Long-Term Mastication Changed Salivary Metabolomic Profiles
Abstract
:1. Introduction
2. Results
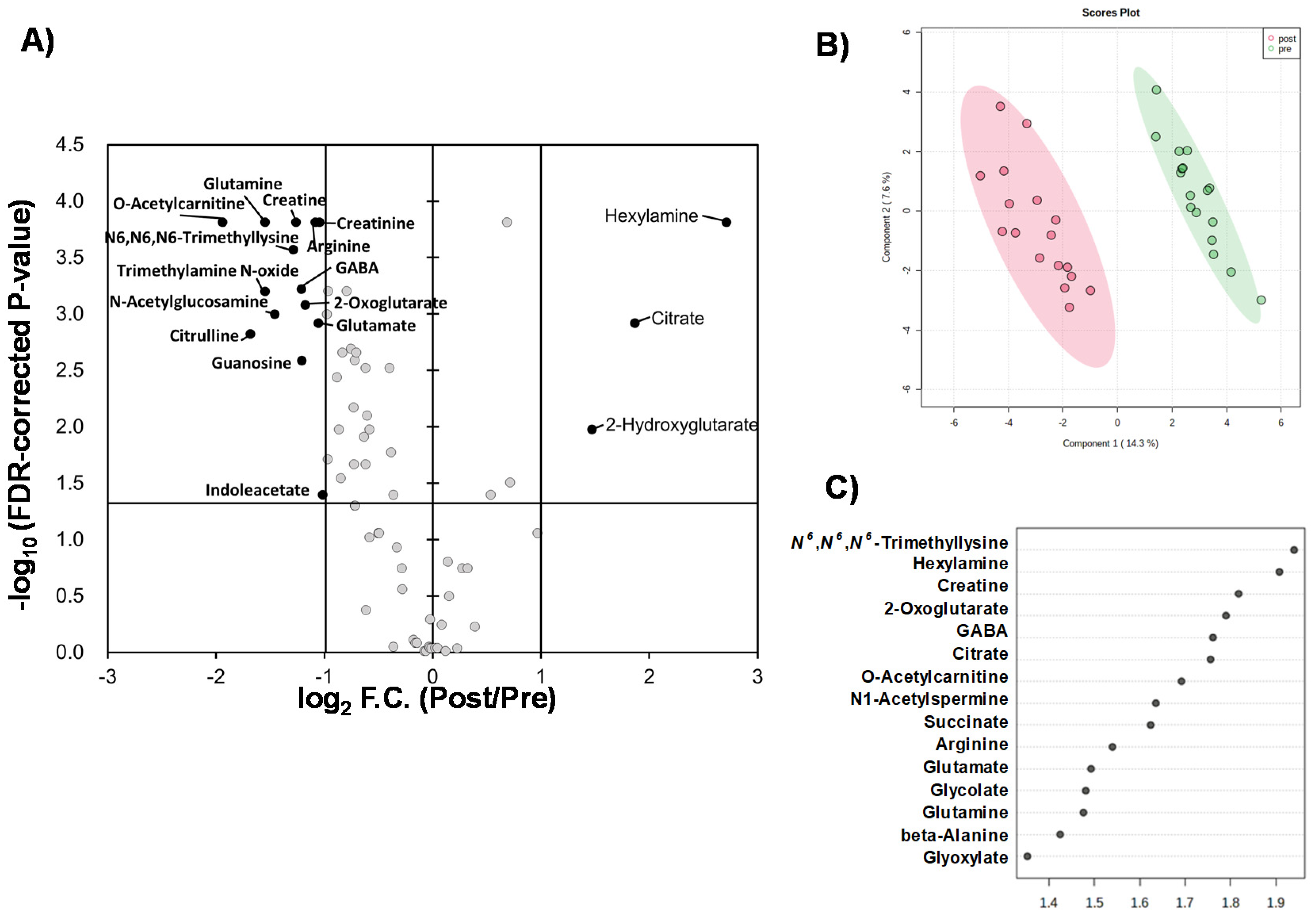
2.1. Overview of Quantified Metabolites
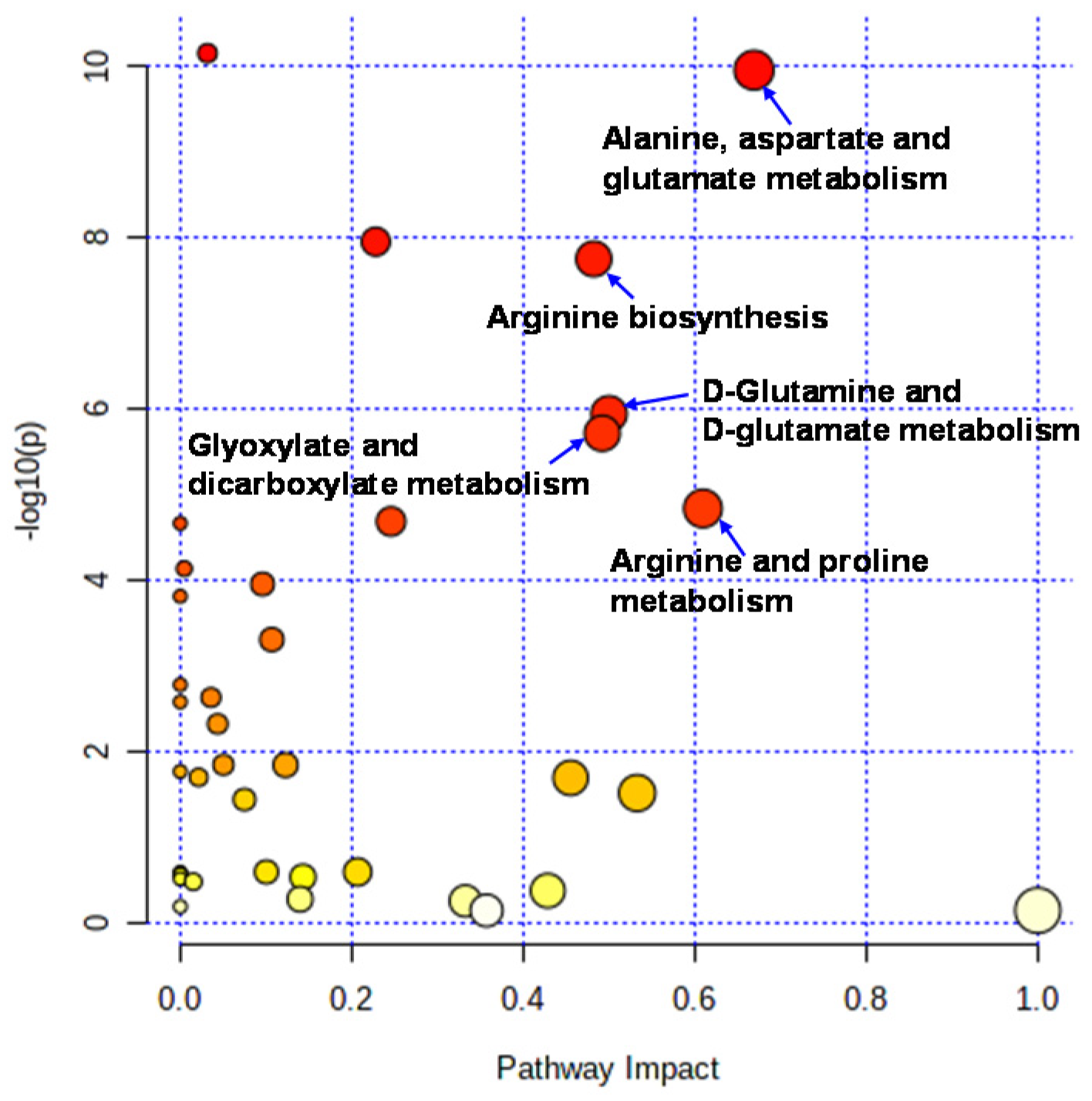
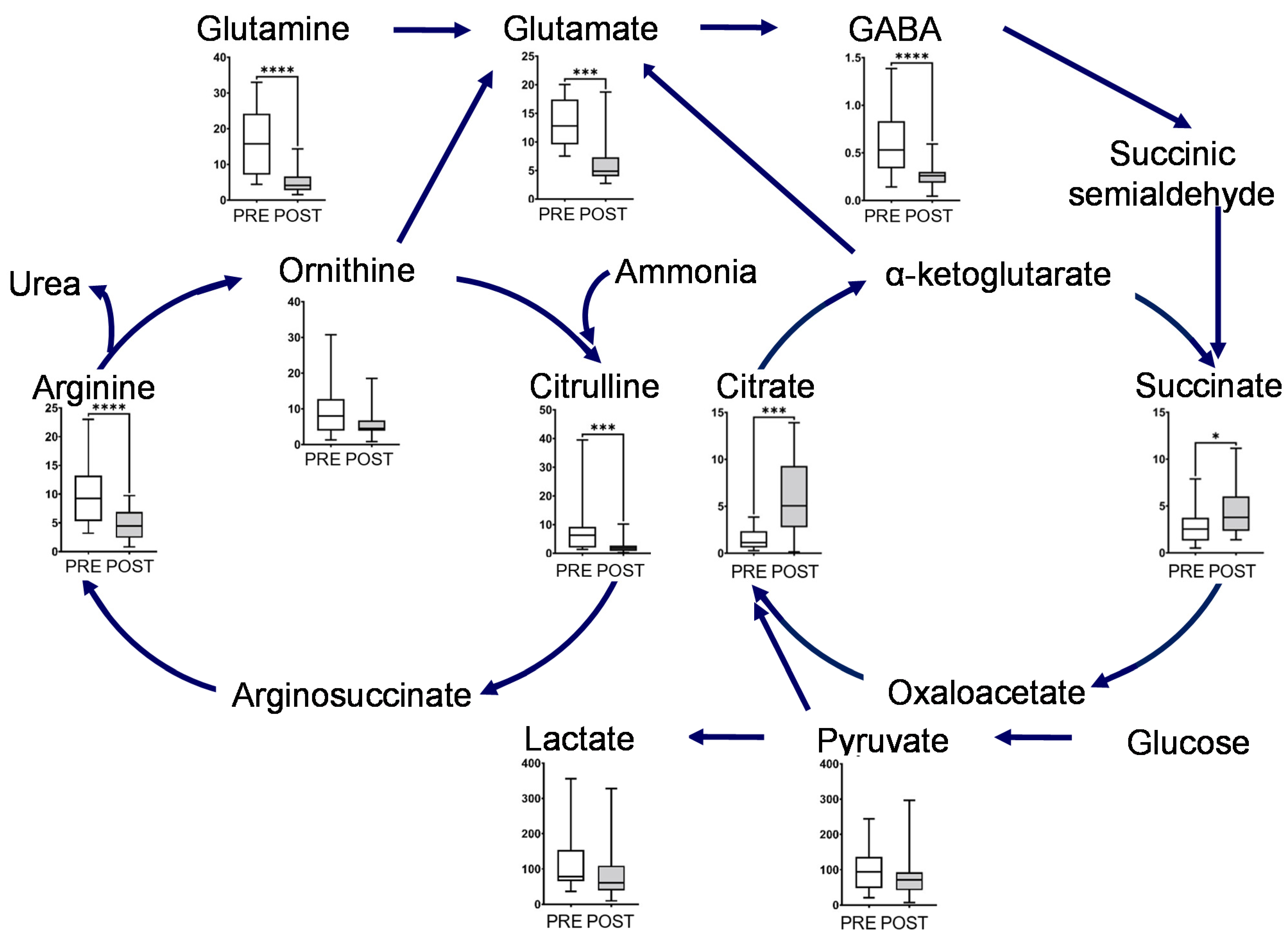
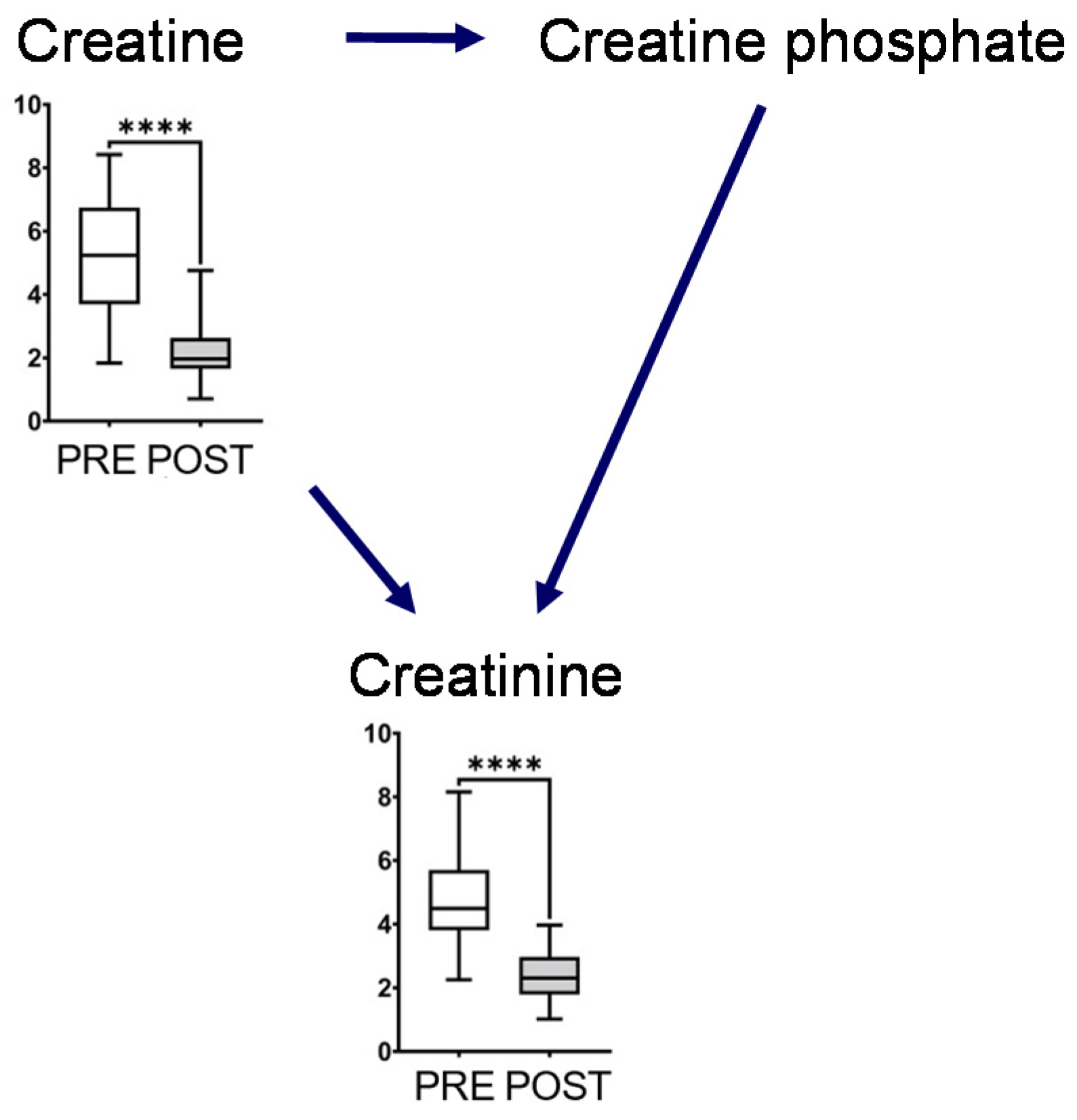
2.2. Effect of Intervention on Individual Pathways
3. Discussion
4. Materials and Methods
4.1. Subject Characteristics
4.2. Saliva Collection
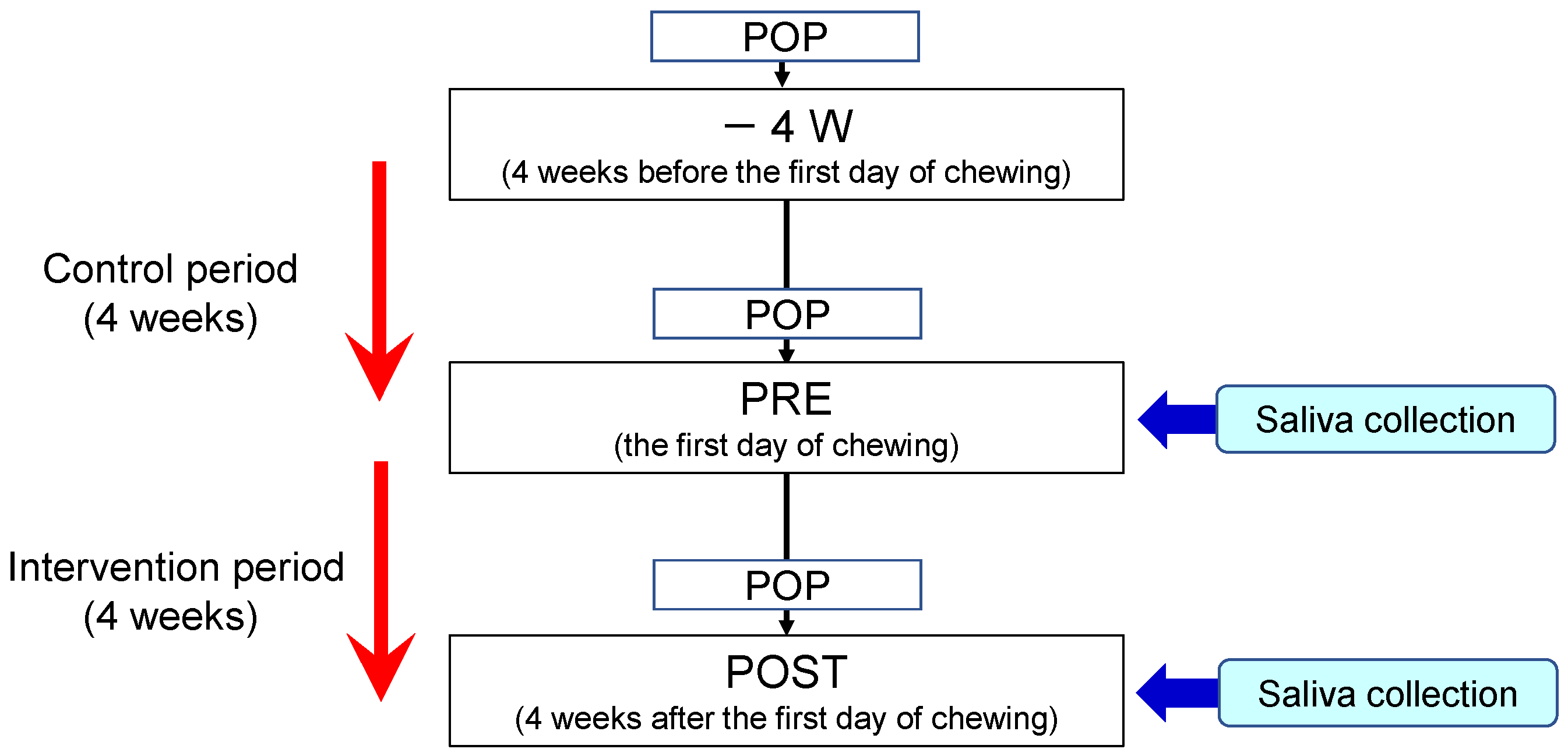
4.3. Study Design
4.4. Metabolomic Analysis
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dodds, M.W.; Johnson, D.A.; Yeh, C.K. Health Benefits of Saliva: A Review. J. Dent. 2005, 33, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, E.; Lamster, I.B. The Diagnostic Applications of Saliva—A Review. Crit. Rev. Oral Biol. Med. 2002, 13, 197–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.M.; Garon, E.; Wong, D.T. Salivary Diagnostics. Orthod. Craniofac. Res. 2009, 12, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, A.; Saeki, Y.; Otani, E.; Kim, M.; Fushimi, A.; Satoh, Y.; Kakegawa, Y.; Arai, H.; Taguchi, N.; Matsukubo, T. Effects of Chewing Gum Base on Oral Hygiene and Mental Health: A Pilot Study. Bull. Tokyo Dent. Coll. 2021, 62, 7–14. [Google Scholar] [CrossRef]
