NMR-Based Metabolomics Identify Metabolic Change in Spleen of Idiopathic Thrombocytopenic Purpura Patients
Abstract
:1. Introduction
2. Results
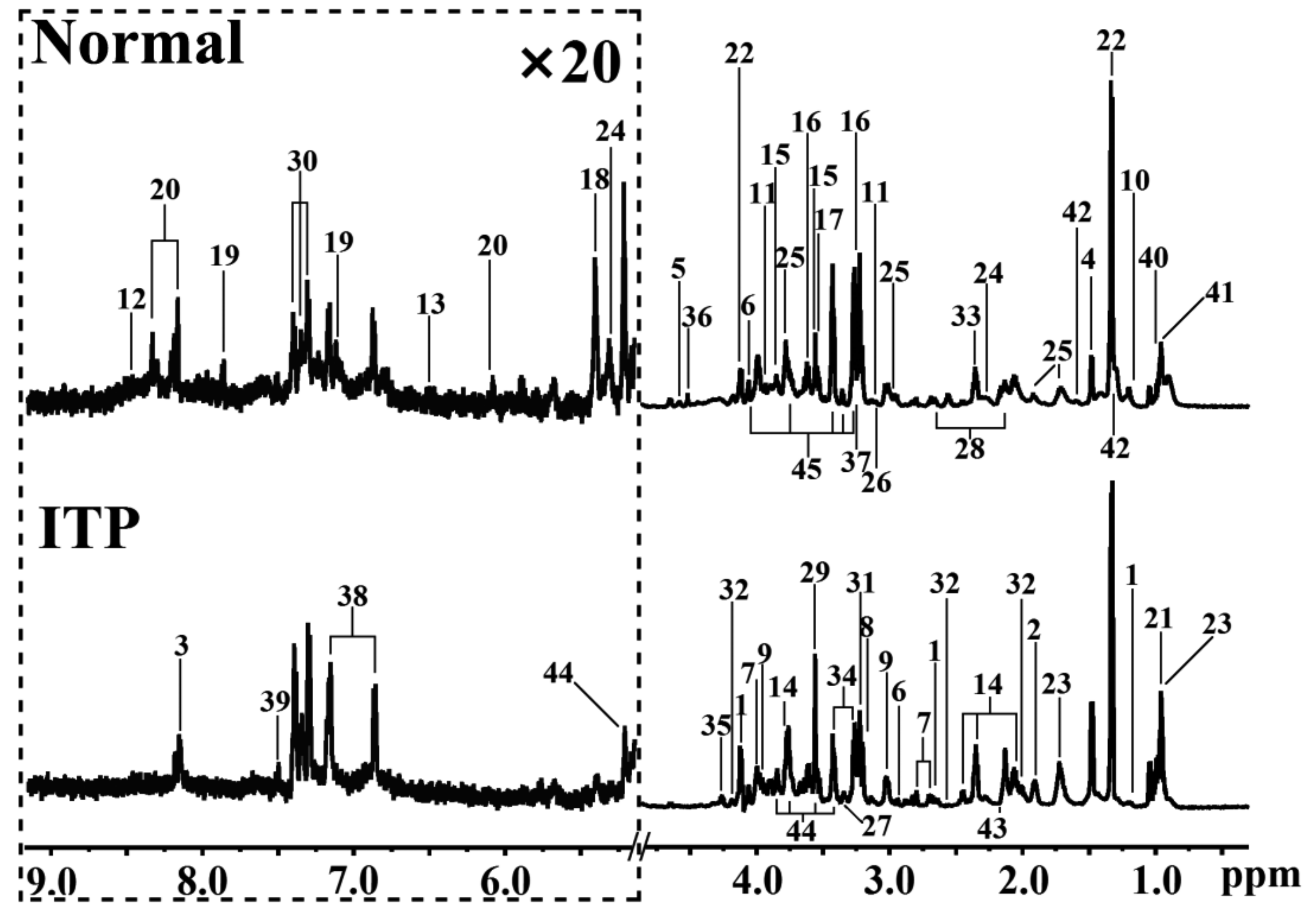
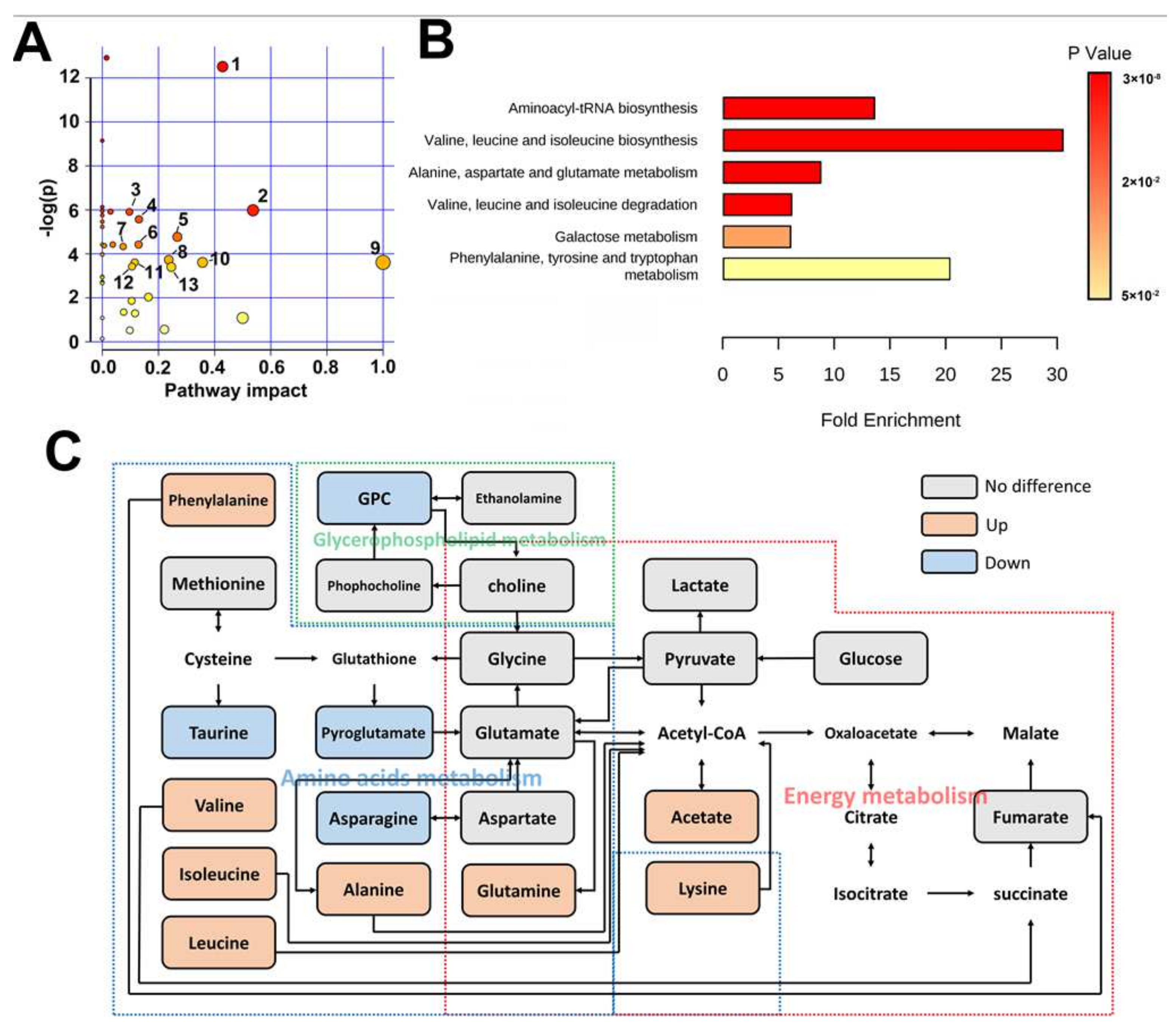
2.1. Metabolomic Difference between ITP and Normal Spleen
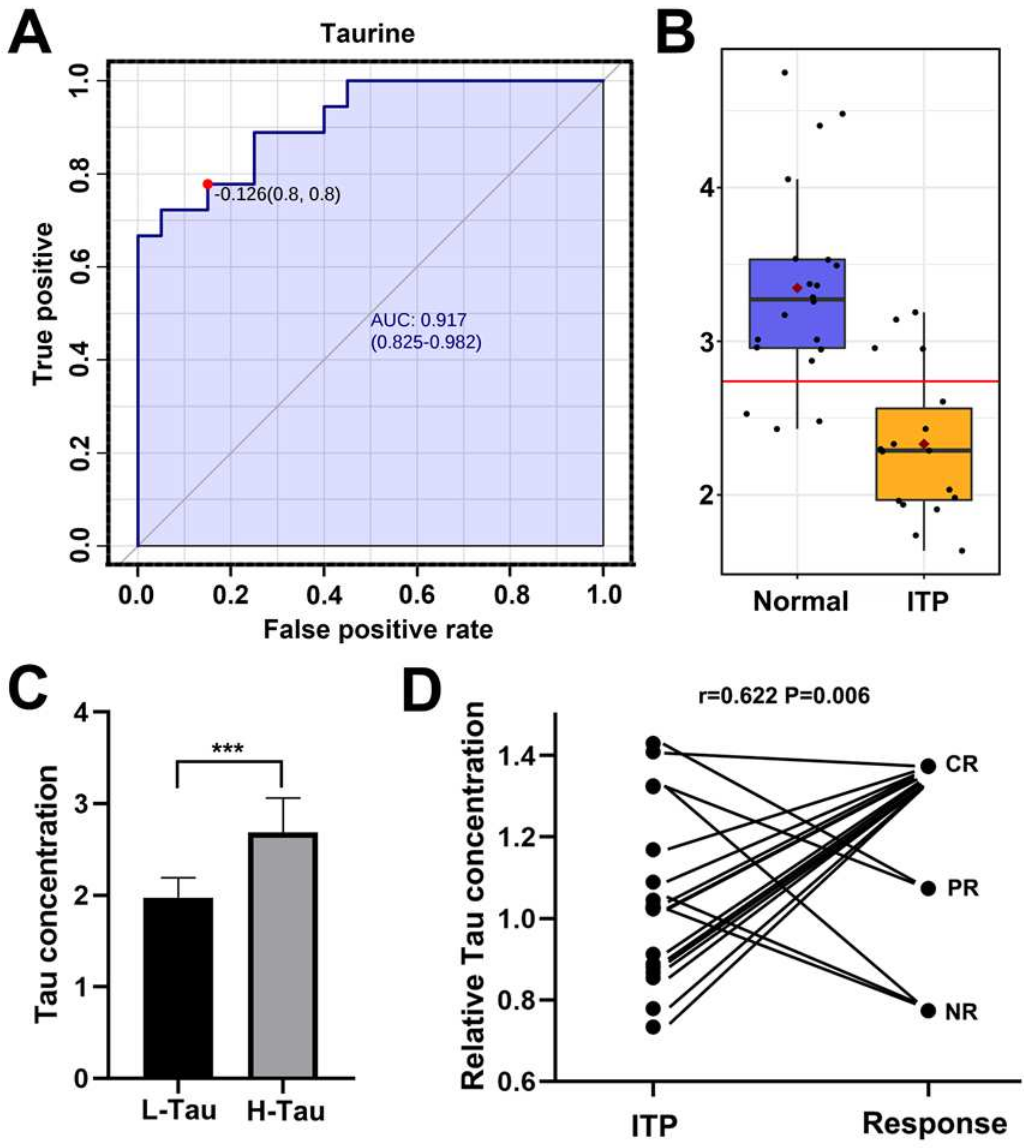
2.2. Taurine Was a Potential Metabolic Marker for Diagnosis of ITP and Efficacy Predictor for Splenectomy
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Sample Preparation and 1H HR–MAS NMR Spectroscopy
4.3. Data Processing
4.4. Statistical Analysis
4.5. Follow Up
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-HB | 3-Hydroxybutyrate |
| Asc | Ascorbate |
| Asn | Asparagine |
| β-Glc | β-Glucose |
| CR | Complete response |
| CRP | C-reactive protein |
| Glu | Glutamate |
| Gln | Glutamine |
| GPC | Glycerophosphocholine |
| Glg | Glycogen |
| HR–MAS | High-resolution magic angle spinning |
| ITP | Immune thrombocytopenia |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MBRole | Metabolites Biological Role |
| m-I | Myo-inositol |
| NMR | Nuclear magnetic resonance |
| NR | No response |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PC | Phosphocholine |
| PCA | Principal component analysis |
| ROC | Receiver operating characteristic |
| PR | Partial response |
| Tau | Taurine |
| VIP | Variable importance in projection |
References
- Cines, D.B.; Blanchette, V.S. Immune thrombocytopenic purpura. N. Engl. J. Med. 2002, 346, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Kelton, J.G.; Gibbons, S. Autoimmune platelet destruction: Idiopathic thrombocytopenic purpura. Semin. Thromb. Hemost. 1982, 8, 83–104. [Google Scholar] [CrossRef] [PubMed]
