Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review
Abstract
1. Introduction
- (a)
- identify common unsaturated fatty acids found in lung tissue;
- (b)
- based on the free radical mechanism of LPO, simulate oxidative cleavage of the identified panel of unsaturated lipids;
- (c)
- list potential aldehyde products of LPO generated by the simulation;
- (d)
- conduct a literature search for reports of the LPO products in exhaled breath and document the analytical techniques used to detect them.
2. Lipid Composition of Lung Tissue
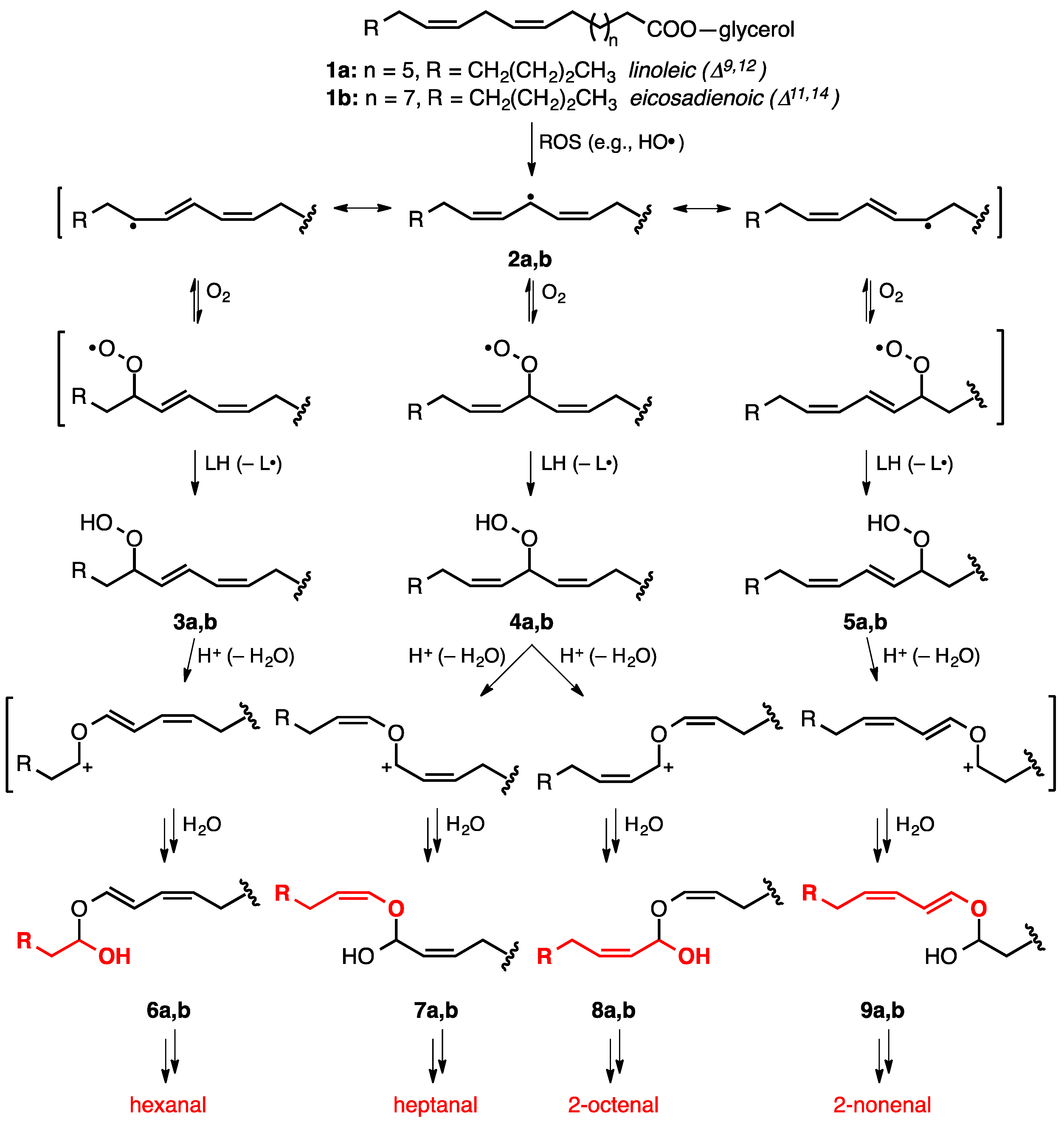
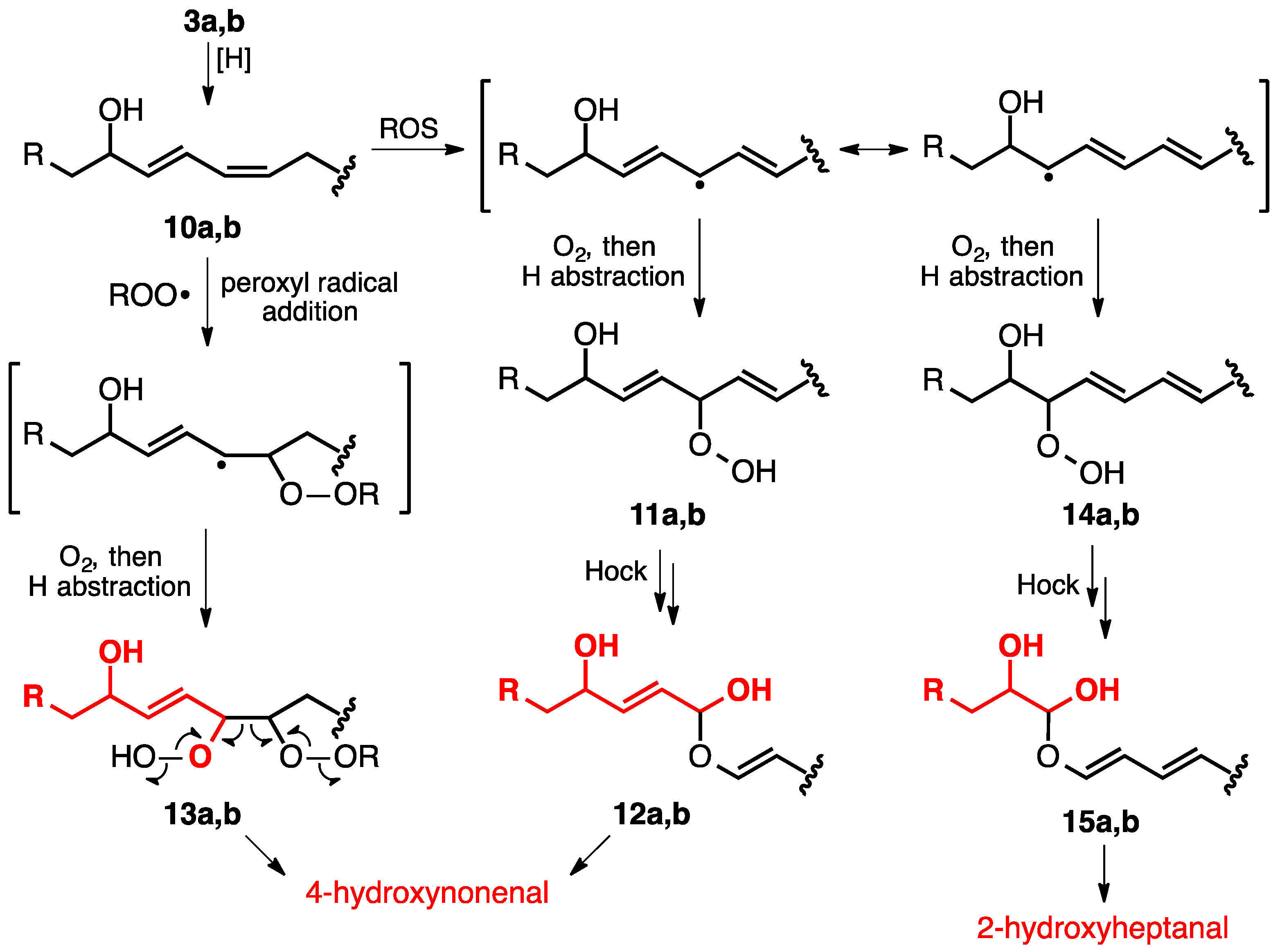
3. Lipid Peroxidation
4. Search Method and Results
5. Aldehydes Observed in the Exhaled Breath of Cancer Patients
6. Saturated Aldehydes
6.1. Propanal
6.2. Butanal
6.3. Pentanal
6.4. Hexanal
6.5. Heptanal
6.6. Octanal
6.7. Nonanal
6.8. Decanal
7. Unsaturated Aldehydes
7.1. 2-Propenal (Acrolein) and 2-Butenal (Crotonaldehyde)
7.2. 2-Hexenal, 2-Heptenal and 2-Nonenal
7.3. 2-Decenal
7.4. 4-Hydroxy-2-Hexenal (4-HHE)
7.5. 4-Hydroxy-2-Nonenal (4-HNE)
7.6. Malondialdehyde (MDA)
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNP | silver nanoparticle |
| ATM | 2-aminooxy-N,N,N-trimethylethan-1-ammonium iodide |
| CAR | Carboxen |
| CRDS | cavity ring-down spectroscopy |
| DNPH | dinitrophenylhydrazine |
| DVB | divinylbenzene |
| EB | exhaled breath |
| e-nose | electronic nose |
| FA | fatty acid |
| FT-ICR-MS | Fourier-transform ion cyclotron resonance mass spectrometry |
| GC-MS | gas chromatography-mass spectrometry |
| HC | healthy control |
| 4-HHE | 4-hydroxy-2-hexenal |
| 4-HNE | 4-hydroxy-2-nonenal |
| IMS | ion mobility spectrometry |
| LC | lung cancer |
| LoD | limit of detection |
| LPO | lipid peroxidation |
| MDA | malondialdehyde |
| MUFA | monounsaturated fatty acid |
| NSCLC | non-small cell lung cancer |
| NR | not reported |
| OS | oxidative stress |
| PC | phosphatidylcholine |
| PDMS | polydimethylsiloxane |
| PE | phosphatidylethanolamine |
| PFBHA | (pentafluorobenzyl)hydroxylamine |
| PG | phosphatidylglycerol |