- Srivastava, S. Emerging Insights into the Metabolic Alterations in Aging Using Metabolomics. Metabolites 2019, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- To, M.; Sugimoto, M.; Saruta, J.; Yamamoto, Y.; Sakaguchi, W.; Kawata, A.; Matsuo, M.; Tsukinoki, K. Cognitive Dysfunction in a Mouse Model of Cerebral Ischemia Influences Salivary Metabolomics. J. Clin. Med. 2021, 10, 1698. [Google Scholar] [CrossRef]
- Asai, Y.; Itoi, T.; Sugimoto, M.; Sofuni, A.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Fujita, M.; et al. Elevated Polyamines in Saliva of Pancreatic Cancer. Cancers 2018, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, M. Salivary Metabolomics for Cancer Detection. Expert Rev. Proteom. 2020, 17, 639–648. [Google Scholar] [CrossRef]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary Electrophoresis Mass Spectrometry-Based Saliva Metabolomics Identified Oral, Breast and Pancreatic Cancer-Specific Profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [Green Version]
- Yatsuoka, W.; Ueno, T.; Miyano, K.; Enomoto, A.; Ota, S.; Sugimoto, M.; Uezono, Y. Time-Course of Salivary Metabolomic Profiles During Radiation Therapy for Head and Neck Cancer. J. Clin. Med. 2021, 10, 2631. [Google Scholar] [CrossRef]
- Sugimoto, M.; Saruta, J.; Matsuki, C.; To, M.; Onuma, H.; Kaneko, M.; Soga, T.; Tomita, M.; Tsukinoki, K. Physiological and Environmental Parameters Associated with Mass Spectrometry-Based Salivary Metabolomic Profiles. Metabolomics 2013, 9, 454–463. [Google Scholar] [CrossRef]
- Okuma, N.; Saita, M.; Hoshi, N.; Soga, T.; Tomita, M.; Sugimoto, M.; Kimoto, K. Effect of Masticatory Stimulation on the Quantity and Quality of Saliva and the Salivary Metabolomic Profile. PLoS ONE 2017, 12, e0183109. [Google Scholar] [CrossRef] [Green Version]
- Wessel, S.W.; van der Mei, H.C.; Maitra, A.; Dodds, M.W.; Busscher, H.J. Potential Benefits of Chewing Gum for the Delivery of Oral Therapeutics and Its Possible Role in Oral Healthcare. Expert Opin. Drug Deliv. 