- Ballem, P.J.; Segal, G.M.; Stratton, J.R.; Gernsheimer, T.; Adamson, J.W.; Slichter, S.J. Mechanisms of thrombocytopenia in chronic autoimmune thrombocytopenic purpura. Evidence of both impaired platelet production and increased platelet clearance. J. Clin. Investig. 1987, 80, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houwerzijl, E.J.; Blom, N.R.; van der Want, J.J.; Esselink, M.T.; Koornstra, J.J.; Smit, J.W.; Louwes, H.; Vellenga, E.; de Wolf, J.T. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenic purpura. Blood 2004, 103, 500–506. [Google Scholar] [CrossRef]
- Cohen, Y.C.; Djulbegovic, B.; Shamai-Lubovitz, O.; Mozes, B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch. Intern. Med. 2000, 160, 1630–1638. [Google Scholar] [CrossRef]
- Sys, J.; Provan, D.; Schauwvlieghe, A.; Vanderschueren, S.; Dierickx, D. The role of splenectomy in autoimmune hematological disorders: Outdated or still worth considering? Blood Rev. 2017, 31, 159–172. [Google Scholar] [CrossRef]
- Kojouri, K.; Vesely, S.K.; Terrell, D.R.; George, J.N. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: A systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood 2004, 104, 2623–2634. [Google Scholar] [CrossRef] [Green Version]
- Neunert, C.; Lim, W.; Crowther, M.; Cohen, A.; Solberg, L., Jr.; Crowther, M.A. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011, 117, 4190–4207. [Google Scholar] [CrossRef] [Green Version]
- Newsholme, E.A.; Newsholme, P.; Curi, R. The role of the citric acid cycle in cells of the immune system and its importance in sepsis, trauma and burns. Biochem. Soc. Symp. 1987, 54, 145–162. [Google Scholar]
