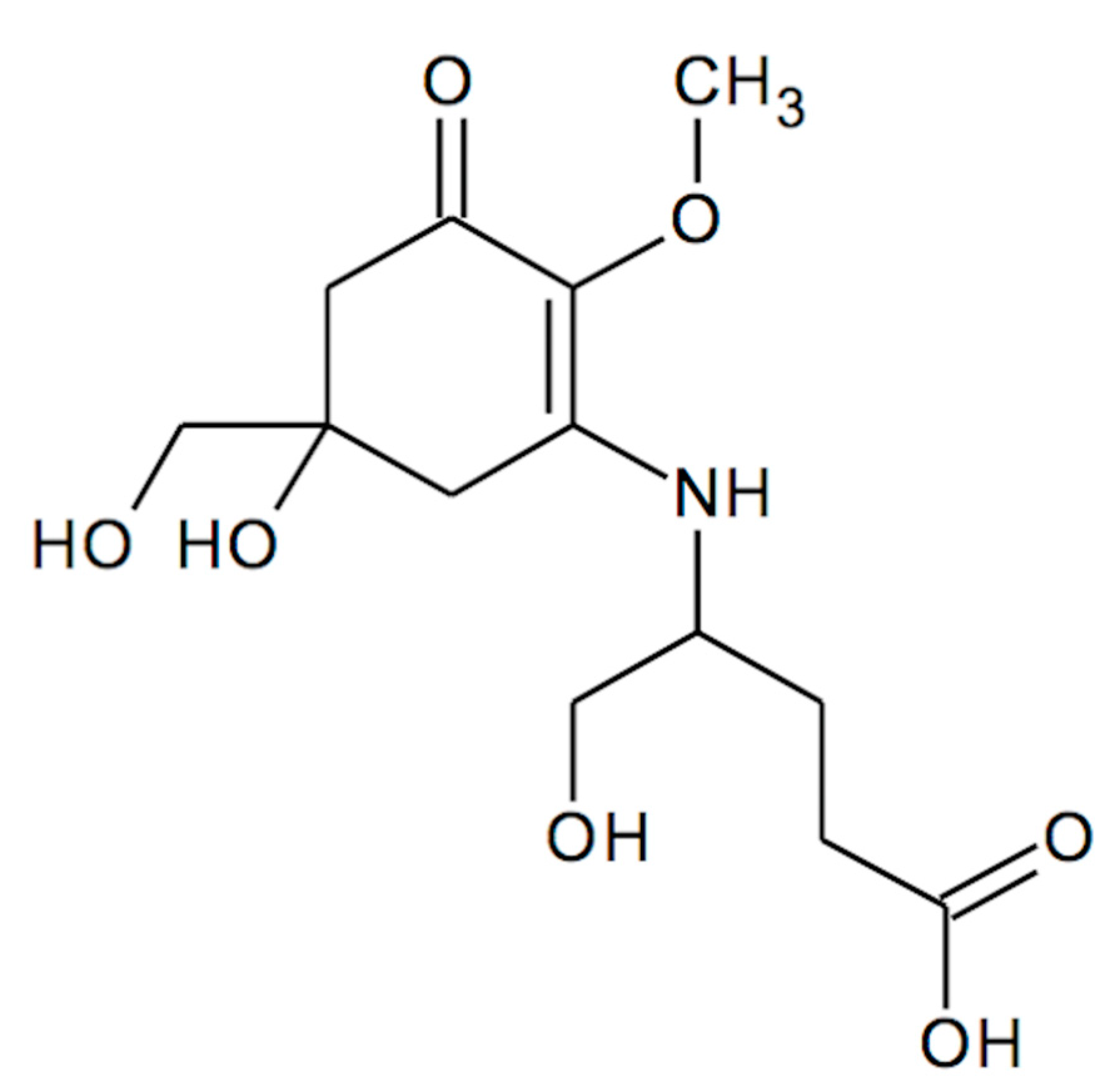
Metabolism of Mycosporine-Glutamicol in the Lichen Cladonia arbuscula subsp. squarrosa under Seasonal Changes and Elevated Exposure to UV-B or PAR Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Organism
2.2. Experimental Conditions
- PAR (λ = 400–700 nm) at the intensity of 1000, 1500, and 2000 µmol m−2 s−1 (two 1000 W Tungsram lamps, The Netherlands, 12 h light: 12 h dark) or darkness (24 h) with continuous access to atmospheric CO2 levels;
- UV-B radiation (λ = 290–315 nm, λmax = 310 nm) of 5 µmol m−2 s−1 (two fluorescence tubes, Philips TL 40W/12, Germany, screened with a 0.13 mm thick cellulose acetate filter paper to remove all radiation below 290 nm);
- PAR of 1000 µmol m−2 s−1 or darkness with simultaneous deprivation of CO2 in the atmosphere surrounding lichen-forming fungus, which was achieved by replacing water in a glass vessel with a 5% Ba(OH)2 solution.
