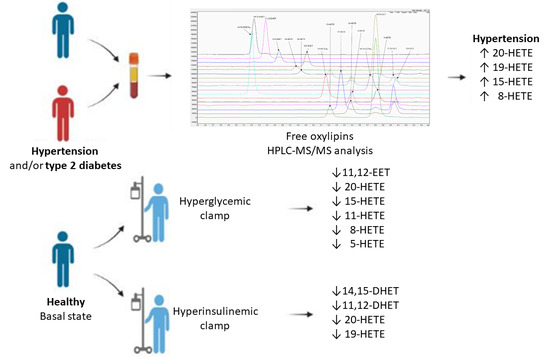
Determination of Lipoxygenase, CYP450, and Non-Enzymatic Metabolites of Arachidonic Acid in Essential Hypertension and Type 2 Diabetes
Abstract
:1. Introduction
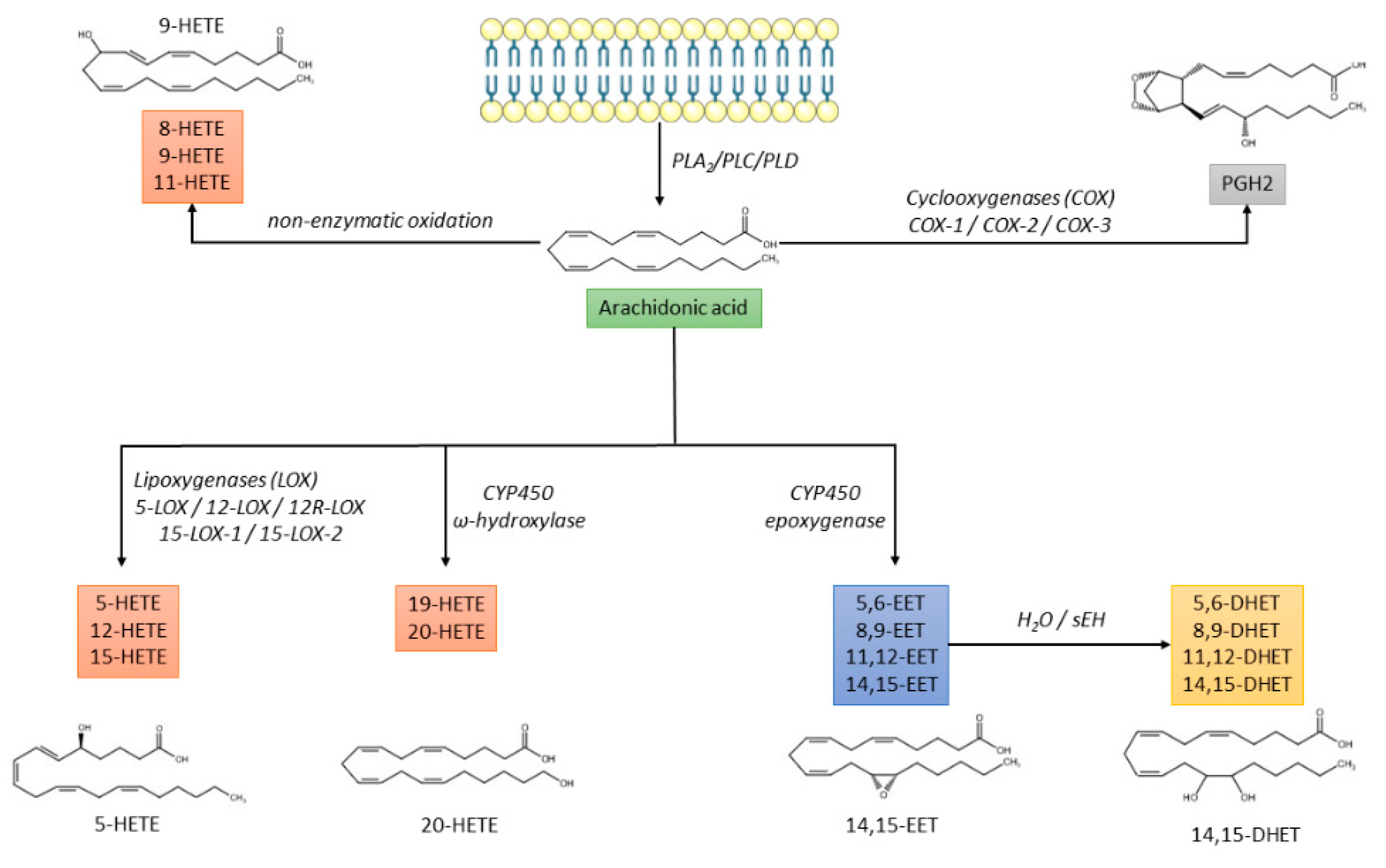
- The cyclo-oxygenase (COX) pathway is responsible for the production of prostaglandin H2 (PGH2), which is further converted into prostaglandins (PGD2, PGE2, PGF2α, PGI2) or thromboxane A2 (TXA2) [13];
- The lipoxygenase (LOX) pathway that produces hydroxyeicosatetraenoic acids (HETEs), leukotrienes, and lipoxins;
- The cytochrome P450 (CYP) pathway that promotes the synthesis of HETEs and epoxyeicosatrienoic acids (EETs), which are further converted by soluble epoxide hydrolase (sEH) into dihydroxieicosatrienoic acids (DHETs) [14];
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. DHETs, HETEs, and EETs Quantitation
2.2.1. Sample Preparation
2.2.2. LC-MS/MS Conditions
2.2.3. Method Validation
- Calibration curves were obtained by spiking the standards at increasing concentrations (0, 10, 20, 50, 100, 200, 500, 1000, 2000, and 5000 pg/mL) with a fixed concentration of the IS (30 ng/mL for both 14,15-DHET-d11, 14,15-EET-d11 and 15-HETE-d8) using a different matrix: phosphate-buffered saline (PBS), BSA (PBS + bovine serum albumin 8%), and plasma;
- Calibration curve linearity, lower limit of quantification (LLOQ), and carryover were assessed according to FDA guidelines on validation of bioanalytical methods for each analyte [22]. Parallelism between the three matrices were also investigated;
- Sample recovery (RE), matrix effect (ME), and process efficiency (PE) were determined according to Matuszewski et al. [23]. Calibration curves (0, 10, 20, 50, 100, 200, 500, 1000 pg/mL) were prepared in MeOH as a reference matrix (Set 1). Bovine serum albumin (BSA), phosphate-buffered saline (PBS) and plasma matrix were spiked post-extraction (Set 2) and pre-extraction (Set 3). BSA and PBS were spiked with the same calibrator levels as Set 1. Plasma matrix was spiked with three calibrator levels (200, 500, and 1000 pg/mL) and baseline signal due to the presence of endogenous analytes was subtracted to obtain the true spiked signal. This allowed for calculation of RE (Set 3/Set 2 × 100), ME (Set 2/Set 1 × 100), and PE (Set 3/Set 1 × 100);