- Curi, R.; Newsholme, P.; Marzuca-Nassr, G.N.; Takahashi, H.K.; Hirabara, S.M.; Cruzat, V.; Krause, M.; de Bittencourt, P.I., Jr. Regulatory principles in metabolism-then and now. Biochem. J. 2016, 473, 1845–1857. [Google Scholar] [CrossRef]
- Jha, A.K.; Huang, S.C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.H.; Olona, A.; Papathanassiu, A.E.; Buang, N.; Park, K.S.; Costa, A.S.H.; Mauro, C.; Frezza, C.; Behmoaras, J. BCAT1 affects mitochondrial metabolism independently of leucine transamination in activated human macrophages. J. Cell. Sci. 2020, 133, jcs24795. [Google Scholar] [CrossRef]
- Papathanassiu, A.E.; Ko, J.H.; Imprialou, M.; Bagnati, M.; Srivastava, P.K.; Vu, H.A.; Cucchi, D.; McAdoo, S.P.; Ananieva, E.A.; Mauro, C.; et al. BCAT1 controls metabolic reprogramming in activated human macrophages and is associated with inflammatory diseases. Nat. Commun. 2017, 8, 16040. [Google Scholar] [CrossRef]
- Silva, L.S.; Poschet, G.; Nonnenmacher, Y.; Becker, H.M.; Sapcariu, S.; Gaupel, A.C.; Schlotter, M.; Wu, Y.; Kneisel, N.; Seiffert, M.; et al. Branched-chain ketoacids secreted by glioblastoma cells via MCT1 modulate macrophage phenotype. EMBO Rep. 2017, 18, 2172–2185. [Google Scholar] [CrossRef]
- Nugent, D.; McMillan, R.; Nichol, J.L.; Slichter, S.J. Pathogenesis of chronic immune thrombocytopenia: Increased platelet destruction and/or decreased platelet production. Br. J. Haematol. 2009, 146, 585–596. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2014, 46, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Schuller-Levis, G.B.; Park, E. Taurine and its chloramine: Modulators of immunity. Neurochem. Res. 2004, 29, 117–126. [Google Scholar] [CrossRef]