2016, 13, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.N.; Edgar, W.M. The Effect of Daily Gum-Chewing on Salivary Flow Rates in Man. J. Dent. Res. 1989, 68, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Dodds, M.W.; Hsieh, S.C.; Johnson, D.A. The Effect of Increased Mastication by Daily Gum-Chewing on Salivary Gland Output and Dental Plaque Acidogenicity. J. Dent. Res. 1991, 70, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Miyaji, A.; Hayashi, N. Effect of Postprandial Gum Chewing on Diet-Induced Thermogenesis. Obesity 2016, 24, 878–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shambaugh, G.E. Ill Urea Biosynthesis I. The Urea Cycle and Relationships to the Citric Acid Cycle. Am. J. Clin. Nutr. 1977, 30, 2083–2087. [Google Scholar]
- Yamano, E.; Sugimoto, M.; Hirayama, A.; Kume, S.; Yamato, M.; Jin, G.; Tajima, S.; Goda, N.; Iwai, K.; Fukuda, S.; et al. Index Markers of Chronic Fatigue Syndrome with Dysfunction of TCA and Urea Cycles. Sci. Rep. 2016, 6, e34990. [Google Scholar]
- Kurosawa, Y.; Kurosawa, Y.; Hamaoka, T.; Katsumura, T.; Kuwamori, M.; Kimura, N.; Sako, T.; Chance, B. Creatine Supplementation Enhances Anaerobic ATP Synthesis during a Single 10-sec Maximal Handgrip Exercise. Mol. Cell. Biochem. 2003, 244, 105–112. [Google Scholar] [CrossRef]