- Oxylipins adsorption during the deproteinization step was performed using standard eppendorf (1.5 mL), Lobind® eppendorf (2 mL), and Chromacol (4 mL). PBS was used as a surrogate matrix and spiked with 10 ng/mL of each compound. Analyses were performed in triplicate;
- Comparative analysis of lithium heparinate and ethylenediaminetetraacetic acid (EDTA) on compound concentrations was performed. Samples were drawn at the same time for the same subject for a 1-on-1 comparison and the concentration of each oxylipin was assessed. Analyses were performed from five subjects randomly selected from the study and for whom an aliquot of plasma from EDTA and heparin tubes remained.
2.3. Population
2.4. Hyperglycemic and Hyperinsulinemic Clamps
2.5. Statistical Analysis
3. Results
3.1. DHETs, HETEs and EETs Analytical Method
3.2. Baseline Popoulation Characteristics
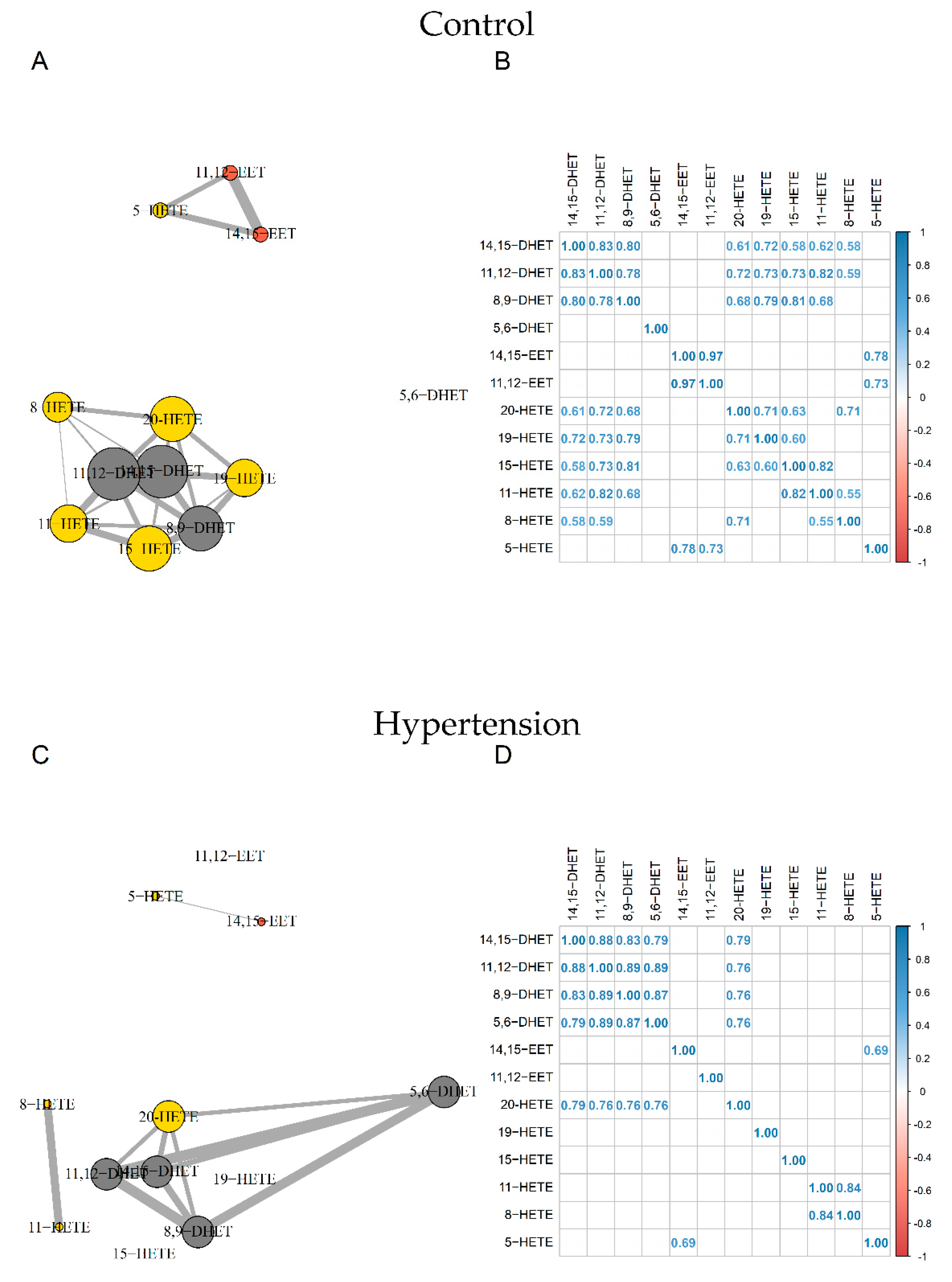
3.3. Analysis of Oxylipin Profiles
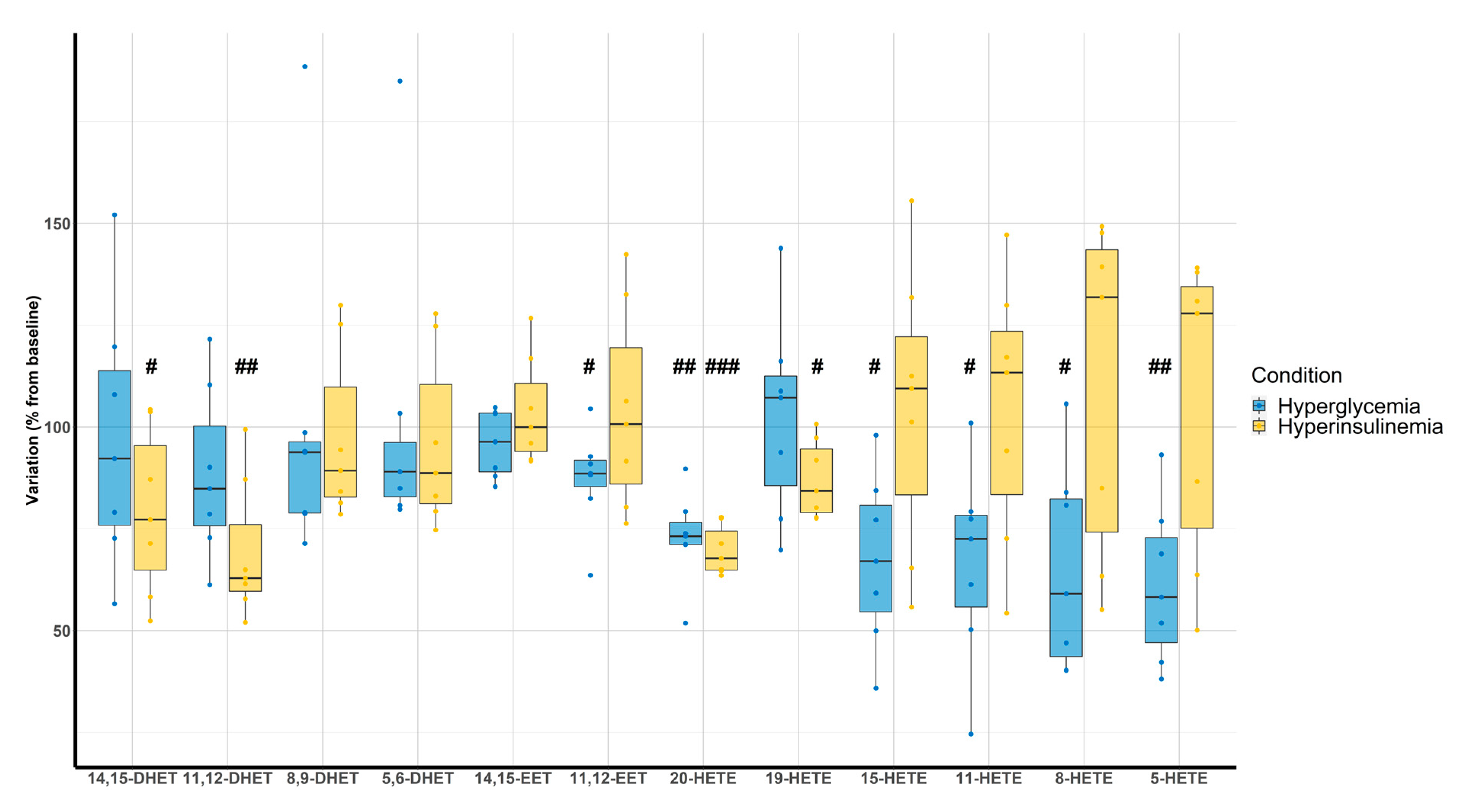
3.4. Clamp Investigations
4. Discussion
4.1. Analytical Method
4.2. Oxylipin Analysis in Pathological Status
4.3. Clamps
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunkara, N.; Ahsan, C.H. Hypertension in diabetes and the risk of cardiovascular disease. Cardiovasc. Endocrinol. 2017, 6, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Pietri, P.; Vyssoulis, G.; Vlachopoulos, C.; Zervoudaki, A.; Gialernios, T.; Aznaouridis, K.; Stefanadis, C. Relationship between low-grade inflammation and arterial stiffness in patients with essential hypertension. J. Hypertens. 2006, 24, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta 2013, 1832, 2216–2231. [Google Scholar] [CrossRef]