- Bouckenooghe, T.; Remacle, C.; Reusens, B. Is taurine a functional nutrient? Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 728–733. [Google Scholar] [CrossRef]
- Kim, C. Accumulation of taurine in tumor and inflammatory lesions. Adv. Exp. Med. Biol. 2006, 583, 213–217. [Google Scholar]
- Kim, C.; Cha, Y.N. Production of reactive oxygen and nitrogen species in phagocytes is regulated by taurine chloramine. Adv. Exp. Med. Biol. 2009, 643, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Schuller-Levis, G.; Mehta, P.D.; Rudelli, R.; Sturman, J. Immunologic consequences of taurine deficiency in cats. J. Leukoc. Biol. 1990, 47, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Akimana, C.; Wang, E.; Ng, C.K. (1)H-MRS Quantitation of Age-Dependent Taurine Changes in Mouse Brain. Mol. Imaging Biol. 2019, 21, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Lodi, A.; Rivera, L.B.; Izquierdo-Garcia, J.L.; Firpo, M.A.; Mulvihill, S.J.; Tempero, M.A.; Bergers, G.; Ronen, S.M. HR-MAS MRS of the pancreas reveals reduced lipid and elevated lactate and taurine associated with early pancreatic cancer. NMR Biomed. 2014, 27, 1361–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provan, D.; Stasi, R.; Newland, A.C.; Blanchette, V.S.; Bolton-Maggs, P.; Bussel, J.B.; Chong, B.H.; Cines, D.B.; Gernsheimer, T.B.; Godeau, B.; et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010, 115, 168–186. [Google Scholar] [CrossRef]
- Cui, Q.; Lewis, I.A.; Hegeman, A.D.; Anderson, M.E.; Li, J.; Schulte, C.F.; Westler, W.M.; Eghbalnia, H.R.; Sussman, M.R.; Markley, J.L. Metabolite identification via the Madison Metabolomics Consortium Database. Nat. Biotechnol. 2008, 26, 162–164. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- Chagoyen, M.; Pazos, F. MBRole: Enrichment analysis of metabolomic data. Bioinformatics 2011, 27, 730–731. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 2014, 42, D199–D205. [Google Scholar] [CrossRef] [Green Version]