2.3. Kinetics of the Changes in Myc-Glu(OH) Concentration
2.4. Sample Preparation
2.5. Analytical Determination
2.6. Reagents and Chemicals
2.7. Statistical Analysis
3. Results and Discussion
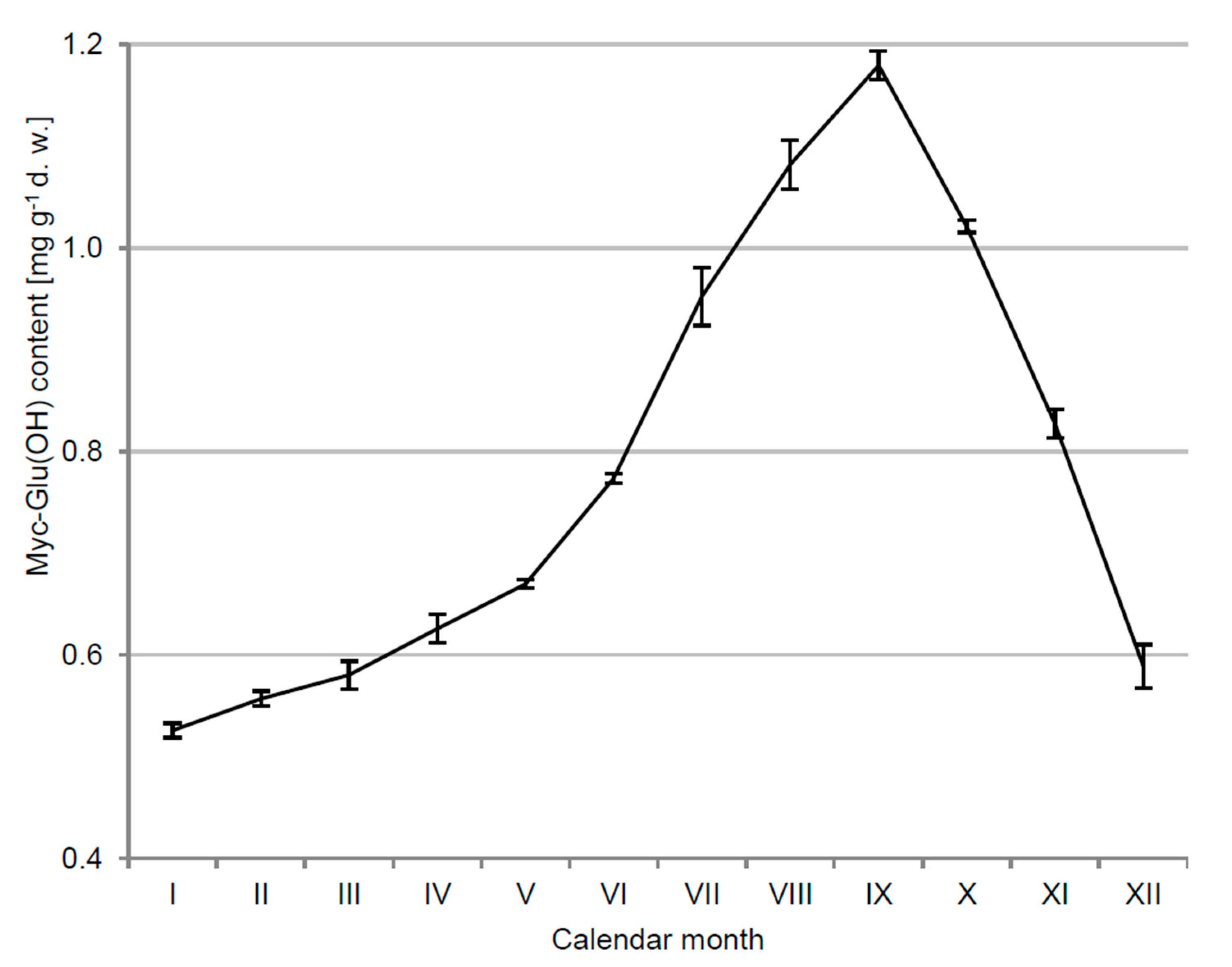
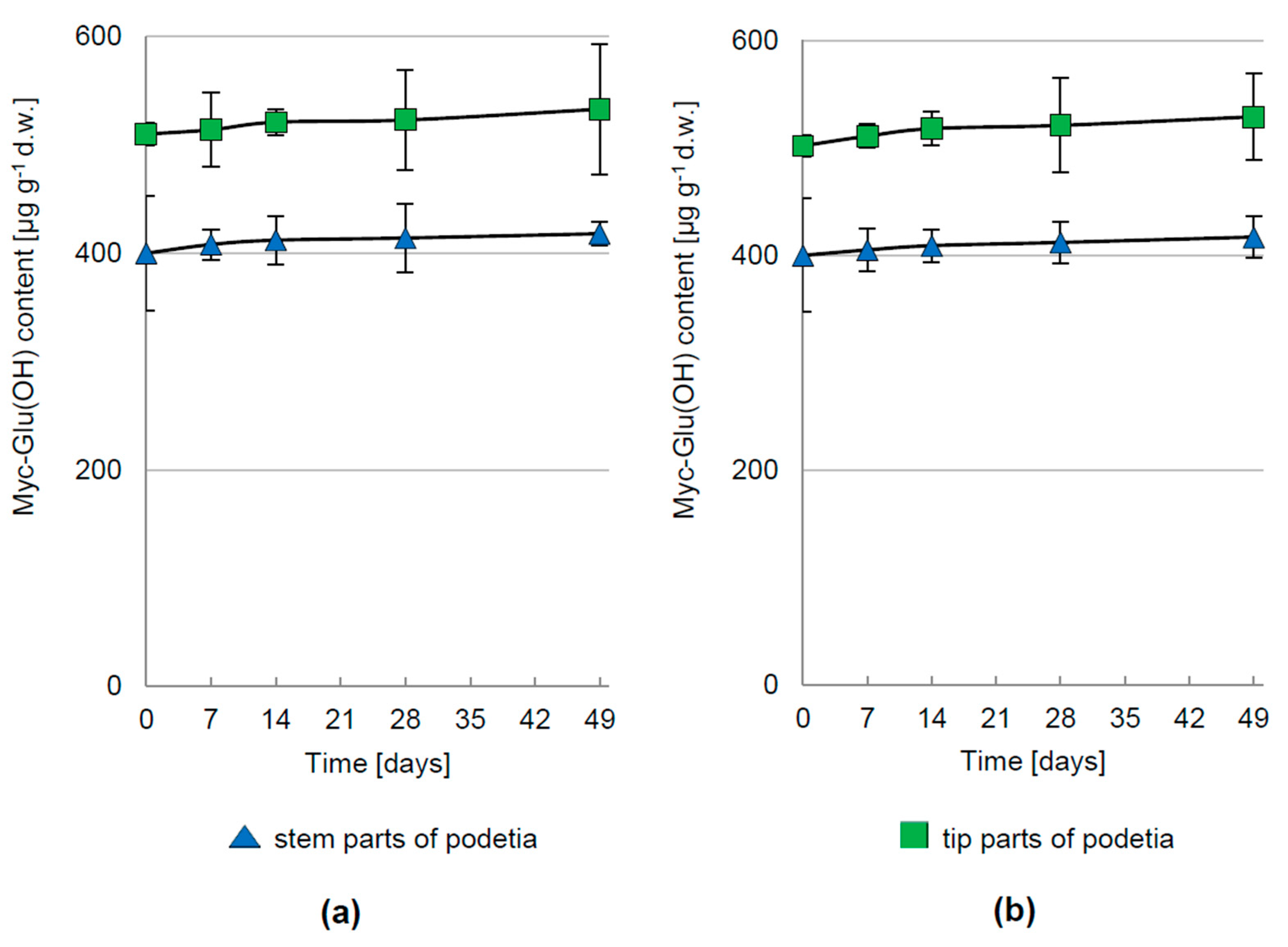
3.1. Kinetics of Myc-Glu(OH) Concentration in C. arbuscula Thalli
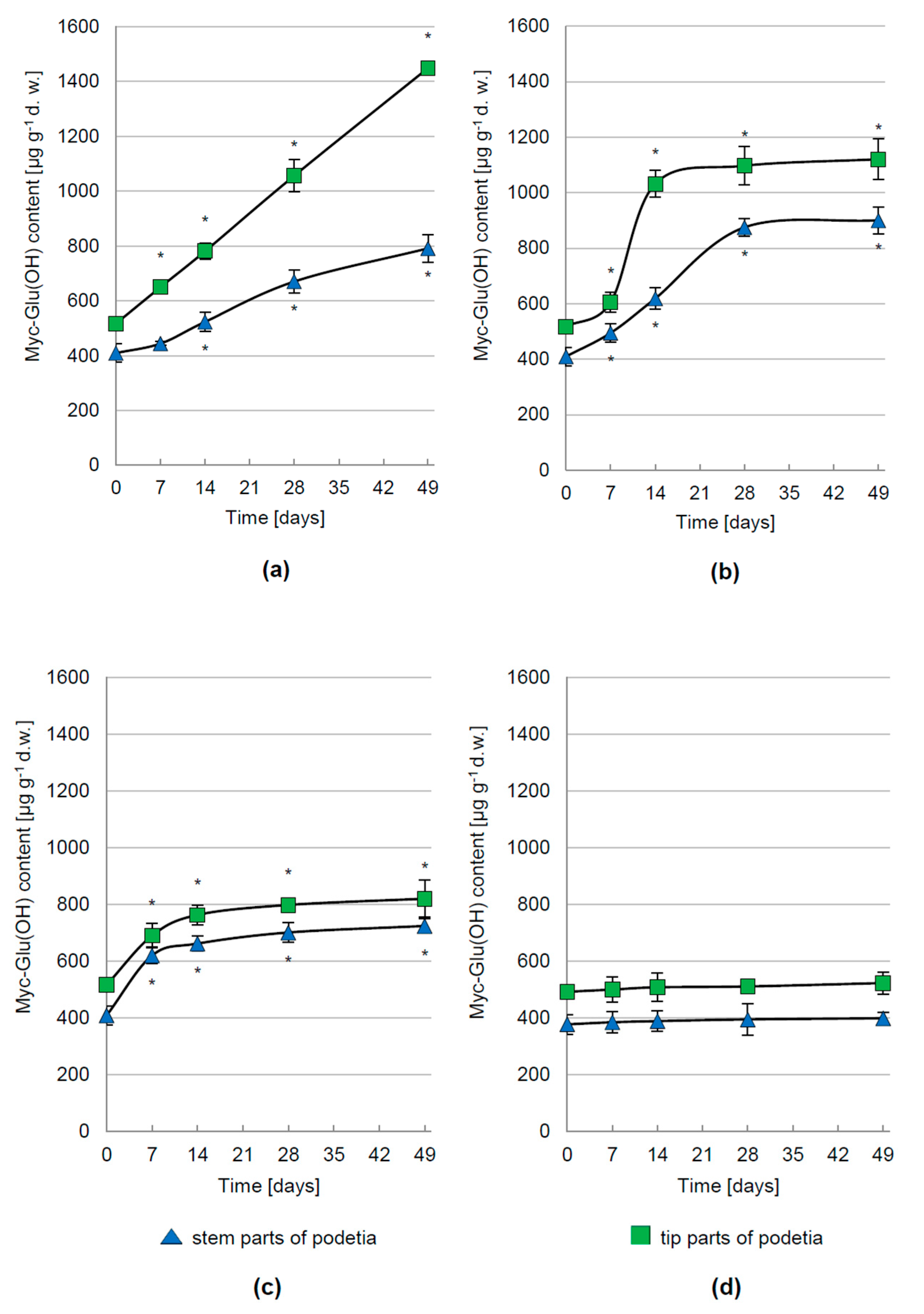
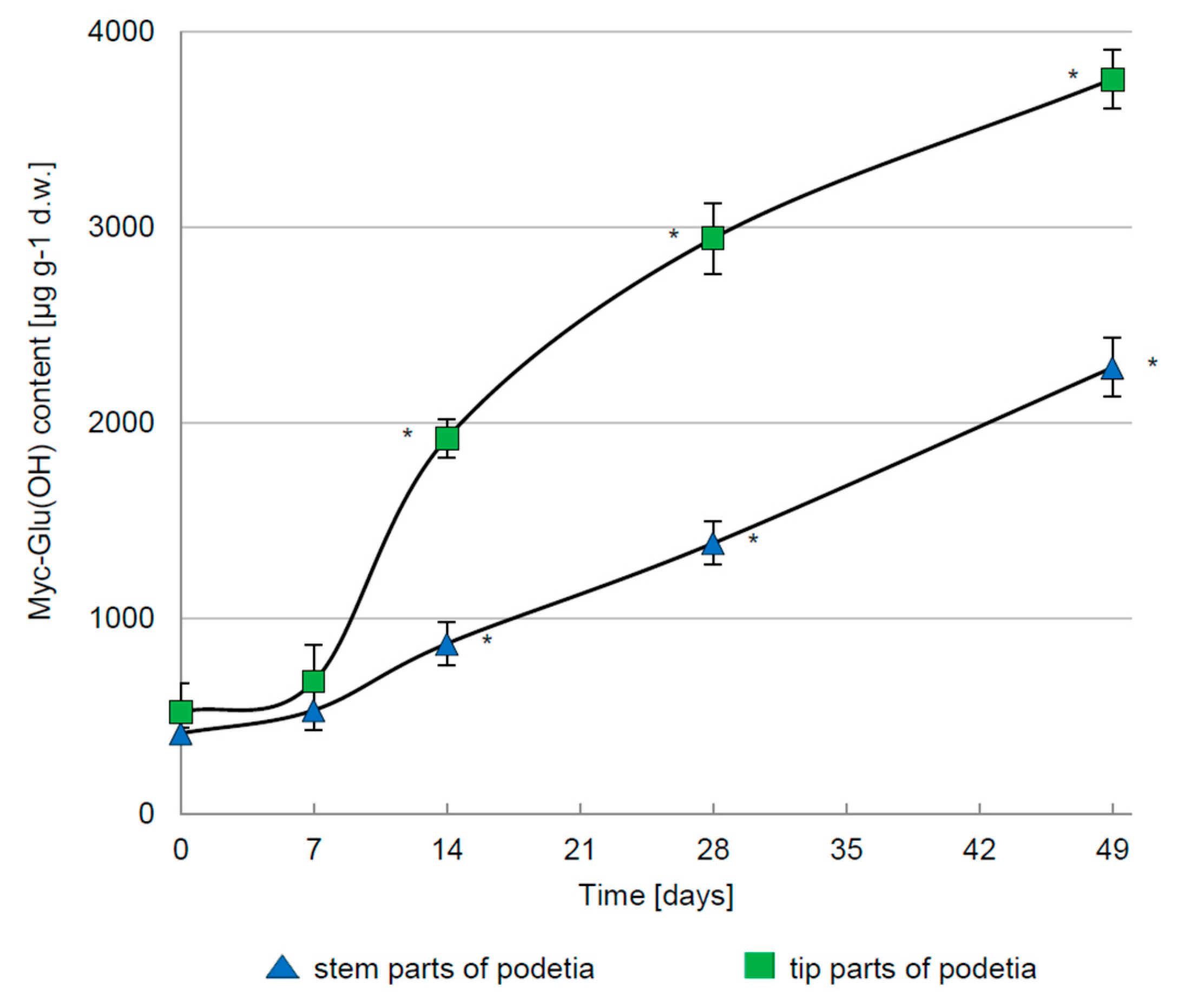
3.2. Regulation of Myc-Glu(OH) Accumulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karentz, D. Chemical defenses of marine organisms against solar radiation exposure: UV-absorbing mycosporine-like amino acids and scytonemin. In Marine Chemical Ecology; McClintock, J.B., Baker, J., Eds.; CRC Press, Inc.: Boca Raton, FL, USA, 2001; pp. 481–520. ISBN 978-0-8493-9064-7. [Google Scholar]
- Rastogi, R.P.; Sinha, R.P.; Singh, S.P.; Häder, D.-P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. 2010, 37, 537–558. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Häder, D.-P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.-P.; Sinha, R.P. Solar ultraviolet radiation-induced DNA damage in aquatic organisms: Potential environmental impact. Mutat. Res. 2005, 571, 221–233. [Google Scholar] [CrossRef]
- Mittler, R.; Tel-Or, E. Oxidative stress responses in the unicellular cyanobacterium Synechococcus PCC 7942. Free Radic. Res. Commun. 1991, 12-13 Pt 2, 845–850. [Google Scholar] [CrossRef]
- Sinha, R.P.; Klisch, M.; Walter Helbling, E.; Häder, D.P. Induction of mycosporine-like amino acids (MAAs) in cyanobacteria by solar ultraviolet-B radiation. J. Photochem. Photobiol. B Biol. 2001, 60, 129–135. [Google Scholar] [CrossRef]