- van Sloten, T.T.; Henry, R.M.; Dekker, J.M.; Nijpels, G.; Unger, T.; Schram, M.T.; Stehouwer, C.D. Endothelial dysfunction plays a key role in increasing cardiovascular risk in type 2 diabetes: The Hoorn study. Hypertension 2014, 64, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Yuyun, M.F.; Ng, L.L.; Ng, G.A. Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy? Microvasc. Res. 2018, 119, 7–12. [Google Scholar] [CrossRef]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef]
- Roman, R.J. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 2002, 82, 131–185. [Google Scholar] [CrossRef]
- Campbell, W.B.; Gebremedhin, D.; Pratt, P.F.; Harder, D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996, 78, 415–423. [Google Scholar] [CrossRef]
- Frömel, T.; Fleming, I. Whatever happened to the epoxyeicosatrienoic Acid-like endothelium-derived hyperpolarizing factor? The identification of novel classes of lipid mediators and their role in vascular homeostasis. Antioxid. Redox Signal. 2015, 22, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef]
- Massey, K.A.; Nicolaou, A. Lipidomics of oxidized polyunsaturated fatty acids. Free Radic. Biol. Med. 2013, 59, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed]
- Palmu, J.; Watrous, J.D.; Mercader, K.; Havulinna, A.S.; Lagerborg, K.A.; Salosensaari, A.; Inouye, M.; Larson, M.G.; Rong, J.; Vasan, R.S.; et al. Eicosanoid Inflammatory Mediators Are Robustly Associated With Blood Pressure in the General Population. J. Am. Heart Assoc. 2020, 9, e017598. [Google Scholar] [CrossRef]
- Tuomisto, K.; Palmu, J.; Long, T.; Watrous, J.D.; Mercader, K.; Lagerborg, K.A.; Andres, A.; Salmi, M.; Jalkanen, S.; Vasan, R.S.; et al. A plasma metabolite score of three eicosanoids predicts incident type 2 diabetes: A prospective study in three independent cohorts. BMJ Open Diabetes Res. Care 2022, 10, e002519. [Google Scholar] [CrossRef]
- Das, U.N. Arachidonic acid in health and disease with focus on hypertension and diabetes mellitus: A review. J. Adv. Res. 2018, 11, 43–55. [Google Scholar] [CrossRef]