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef]

| No. | Gender | Age | PPC 1 | PCD 2 | DPC 3 | Complication | Resp.4 |
|---|---|---|---|---|---|---|---|
| 1 | Female | 63 | 161 | 489 | >100 | CR | |
| 2 | Male | 41 | 8 | 59 | 30–100 | PR | |
| 3 | Female | 21 | 25 | 235 | 30–100 | PR | |
| 4 | Female | 23 | 276 | 503 | >100 | CR | |
| 5 | Female | 28 | 183 | 157 | <30 | bleeding | NR |
| 6 | Male | 58 | 171 | 426 | >100 | CR | |
| 7 | Female | 78 | 31 | 345 | >100 | CR | |
| 8 | Male | 22 | 453 | 463 | <30 | bleeding | NR |
| 9 | Female | 55 | 108 | 408 | >100 | CR | |
| 10 | Female | 20 | 149 | 215 | >100 | CR | |
| 11 | Female | 21 | 68 | 990 | >100 | CR | |
| 12 | Male | 21 | 288 | 228 | >100 | CR | |
| 13 | Male | 60 | 49 | 38 | <30 | NR | |
| 14 | Male | 38 | 66 | 150 | >100 | CR | |
| 15 | Male | 25 | 63 | 87 | >100 | CR | |
| 16 | Male | 21 | 289 | 311 | >100 | CR | |
| 17 | Male | 19 | 157 | 144 | >100 | CR | |
| 18 | Female | 22 | 60 | 421 | >100 | CR |
| Metabolites | ITP vs. Normal | |||
|---|---|---|---|---|
| Pcorr 1 | FC 2 | p 3 | VIP 4 | |
| Taurine | −0.765 | 0.696 | 3.7 × 10−6 | 2.689 |
| 3-Hydroxybutyric acid | −0.603 | 0.553 | 1.1 × 10−4 | 2.213 |
| Glycogen | −0.524 | 0.198 | 4.4 × 10−4 | 2.146 |
| Ascorbate | −0.634 | 0.423 | 1.3 × 10−3 | 2.072 |
| Alanine | 0.511 | 1.497 | 2.2 × 10−3 | 1.811 |
| Lysine | 0.592 | 1.362 | 2.6 × 10−3 | 2.029 |
| Asparagine | −0.623 | 0.717 | 2.6 × 10−3 | 1.907 |
| Glycerophosphocholine | −0.614 | 0.772 | 3.2 × 10−3 | 1.886 |