- Guimarães-Ferreira, L. Role of the Phosphocreatine System on Energetic Homeostasis in Skeletal and Cardiac Muscles. Einstein 2014, 12, 126–131. [Google Scholar]
- Belstrøm, D. The Salivary Microbiota in Health and Disease. J. Oral Microbiol. 2020, 12, 1723975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.Z.; Chen, G.; Hong, Q.; Huang, S.; Smith, H.M.; Shah, R.D.; Scholz, M.; Ferguson, J.F. Multi-Omic Analysis of the Microbiome and Metabolome in Healthy Subjects Reveals Microbiome-Dependent Relationships Between Diet and Metabolites. Front. Gen. 2019, 10, 454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomasson, R.; Baillot, A.; Jollin, L.; Lecoq, A.-M.; Amiot, V.; Lasne, F.; Collomp, F. Correlation between plasma and saliva adrenocortical hormones in response to submaximal exercise. J. Physiol. Sci. 2010, 60, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Tu, M.; Sugano, A.; Yamamori, I.; Iba, A.; Yusa, K.; Kaneko, M.; Ota, S.; et al. Effect of Timing of Collection of Salivary Metabolomic Biomarkers on Oral Cancer Detection. Amino Acids 2017, 49, 761–770. [Google Scholar] [CrossRef]