- Sinha, R.P.; Klisch, M.; Gröniger, A.; Häder, D.-P. Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J. Photochem. Photobiol. B Biol. 1998, 47, 83–94. [Google Scholar] [CrossRef]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential Health and Beauty Ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.-L.; Zhang, Z.-C.; Yin, X.-Y.; Chen, M.; Hao, F.-H.; Wang, K.; Feng, J.-L.; Xu, H.-F.; Yin, Y.-C.; Tang, H.-R.; et al. UV-B induced biosynthesis of a novel sunscreen compound in solar radiation and desiccation tolerant cyanobacteria. Environ. Microbiol. 2018, 20, 200–213. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Mycosporine-like amino acids profile and their activity under PAR and UVR in a hot-spring cyanobacterium Scytonema sp. HKAR-3. Aust. J. Bot. 2010, 58, 286–293. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J. Exp. Biol. 2008, 46, 7–17. [Google Scholar] [PubMed]
- Chrapusta-Srebrny, E.; Białczyk, J.; Duchnik, K. Kinetics of mycosporine-glutamicol biosynthesis in vivo in Cladonia arbuscula (Wallr.) Flot subsp. squarrosa (Wallr.) Ruoss thalli. In Abstracts of Lectures and Posters of the 58th PBS Congress Botany without Borders—58th Congress of the Polish Botanical Society; Frey, L., Ed.; Polish Botanical Society: Kraków, Poland, 2019; p. 173. ISBN 978-83-954123-0-1. [Google Scholar]
- Nash, T.H., III (Ed.) Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Orange, A.; James, P.W.; White, F.J. Microchemical Methods for the Identification of Lichens; British Lichen Society: London, UK, 2001. [Google Scholar]
- Tartarotti, B.; Sommaruga, R. The effect of different methanol concentrations and temperatures on the extraction of mycosporine-like amino acids (MAAs) in algae and zooplankton. Arch. fur Hydrobiol. 2002, 1544, 691–703. [Google Scholar] [CrossRef]
- Stanisz, A. Przystępny kurs statystyki z zastosowaniem Statistica PL na przykładach z medycyny; StatSoft Polska: Kraków, Poland, 2006; ISBN 83-88724-18-5. [Google Scholar]
- Ranković, B.; Kosanić, M. Lichens as a Potential Source of Bioactive Secondary Metabolites. In Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential; Ranković, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–26. ISBN 978-3-319-13374-4. [Google Scholar]