| Acetate | 0.523 | 1.400 | 3.5 × 10−3 | 1.822 |
| LDL-1 | −0.703 | 0.695 | 3.8 × 10−3 | 2.011 |
| Valine | 0.548 | 1.525 | 3.9 × 10−3 | 1.948 |
| Isoleucine | 0.583 | 1.523 | 5.0 × 10−3 | 1.953 |
| Pyroglutamate | 0.594 | 0.497 | 6.4 × 10−3 | 1.782 |
| myo-Inositol | −0.607 | 0.849 | 1.2 × 10−2 | 1.937 |
| Glutamine | 0.589 | 1.246 | 1.3 × 10−2 | 1.656 |
| Phenylalanine | 0.496 | 1.282 | 2.1 × 10−2 | 1.876 |
| Glycerol | 0.478 | 1.188 | 2.4 × 10−2 | 1.742 |
| Leucine | 0.582 | 1.257 | 2.6 × 10−2 | 2.056 |
| Ethanol | −0.509 | 0.809 | 3.6 × 10−2 | 1.739 |
| Methanol | 0.500 | 1.190 | 3.9 × 10−2 | 1.676 |
| Malonate | −0.491 | 0.810 | 4.4 × 10−2 | 1.654 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, S.; Wang, Z.; Feng, J.; Yang, Y.; Lin, X.; Huang, H. NMR-Based Metabolomics Identify Metabolic Change in Spleen of Idiopathic Thrombocytopenic Purpura Patients. Metabolites 2022, 12, 565. https://doi.org/10.3390/metabo12060565
Wen S, Wang Z, Feng J, Yang Y, Lin X, Huang H. NMR-Based Metabolomics Identify Metabolic Change in Spleen of Idiopathic Thrombocytopenic Purpura Patients. Metabolites. 2022; 12(6):565. https://doi.org/10.3390/metabo12060565
Chicago/Turabian StyleWen, Shi, Zhenzhao Wang, Jianghua Feng, Yuanyuan Yang, Xianchao Lin, and Heguang Huang. 2022. "NMR-Based Metabolomics Identify Metabolic Change in Spleen of Idiopathic Thrombocytopenic Purpura Patients" Metabolites 12, no. 6: 565. https://doi.org/10.3390/metabo12060565
APA StyleWen, S., Wang, Z., Feng, J., Yang, Y., Lin, X., & Huang, H. (2022). NMR-Based Metabolomics Identify Metabolic Change in Spleen of Idiopathic Thrombocytopenic Purpura Patients. Metabolites, 12(6), 565. https://doi.org/10.3390/metabo12060565