- Duflot, T.; Moreau-Grangé, L.; Roche, C.; Iacob, M.; Wils, J.; Rémy-Jouet, I.; Cailleux, A.; Leuillier, M.; Renet, S.; Li, D.; et al. Altered bioavailability of epoxyeicosatrienoic acids is associated with conduit artery endothelial dysfunction in type 2 diabetic patients. Cardiovasc. Diabetol. 2019, 18, 35. [Google Scholar] [CrossRef] [Green Version]
- Shearer, G.C.; Newman, J.W. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 215–222. [Google Scholar] [CrossRef] [PubMed]
- FDA Guidance: Guidance for Industry. Bioanalytical Method Validation. 2018. Available online: https://www.fda.gov/media/70858/download (accessed on 24 August 2022).
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 20 July 2022).
- Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R Package Version 0.9-81-2. 2022. Available online: https://CRAN.R-project.org/package=RVAideMemoire (accessed on 20 July 2022).
- Xiao, N. ggsci: Scientific Journal and Sci-Fi Themed Color Palettes for ‘ggplot2’. R Package Version 2.9. 2018. Available online: https://CRAN.R-project.org/package=ggsci (accessed on 20 July 2022).
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 20 July 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0. 2021. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 20 July 2022).
- Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.6.1. 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 20 July 2022).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed EffectsModels. R Package Version 3.1-152. 2021. Available online: https://CRAN.R-project.org/package=nlme (accessed on 20 July 2022).
- Wei, T.; Simko, V. R Package “corrplot”: Visualization of a Correlation Matrix (Version 0.88). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 20 July 2022).
- Wickham, H. Reshaping Data with the reshape Package. J. Stat. Softw. 2007, 21, 1–20. Available online: http://www.jstatsoft.org/v21/i12/ (accessed on 20 July 2022). [CrossRef]
- Csardi, G.; Nepusz, T. The Igraph Software Package for Complex Network Research, InterJournal, Complex Systems 1695. 2006. Available online: https://igraph.org (accessed on 20 July 2022).