- Karentz, D.; Dunlap, W.C.; Bosch, I. Temporal and spatial occurrence of UV-absorbing mycosporine-like amino acids in tissues of the antarctic sea urchin Sterechinus neumayeri during springtime ozone-depletion. Mar. Biol. 1997, 129, 343–353. [Google Scholar] [CrossRef]
- Karsten, U.; Wiencke, C. Factors controlling the formation of UV-absorbing mycosporine-like amino acids in the marine red alga Palmaria palmata. J. Plant Physiol. 1999, 155, 407–415. [Google Scholar] [CrossRef]
- Llewellyn, C.A.; Harbour, D.S. A temporal study of mycosporine-like amino acids in surface water phytoplankton from the English Channel and correlation with solar irradiation. J. Mar. Biol. Assoc. United Kingdom 2003, 83, 1–9. [Google Scholar] [CrossRef]
- Michalek-Wagner, K. Seasonal and sex-specific variations in levels of photo-protecting mycosporine-like amino acids (MAAs) in soft corals. Mar. Biol. 2001, 139, 651–660. [Google Scholar] [CrossRef]
- Tartarotti, B.; Sommaruga, R. Seasonal and ontogenetic changes of mycosporine-like amino acids in planktonic organisms from an alpine lake. Limnol. Oceanogr. 2006, 51, 1530–1541. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, R.F.; De Mora, S.J.; Demers, S. Enhanced UV radiation—A new problem for the marine environment. In The Effects of UV Radiation in the Marine Environment; De Mora, S.J., Demers, S., Vernet, M., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 1–34. [Google Scholar]
- Drollet, J.H.; Teai, T.; Faucon, M.; Martin, P.M. V Field study of compensatory changes in UV-absorbing compounds in the mucus of the solitary coral Fungia repanda (Scleractinia: Fungiidae) in relation to solar UV radiation, sea-water temperature, and other coincident physico-chemical parameters. Mar. Freshw. Res. 1997, 48, 329–333. [Google Scholar] [CrossRef]
- Rautio, M.; Korhola, A. UV-induced pigmentation in subarctic Daphnia. Limnol. Oceanogr. 2002, 47, 295–299. [Google Scholar] [CrossRef]