| PL | phospholipid |
| POSS | polyhedral oligomeric silsesquioxane |
| PS | phosphatidylserine |
| PTR-MS | proton transfer reaction-mass spectrometry |
| PUFA | polyunsaturated fatty acid |
| ROS | reactive oxygen species |
| SAW | surface acoustic wave |
| SCLC | small cell lung cancer |
| Sph | sphingomyelin |
| SPME | solid-phase microextraction |
| TBA | thiobarbituric acid |
| VOC | volatile organic compound |
References
- Bargagli, E.; Olivieri, C.; Bennett, D.; Prasse, A.; Muller-Quernheim, J.; Rottoli, P. Oxidative stress in the pathogenesis of diffuse lung diseases: A review. Respir. Med. 2009, 103, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.H., I; Islam, M.M.T.; Shekhar, H.U. Oxidative stress and human health. J. Avd. Biol. Biotechnol. 2012, 3, 997–1019. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Wang, Z.; Pu, X.; Zhou, S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol. Appl. Pharmacol. 2011, 254, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Hauck, A.K.; Bernlohr, D.A. Oxidative stress and lipotoxicity. J. Lipid Res. 2016, 57, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Gueraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, M.; Han, X. Shotgun lipidomics in substantiating lipid peroxidation in redox biology: Methods and applications. Redox Biol. 2017, 12, 946–955. [Google Scholar] [CrossRef]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME–GC/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2643–2651. [Google Scholar] [CrossRef]
- United States Cancer Statistics Lung Cancer Stat Bite. Available online: https://www.cdc.gov/cancer/uscs/about/stat-bites/stat-bite-lung.htm (accessed on 24 May 2022).
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Krilaviciute, A.; Heiss, J.A.; Leja, M.; Kupcinskas, J.; Haick, H.; Brenner, H. Detection of cancer through exhaled breath: A systematic review. Oncotarget 2015, 6, 38643–38657. [Google Scholar] [CrossRef]