- Yuan, Z.X.; Majchrzak-Hong, S.; Keyes, G.S.; Iadarola, M.J.; Mannes, A.J.; Ramsden, C.E. Lipidomic profiling of targeted oxylipins with ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 6009–6029. [Google Scholar] [CrossRef]
- Liakh, I.; Pakiet, A.; Sledzinski, T.; Mika, A. Modern Methods of Sample Preparation for the Analysis of Oxylipins in Biological Samples. Molecules 2019, 24, 1639. [Google Scholar] [CrossRef]
- Jonasdottir, H.S.; Brouwers, H.; Toes, R.E.M.; Ioan-Facsinay, A.; Giera, M. Effects of anticoagulants and storage conditions on clinical oxylipid levels in human plasma. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1511–1522. [Google Scholar] [CrossRef]
- Jones, B.R.; Schultz, G.A.; Eckstein, J.A.; Ackermann, B.L. Surrogate matrix and surrogate analyte approaches for definitive quantitation of endogenous biomolecules. Bioanalysis 2012, 4, 2343–2356. [Google Scholar] [CrossRef]
- Mainka, M.; Dalle, C.; Pétéra, M.; Dalloux-Chioccioli, J.; Kampschulte, N.; Ostermann, A.I.; Rothe, M.; Bertrand-Michel, J.; Newman, J.W.; Gladine, C.; et al. Harmonized procedures lead to comparable quantification of total oxylipins across laboratories. J. Lipid Res. 2020, 61, 1424–1436. [Google Scholar] [CrossRef] [PubMed]
- Soler, A.; Hunter, I.; Joseph, G.; Hutcheson, R.; Hutcheson, B.; Yang, J.; Zhang, F.F.; Joshi, S.R.; Bradford, C.; Gotlinger, K.H.; et al. Elevated 20-HETE in metabolic syndrome regulates arterial stiffness and systolic hypertension via MMP12 activation. J. Mol. Cell. Cardiol. 2018, 117, 88–99. [Google Scholar] [CrossRef]
- Williams, J.M.; Murphy, S.; Burke, M.; Roman, R.J. 20-hydroxyeicosatetraeonic acid: A new target for the treatment of hypertension. J. Cardiovasc. Pharmacol. 2010, 56, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Shoieb, S.M.; El-Kadi, A.O.S. S-Enantiomer of 19-Hydroxyeicosatetraenoic Acid Preferentially Protects Against Angiotensin II-Induced Cardiac Hypertrophy. Drug Metab. Dispos. 2018, 46, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Dakarapu, R.; Errabelli, R.; Manthati, V.L.; Adebesin, A.M.; Barma, D.K.; Barma, D.; Garcia, V.; FanZhang; Schwartzman, M.L.; Falck, J.R. 19-Hydroxyeicosatetraenoic acid analogs: Antagonism of 20-hydroxyeicosatetraenoic acid-induced vascular sensitization and hypertension. Bioorg. Med. Chem. Lett. 2019, 29, 126616. [Google Scholar] [CrossRef] [PubMed]
- Chawengsub, Y.; Gauthier, K.M.; Campbell, W.B. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H495–H507. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Spitzbarth, N.; Kuhn, H.; Chaitidis, P.; Campbell, W.B. Interleukin-13 upregulates vasodilatory 15-lipoxygenase eicosanoids in rabbit aorta. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Miyatake, K.; Dahlén, S.E. Pharmacodynamics of 15(S)-hydroperoxyeicosatetraenoic (15-HPETE) and 15(S)-hydroxyeicosatetraenoic acid (15-HETE) in isolated arteries from guinea pig, rabbit, rat and human. J. Pharmacol. Exp. Ther. 1995, 273, 1182–1189. [Google Scholar]
- Van Diest, M.J.; Verbeuren, T.J.; Herman, A.G. 15-lipoxygenase metabolites of arachidonic acid evoke contractions and relaxations in isolated canine arteries: Role of thromboxane receptors, endothelial cells and cyclooxygenase. J. Pharmacol. Exp. Ther. 1991, 256, 194–203. [Google Scholar]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta 2015, 1851, 340–355. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Abdelhamid, G.; El-Kadi, A.O. Development of cellular hypertrophy by 8-hydroxyeicosatetraenoic acid in the human ventricular cardiomyocyte, RL-14 cell line, is implicated by MAPK and NF-κB. Cell Biol. Toxicol. 2015, 31, 241–259. [Google Scholar] [CrossRef]