- Gauslaa, Y.; McEvoy, M. Seasonal changes in solar radiation drive acclimation of the sun-screening compound parietin in the lichen Xanthoria parietina. Basic Appl. Ecol. 2005, 6, 75–82. [Google Scholar] [CrossRef]
- de la Coba, F.; Aguilera, J.; Figueroa, F.L.; de Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Büdel, B.; Karsten, U.; Garcia-Pichel, F. Ultraviolet-absorbing scytonemin and mycosporine-like amino acid derivatives in exposed, rock-inhabiting cyanobacterial lichens. Oecologia 1997, 112, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Maguas, C.; Adams, W.W., 3rd; Meyer, A.; Kilian, E.; Lange, O.L. Effect of high light on the efficiency of photochemical energy conversion in a variety of lichen species with green and blue-green phycobionts. Planta 1990, 180, 400–409. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O.; Daleo, G.; De Marco, S.G. Occurrence of mycosporine-like amino acids in the red-tide dinoflagellate Alexandrium excavatum: UV-photoprotective compounds? J. Plankton Res. 1990, 12, 909–921. [Google Scholar] [CrossRef]
- Neale, P.J.; Banaszak, A.T.; Jarriel, C.R. Ultraviolet sunscreens in Gymnodinium sanguineum (Dynophyceae): Mycosporine-like amino acids protect against inhibition of photosynthesis. J. Phycol. 1998, 938, 928–938. [Google Scholar] [CrossRef] [Green Version]
- Korbee, N.; Figueroa, F.L.; Aguilera, J. Effect of light quality on the accumulation of photosynthetic pigments, proteins and mycosporine-like amino acids in the red alga Porphyra leucosticta (Bangiales, Rhodophyta). J. Photochem. Photobiol. B Biol. 2005, 80, 71–78. [Google Scholar] [CrossRef]
- Riegger, L.; Robinson, D. Photoinduction of UV-absorbing compounds in Antarctic diatoms and Phaeocystis antarctica. Mar. Ecol. Prog. Ser. 1997, 160, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Laurion, I.; Roy, S. Growth and photoprotection in three dinoflagellates (including two strains of Alexandrium tamarense) and one diatom exposed to four weeks of natural and enhanced ultraviolet-B radiation. J. Phycol. 2009, 45, 16–33. [Google Scholar] [CrossRef]
