 460.1648 | Carnosine * | C9H14N4O3 | 0.4 | HMDB0000033 | ↓ |
| Vs | [M+DC]+ | 353.1160 | Threonine * | C4H9NO3 | 3.1 | HMDB0000167 | ↑ |
| Ions | Modes of Ion Detection | m/z of Detection | Identity | Formula | ∆ppm | Database ID | Effects of OCO |
|---|---|---|---|---|---|---|---|
| IL | [M-H]− | 175.0236 | Ascorbic acid * | C6H8O6 | −4.5 | HMDB0000044 | ↓ |
| IIL | [M+DC]+ | 323.1055 | Alanine * | C3H7NO2 | −3.4 | HMDB0000161 | ↓ |
| IIIL | [M−H]− [M+H]+ | 346.0551 348.0691 | AMP * | C10H14N5O7P | −0.6 −5.2 | HMDB0000045 | ↑ |
| IVL | [M−H]− [M+H]+ | 611.1444 613.1590 | GSSG * | C20H32N6O12S2 | 0.7 −1.3 | HMDB0003337 | ↑ |
| VL | [M−H]− | 306.0758 | GSH * | C10H17N3O6S | −4.8 | HMDB0000125 | ↑ |
| VIL | [M+H]+ | 664.1165 | NAD * | C21H27N7O14P2 | −0.1 | HMDB0000902 | ↑ |
| VIIL | [M+H]+ | 130.0499 | Pyroglutamate * | C5H7NO3 | −2.5 | HMDB0000267 | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wang, L.; Hanson, A.; Urriola, P.E.; Shurson, G.C.; Chen, C. Identification of Protective Amino Acid Metabolism Events in Nursery Pigs Fed Thermally Oxidized Corn Oil. Metabolites 2023, 13, 103. https://doi.org/10.3390/metabo13010103
Guo Y, Wang L, Hanson A, Urriola PE, Shurson GC, Chen C. Identification of Protective Amino Acid Metabolism Events in Nursery Pigs Fed Thermally Oxidized Corn Oil. Metabolites. 2023; 13(1):103. https://doi.org/10.3390/metabo13010103
Chicago/Turabian StyleGuo, Yue, Lei Wang, Andrea Hanson, Pedro E. Urriola, Gerald C. Shurson, and Chi Chen. 2023. "Identification of Protective Amino Acid Metabolism Events in Nursery Pigs Fed Thermally Oxidized Corn Oil" Metabolites 13, no. 1: 103. https://doi.org/10.3390/metabo13010103
APA StyleGuo, Y., Wang, L., Hanson, A., Urriola, P. E., Shurson, G. C., & Chen, C. (2023). Identification of Protective Amino Acid Metabolism Events in Nursery Pigs Fed Thermally Oxidized Corn Oil. Metabolites, 13(1), 103. https://doi.org/10.3390/metabo13010103