- Zu, L.; Guo, G.; Zhou, B.; Gao, W. Relationship between metabolites of arachidonic acid and prognosis in patients with acute coronary syndrome. Thromb. Res. 2016, 144, 192–201. [Google Scholar] [CrossRef]
- Garcia, V.; Gilani, A.; Shkolnik, B.; Pandey, V.; Zhang, F.F.; Dakarapu, R.; Gandham, S.K.; Reddy, N.R.; Graves, J.P.; Gruzdev, A.; et al. 20-HETE Signals Through G-Protein-Coupled Receptor GPR75 (Gq) to Affect Vascular Function and Trigger Hypertension. Circ. Res. 2017, 120, 1776–1788. [Google Scholar] [CrossRef]
- Akbari, P.; Gilani, A.; Sosina, O.; Kosmicki, J.A.; Khrimian, L.; Fang, Y.; Persaud, T.; Garcia, V.; Sun, D.; Li, A.; et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science 2021, 373, eabf8683. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.; Agostinucci, K.; Hossain, S.; Pascale, J.V.; Garcia, V.; Adebesin, A.M.; Falck, J.R.; Schwartzman, M.L. 20-HETE interferes with insulin signaling and contributes to obesity-driven insulin resistance. Prostaglandins Other Lipid Mediat. 2021, 152, 106485. [Google Scholar] [CrossRef] [PubMed]
- Tunaru, S.; Bonnavion, R.; Brandenburger, I.; Preussner, J.; Thomas, D.; Scholich, K.; Offermanns, S. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat. Commun. 2018, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lai, G.; Wu, J.; Sun, R.; Xu, R.; Yang, X.; Qi, Y.; Zhao, Y. 20-HETE attenuates the response of glucose-stimulated insulin secretion through the AKT/GSK-3β/Glut2 pathway. Endocrine 2016, 54, 371–382. [Google Scholar] [CrossRef]
- Laffer, C.L.; Laniado-Schwartzman, M.; Nasjletti, A.; Elijovich, F. 20-HETE and circulating insulin in essential hypertension with obesity. Hypertension 2004, 43, 388–392. [Google Scholar] [CrossRef] [Green Version]

| Analyte and IS | Recovery | Matrix Effect | Process Efficiency | Analyte/IS Process Efficiency | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BSA | PBS | Plasma | BSA | PBS | Plasma | BSA | PBS | Plasma | BSA | PBS | Plasma | |
| 14,15-DHET | 35.9 ± 10.2 (28%) | 35.3 ± 6.0 (17%) | 32.3 ± 9.4 (29%) | 109.2 ± 30.6 (28%) | 98.5 ± 18.1 (18%) | 54.3 ± 6.9 (13%) | 37.1 ± 7.9 (21%) | 34.6 ± 7.2 (21%) | 17.1 ± 3.6 (21%) | 82.6 ± 13.0 (16%) | 88.6 ± 18.2 (21%) | 122.0 ± 29.1 (24%) |
| 11,12-DHET | 47.7 ± 10.5 (22%) | 45.7 ± 6.8 (15%) | 43.7 ± 11.1 (25%) | 90.7 ± 10.7 (12%) | 88.8 ± 7.6 (9%) | 61.9 ± 9.3 (15%) | 43.1 ± 10.2 (24%) | 40.5 ± 6.3 (16%) | 26.5 ± 4.8 (18%) | 96.0 ± 16.9 (18%) | 103.7 ± 15.6 (15%) | 189.1 ± 40.8 (22%) |
| 8,9-DHET | 65.2 ± 12.4 (19%) | 74.5 ± 17.4 (23%) | 53.5 ± 5.1 (10%) | 87.6 ± 11.5 (13%) | 89.6 ± 9.8 (11%) | 60.0 ± 8.3 (14%) | 57.0 ± 120 (21%) | 65.8 ± 11.2 (17%) | 32.2 ± 6.2 (19%) | 128.5 ± 27.0 (21%) | 169.1 ± 30.8 (18%) | 233.5 ± 66.3 (28%) |
| 5,6-DHET | 68.0 ± 9.8 (14%) | 79.8 ± 12.5 (16%) | 67.9 ± 16.0 (24%) | 99.1 ± 13.0 (13%) | 92.4 ± 8.9 (10%) | 71.3 ± 6.9 (10%) | 66.5 ± 7.1 (11%) | 73.6 ± 12.4 (17%) | 47.8 ± 9.2 (19%) | 150.1 ± 21.7 (14%) | 189.3 ± 35.2 (19%) | 336.7 ± 52.4 (16%) |
| 14,15-EET | 68.3 ± 10.8 (16%) | 83.0 ± 11.9 (14%) | 67.1 ± 13.1 (19%) | 91.5 ± 10.5 (12%) | 93.2 ± 10.1 (11%) | 72.0 ± 7.0 (10%) | 61.9 ± 9.0 (15%) | 77.1 ± 12.4 (16%) | 48.3 ± 10.0 (21%) | 82.5 ± 14.0 (17%) | 85.7 ± 12.4 (15%) | 92.9 ± 15.5 (17%) |