- Singh, S.P.; Klisch, M.; Sinha, R.P.; Häder, D.-P. Effects of abiotic stressors on synthesis of the mycosporine-like amino acid shinorine in the cyanobacterium Anabaena variabilis PCC 7937. Photochem. Photobiol. 2008, 84, 1500–1505. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A. Analysis of UV-absorbing photoprotectant mycosporine-like amino acid (MAA) in the cyanobacterium Arthrospira sp. CU2556. Photochem. Photobiol. Sci. 2014, 13, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Incharoensakdi, A. Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. Ecol. 2014, 87, 244–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsten, U.; Bischof, K.; Hanelt, D.; Tüg, H.; Wiencke, C. The effect of ultraviolet radiation on photosynthesis and ultraviolet-absorbing substances in the endemic Arctic macroalga Devaleraea ramentacea (Rhodophyta). Physiol. Plant. 1999, 105, 58–66. [Google Scholar] [CrossRef]
- BeGora, M.D.; Fahselt, D. Usnic acid and atranorin concentrations in lichens in relation to bands of UV irradiance. Bryologist 2001, 104, 134–140. [Google Scholar] [CrossRef]
- Hannach, G.; Sigleo, A.C. Photoinduction of UV-absorbing compounds in six species of marine phytoplankton. Mar. Ecol. Prog. Ser. 1998, 174, 207–222. [Google Scholar] [CrossRef] [Green Version]
- Hernando, M.; Carreto, J.I.; Carignan, M.O.; Ferreyra, G.A.; Gross, C. Effects of solar radiation on growth and mycosporine-like amino acids content in Thalassiosira sp, an Antarctic diatom. Polar Biol. 2002, 25, 12–20. [Google Scholar]
- Sinha, R.P.; Klisch, M.; Gröniger, A.; Häder, D.-P.; Groniger, A.; Häder, D.-P. Mycosporine-like amino acids in the marine red alga Gracilaria cornea—Effects of UV and heat. Environ. Exp. Bot. 2000, 43, 33–43. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef]