- Barallobre-Barreiro, J.; Chung, Y.L.; Mayr, M. Proteomics and metabolomics for mechanistic insights and biomarker discovery in cardiovascular disease. Rev. Esp. Cardiol. 2013, 66, 657–661. [Google Scholar] [CrossRef]
- Tomita, A.; Mori, M.; Hiwatari, K.; Yamaguchi, E.; Itoi, T.; Sunamura, M.; Soga, T.; Tomita, M.; Sugimoto, M. Effect of Storage Conditions on Salivary Polyamines Quantified Via Liquid Chromatography-Mass Spectrometry. Sci. Rep. 2018, 8, 12075. [Google Scholar] [CrossRef]
- Shimizu, H.; Usui, Y.; Asakage, M.; Nezu, N.; Wakita, R.; Tsubota, K.; Sugimoto, M.; Goto, H. Serum Metabolomic Profiling of Patients with Non-Infectious Uveitis. J. Clin. Med. 2020, 9, 3955. [Google Scholar] [CrossRef]
- Sugimoto, M.; Kawakami, M.; Robert, M.; Soga, T.; Tomita, M. Bioinformatics Tools for Mass Spectroscopy-Based Metabolomic Data Processing and Analysis. Curr. Bioinform. 2012, 7, 96–108. [Google Scholar] [CrossRef]
- Sugimoto, M.; Ota, S.; Kaneko, M.; Enomoto, A.; Soga, T. Quantification of Salivary Charged Metabolites Using Capillary Electrophoresis Time-of-Flight-Mass Spectrometry. Bio-Protocol 2020, 10, e3797. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.; Li, S.; Xia, J. Metaboanalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, w388–w396. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeki, Y.; Takenouchi, A.; Otani, E.; Kim, M.; Aizawa, Y.; Aita, Y.; Tomita, A.; Sugimoto, M.; Matsukubo, T. Long-Term Mastication Changed Salivary Metabolomic Profiles. Metabolites 2022, 12, 660. https://doi.org/10.3390/metabo12070660
Saeki Y, Takenouchi A, Otani E, Kim M, Aizawa Y, Aita Y, Tomita A, Sugimoto M, Matsukubo T. Long-Term Mastication Changed Salivary Metabolomic Profiles. Metabolites. 2022; 12(7):660. https://doi.org/10.3390/metabo12070660
Chicago/Turabian StyleSaeki, Yoji, Akane Takenouchi, Etsuyo Otani, Minji Kim, Yumi Aizawa, Yasuko Aita, Atsumi Tomita, Masahiro Sugimoto, and Takashi Matsukubo. 2022. "Long-Term Mastication Changed Salivary Metabolomic Profiles" Metabolites 12, no. 7: 660. https://doi.org/10.3390/metabo12070660
APA StyleSaeki, Y., Takenouchi, A., Otani, E., Kim, M., Aizawa, Y., Aita, Y., Tomita, A., Sugimoto, M., & Matsukubo, T. (2022). Long-Term Mastication Changed Salivary Metabolomic Profiles. Metabolites, 12(7), 660. https://doi.org/10.3390/metabo12070660