| 11,12-EET | 66.5 ± 30.4 (46%) | 95.6 ± 44.9 (47%) | 42.1 ± 12.3 (29%) | 53.9 ± 20.3 (38%) | 80.5 ± 22.0 (27%) | 21.6 ± 5.6 (26%) | 33.4 ± 12.7 (38%) | 72.0 ± 26.8 (37%) | 9.3 ± 4.6 (49%) | 44.4 ± 16.4 (37%) | 80.2 ± 30.8 (38%) | 18.1 ± 9.0 (50%) |
| 8,9-EET | 59.4 ± 23.4 (39%) | 71.8 ± 14.6 (20%) | 43.6 ± 16.6 (38%) | 60.6 ± 14.3 (24%) | 88.5 ± 18.0 (20%) | 12.7 ± 4.8 (38%) | 37.3 ± 16.8 (45%) | 63.5 ± 16.3 (26%) | 6.0 ± 4.3 (71%) | 48.5 ± 18.6 (38%) | 72.8 ± 19.1 (26%) | 11.5 ± 7.7 (67%) |
| 20-HETE | 79.3 ± 27.9 (35%) | 79.3 ± 23.6 (30%) | 61.2 ± 13.7 (22%) | 89.8 ± 13.2 (15%) | 90.3 ± 19.4 (22%) | 72.4 ± 13.4 (19%) | 70.5 ± 23.9 (34%) | 68.8 ± 14.5 (21%) | 43.5 ± 9.3 (21%) | 91.1 ± 30.6 (34%) | 77.9 ± 11.1 (14%) | 74.8 ± 20.0 (27%) |
| 19-HETE | 62.2 ± 22.1 (36%) | 72.3 ± 13.9 (19%) | 60.2 ± 18.7 (31%) | 106.1 ± 30.8 (29%) | 91.8 ± 10.4 (11%) | 82.7 ± 15.3 (18%) | 65.6 ± 30.5 (46%) | 65.7 ± 11.1 (17%) | 47.6 ± 8.6 (18%) | 82.3 ± 33.4 (41%) | 77.3 ± 17.0 (22%) | 80.6 ± 12.3 (15%) |
| 15-HETE | 67.8 ± 14.0 (21%) | 77.8 ± 15.3 (20%) | 64.9 ± 15.9 (24%) | 99.7 ± 19.0 (19%) | 92.5 ± 10.9 (12%) | 90.5 ± 10.3 (11%) | 68.1 ± 25.0 (37%) | 71.1 ± 11.4 (16%) | 57.6 ± 9.8 (17%) | 89.8 ± 40.0 (45%) | 80.9 ± 9.2 (11%) | 98.4 ± 20.6 (21%) |
| 12-HETE | 73.0 ± 8.7 (12%) | 80.1 ± 7.1 (9%) | 57.9 ± 17.3 (30%) | 94.1 ± 14.6 (15%) | 97.2 ± 7.7 (8%) | 91.3 ± 26.0 (28%) | 68.0 ± 8.7 (13%) | 77.7 ± 7.3 (9%) | 50.8 ± 16.9 (33%) | 88.1 ± 14.7 (17%) | 89.6 ± 10.8 (12%) | 87.4 ± 29.0 (33%) |
| 11-HETE | 77.1 ± 10.6 (14%) | 85.8 ± 10.4 (12%) | 64.5 ± 12.6 (20%) | 105.4 ± 15.2 (14%) | 102.9 ± 12.9 (13%) | 77.2 ± 15.7 (20%) | 81.1 ± 15.1 (19%) | 88.2 ± 14.1 (16%) | 49.3 ± 11.5 (23%) | 104.8 ± 15.6 (15%) | 100.6 ± 14.8 (15%) | 85.4 ± 24.9 (29%) |
| 8-HETE | 72.8 ± 12.1 (17%) | 77 ± 9.5 (12%) | 62.1 ± 20 (32%) | 89.2 ± 16.8 (19%) | 86.9 ± 12 (14%) | 72.4 ± 20.1 (28%) | 64.1 ± 12.4 (19%) | 66.8 ± 12.4 (19%) | 43 ± 12 (28%) | 82.4 ± 15.9 (19%) | 77.9 ± 12.5 (16%) | 75.3 ± 27.1 (36%) |
| 5-HETE | 75.6 ± 11 (15%) | 78.6 ± 5.6 (7%) | 76.7 ± 19.3 (25%) | 100.6 ± 12.2 (12%) | 100.3 ± 8.2 (8%) | 53.4 ± 11 (21%) | 75.5 ± 11 (15%) | 78.7 ± 7.1 (9%) | 39.6 ± 7.1 (18%) | 97.5 ± 16.4 (17%) | 92.7 ± 14.3 (15%) | 67.3 ± 11.4 (17%) |
| 14,15-DHET-d11 | 43.6 ± 7.5 (17%) | 39.6 ± 4.7 (12%) | 24.6 ± 7.2 (29%) | 102.3 ± 9.1 (9%) | 100.6 ± 6.9 (7%) | 62.5 ± 19 (30%) | 45 ± 7.4 (16%) | 39.3 ± 5.3 (14%) | 14.2 ± 2.1 (15%) | - | - | - |
| 14,15-EET-d11 | 76.8 ± 10.8 (14%) | 89 ± 15.1 (17%) | 72.2 ± 12.4 (17%) | 98.8 ± 9.2 (9%) | 103.5 ± 8.5 (8%) | 73.2 ± 10.9 (15%) | 76.1 ± 11.2 (15%) | 90.9 ± 14.3 (16%) | 52.4 ± 9.7 (18%) | - | - | - |
| 15-HETE-d8 | 80.2 ± 14.1 (18%) | 90.1 ± 11.4 (13%) | 69.7 ± 8.2 (12%) | 97.9 ± 10.4 (11%) | 98.1 ± 6.8 (7%) | 85.5 ± 9.6 (11%) | 77.9 ± 12.4 (16%) | 88.4 ± 12.8 (14%) | 59.2 ± 6.9 (12%) | - | - | - |
| Parameters | Control (n = 12) | HTN (n = 8) | T2D (n = 7) | HTN + T2D (n = 17) |
|---|---|---|---|---|
| Age, years | 56 [53–61] | 57 [54–61] | 58 [55–63] | 60 [58–66] |
| Male, n (%) | 5 (42%) | 4 (50%) | 2 (29%) | 11 (65%) |
| Body mass index (kg/m2) | 25 [23–26] | 25 [25–27] | 26 [25–30] | 31 [29–34] *,† |
| Smoking status, n | ||||
| Current/Past/Never | 1/4/7 | 0/0/8 | 1/1/5 | 0/9/8 |
| SBP, mmHg | 125 [119–133] | 140 [130–146] | 129 [127–140] | 135 [132–141] |
| DBP, mmHg | 77 [74–80] | 86 [82–90] | 81 [79–89] | 81 [72–91] |
| MBP, mmHg | 92 [91–95] | 106 [97–109] | 95 [95–105] | 100 [93–108] |