- García-Pichel, F.; Wingard, C.E.; Castenholz, R.W. Evidence regarding the UV sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp. Appl. Environ. Microbiol. 1993, 59, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Michalek-Wagner, K.; Willis, B.L. Impacts of bleaching on the soft coral Lobophytum compactum. II. Biochemical changes in adults and their eggs. Coral Reefs 2001, 19, 240–246. [Google Scholar] [CrossRef]
- Mirando, M.; Fahselt, D. The effect of thallus age and drying procedure on extractable lichen substances. Can. J. Bot. 1978, 56, 1499–1504. [Google Scholar] [CrossRef]
- Buffoni Hall, R.S.; Bornman, J.F.; Bjorn, L.O. UV-induced changes in pigment content and light penetration in the fruticose lichen Cladonia arbuscula ssp. mitis. J. Photochem. Photobiol. B Biol. 2002, 66, 13–20. [Google Scholar] [CrossRef]
- Bjerke, J.W.; Lerfall, K.; Elvebakk, A. Effects of ultraviolet radiation and PAR on the content of usnic and divaricatic acids in two arctic-alpine lichen. Photochem. Photobiol. Sci. 2002, 1, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Bouillant, M.-L.; Pittet, J.-L.; Bernillon, J.; Favre-Bonvin, J.; Arpin, N. Mycosporins from Ascochyta pisi, Cladosporium herbarum and Septoria nodorum. Phytochemistry 1981, 20, 2705–2707. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Volkmann, M.; Gorbushina, A.A. A broadly applicable method for extraction and characterization of mycosporines and mycosporine-like amino acids of terrestrial, marine and freshwater origin. FEMS Microbiol. Lett. 2006, 255, 286–295. [Google Scholar] [CrossRef]
- Arpin, N.; Favre-Bonvin, J.; Thivend, S. Structure de la mycosporine 2, nouvelle molécule isolée de Botrytis cinereaitle. Tetrahedron Lett. 1977, 18, 819–820. [Google Scholar] [CrossRef]
- Sinha, R.P.; Singh, S.P.; Häder, D.-P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B Biol. 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Torres, A.; Hochberg, M.; Pergament, I.; Smoum, R.; Niddam, V.; Dembitsky, V.M.; Temina, M.; Dor, I.; Lev, O.; Srebnik, M.; et al. A new UV-B absorbing mycosporine with photo protective activity from the lichenized ascomycete Collema cristatum. Eur. J. Biochem. 2004, 271, 780–784. [Google Scholar] [CrossRef]
- Roullier, C.; Chollet-Krugler, M.; Pferschy-Wenzig, E.M.; Maillard, A.; Rechberger, G.N.; Legouin-Gargadennec, B.; Bauer, R.; Boustie, J. Characterization and identification of mycosporines-like compounds in cyanolichens. Isolation of mycosporine hydroxyglutamicol from Nephroma laevigatum Ach. Phytochemistry 2011, 72, 1348–1357. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrapusta-Srebrny, E.; Bialczyk, J.; Duchnik, K.; Bober, B. Metabolism of Mycosporine-Glutamicol in the Lichen Cladonia arbuscula subsp. squarrosa under Seasonal Changes and Elevated Exposure to UV-B or PAR Irradiation. Metabolites 2022, 12, 632. https://doi.org/10.3390/metabo12070632
Chrapusta-Srebrny E, Bialczyk J, Duchnik K, Bober B. Metabolism of Mycosporine-Glutamicol in the Lichen Cladonia arbuscula subsp. squarrosa under Seasonal Changes and Elevated Exposure to UV-B or PAR Irradiation. Metabolites. 2022; 12(7):632. https://doi.org/10.3390/metabo12070632
Chicago/Turabian StyleChrapusta-Srebrny, Ewelina, Jan Bialczyk, Kornelia Duchnik, and Beata Bober. 2022. "Metabolism of Mycosporine-Glutamicol in the Lichen Cladonia arbuscula subsp. squarrosa under Seasonal Changes and Elevated Exposure to UV-B or PAR Irradiation" Metabolites 12, no. 7: 632. https://doi.org/10.3390/metabo12070632
APA StyleChrapusta-Srebrny, E., Bialczyk, J., Duchnik, K., & Bober, B. (2022). Metabolism of Mycosporine-Glutamicol in the Lichen Cladonia arbuscula subsp. squarrosa under Seasonal Changes and Elevated Exposure to UV-B or PAR Irradiation. Metabolites, 12(7), 632. https://doi.org/10.3390/metabo12070632