| Heart rate, bpm | 64 [60–71] | 58 [56–72] | 71 [61–81] | 72 [64–76] |
| LDL cholesterol, g/L | 1.48 [1.37–1.66] | 1.17 [1.07–1.24] | 1.25 [1.02–1.38] | 0.80 [0.62–1.04] * |
| HDL cholesterol, g/L | 0.59 [0.48–0.70] | 0.54 [0.47–0.69] | 0.70 [0.47–0.73] | 0.53 [0.40–0.61] |
| Triglycerides, g/L | 0.74 [0.70–0.91] | 0.88 [0.72–1.5] | 0.99 [0.81–1.42] | 1.00 [0.81–1.35] |
| Fasting glycemia, mg/dL | 1.00 [0.88–1.00] | 0.93 [0.90–1.01] | 1.40 [1.30–1.58] *,† | 1.35 [1.18–1.45] *,† |
| Insulinemia, pmol/L | 55 [37–78] | 71 [51–98] | 69 [62–78] | 90 [67–147] * |
| Hb1Ac, mmol/L | - | - | 6.9 [6.7–7.4] | 6.6 [6.2–6.9] |
| Creatinemia, µmol/L | 70 [61–73] | 75 [62–86] | 67 [61–73] | 65 [56–79] |
| Statins, n (%) | 1 (8%) | 2 (25%) | 2 (29%) | 13 (76%) * |
| Antihypertensive agents, n (%) | ||||
| ACEi/ARB | - | 7 (88%) | - | 12 (72%) |
| CCB | - | 2 (25%) | - | 11 (65%) |
| Beta-blokers | - | 0 (0%) | - | 2 (12%) |
| Diuretics | - | 0 (0%) | - | 7 (41%) |
| Hypoglycemic agents, n (%) | ||||
| Metformin | - | - | 7 (100%) | 14 (82%) |
| Sulfamides/glinides | - | - | 1 (14%) | 7 (41%) |
| DPP-4 inhibitors/GLP-1 agonists | - | - | 2 (29%) | 8 (47%) |
| Analyte (pg/mL) | Control | HTN | T2D |
|---|---|---|---|
| 14,15-DHET | 134 [129–169] | 156 [129–190] | 152 [128–172] |
| 11,12-DHET | 134 [122–163] | 149 [116–171] | 120 [116–158] |
| 8,9-DHET | 66 [58–72] | 68.3 [52.9–87.5] | 71.1 [57.0–89.2] |
| 5,6-DHET | 114 [88–155] | 108 [77–174] | 110 [80–214] |
| 14,15-EET | 16.2 [11.8–19.2] | 20.3 [15.4–40.2] | 19.3 [14.7–42.2] |
| 11,12-EET | 19.7 [18.3–21.7] | 25.0 [21.9–33.7] | 23.2 [18.1–32.4] |
| 8,9-EET | <LLOQ | <LLOQ | <LLOQ |
| 20-HETE | 98.9 [81.7–148.1] | 138 [112–202] ** | 118 [95.9–184] |
| 19-HETE | 89.4 [71.8–99.5] | 112 [91–148] * | 108 [85.6–136] |
| 15-HETE | 213 [202–254] | 261 [232–331] * | 242 [196–325] |
| 12-HETE | >ULOQ | >ULOQ | >ULOQ |
| 11-HETE | 95.9 [92.4–102.3] | 107.0 [93.4–147.9] | 103 [88.0–143] |
| 8-HETE | 75.7 [66.8–86.6] | 98.2 [77.3–132.7] * | 84.1 [61.5–106] |
| 5-HETE | 121 [112–189] | 211 [142–270] | 204 [138–239] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feugray, G.; Pereira, T.; Iacob, M.; Moreau-Grangé, L.; Prévost, G.; Brunel, V.; Joannidès, R.; Bellien, J.; Duflot, T. Determination of Lipoxygenase, CYP450, and Non-Enzymatic Metabolites of Arachidonic Acid in Essential Hypertension and Type 2 Diabetes. Metabolites 2022, 12, 859. https://doi.org/10.3390/metabo12090859
Feugray G, Pereira T, Iacob M, Moreau-Grangé L, Prévost G, Brunel V, Joannidès R, Bellien J, Duflot T. Determination of Lipoxygenase, CYP450, and Non-Enzymatic Metabolites of Arachidonic Acid in Essential Hypertension and Type 2 Diabetes. Metabolites. 2022; 12(9):859. https://doi.org/10.3390/metabo12090859
Chicago/Turabian StyleFeugray, Guillaume, Tony Pereira, Michèle Iacob, Lucile Moreau-Grangé, Gaëtan Prévost, Valéry Brunel, Robinson Joannidès, Jérémy Bellien, and Thomas Duflot. 2022. "Determination of Lipoxygenase, CYP450, and Non-Enzymatic Metabolites of Arachidonic Acid in Essential Hypertension and Type 2 Diabetes" Metabolites 12, no. 9: 859. https://doi.org/10.3390/metabo12090859
APA StyleFeugray, G., Pereira, T., Iacob, M., Moreau-Grangé, L., Prévost, G., Brunel, V., Joannidès, R., Bellien, J., & Duflot, T. (2022). Determination of Lipoxygenase, CYP450, and Non-Enzymatic Metabolites of Arachidonic Acid in Essential Hypertension and Type 2 Diabetes. Metabolites, 12(9), 859. https://doi.org/10.3390/metabo12090859