- Pauling, L.; Robinson, A.B.; Teranishi, R.; Cary, P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc. Natl. Acad. Sci. USA 1971, 68, 2374–2376. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Arcaro, A.; Pizzimenti, S.; Daga, M.; Cetrangolo, G.P.; Dianzani, C.; Lepore, A.; Graf, M.; Ames, P.R.J.; Barrera, G. DNA damage by lipid peroxidation products: Implications in cancer, inflammation and autoimmunity. AIMS Genet. 2017, 4, 103–137. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, N.; Wieczorek, T.; Drabinska, N.; Gould, O.; Osborne, A.; De Lacy Costello, B. A mechanistic study and review of volatile products from peroxidation of unsaturated fatty acids: An aid to understanding the origins of volatile organic compounds from the human body. J. Breath Res. 2020, 14, 34001. [Google Scholar] [CrossRef] [PubMed]
- Van Gossum, A.; Decuyper, J. Breath alkanes as an index of lipid peroxidation. Eur. Respir. J. 1989, 2, 787–791. [Google Scholar] [PubMed]
- Fisher, A.B. Chapter 22—Lung Lipid Composition and Surfactant Biology. In Comparative Biology of the Normal Lung, 2nd ed.; Parent, R.A., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 423–466. [Google Scholar]
- Kyle, J.E.; Clair, G.; Bandyopadhyay, G.; Misra, R.S.; Zink, E.M.; Bloodsworth, K.J.; Shukla, A.K.; Du, Y.; Lillis, J.; Myers, J.R.; et al. Cell type-resolved human lung lipidome reveals cellular cooperation in lung function. Sci. Rep. 2018, 8, 13455. [Google Scholar] [CrossRef]
- Zemski Berry, K.A.; Murphy, R.C.; Kosmider, B.; Mason, R.J. Lipidomic characterization and localization of phospholipids in the human lung. J. Lipid Res. 2017, 58, 926–933. [Google Scholar] [CrossRef]
- Veldhuizen, R.; Nag, K.; Orgeig, S.; Possmayer, F. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta 1998, 1408, 90–108. [Google Scholar] [CrossRef]
- Stachowicz-Kusnierz, A.; Cwiklik, L.; Korchowiec, J.; Rogalska, E.; Korchowiec, B. The impact of lipid oxidation on the functioning of a lung surfactant model. Phys. Chem. Chem. Phys. 2018, 20, 24968–24978. [Google Scholar] [CrossRef]
- Schmidt, R.; Meier, U.; Markart, P.; Grimminger, F.; Velcovsky, H.G.; Morr, H.; Seeger, W.; Gunther, A. Altered fatty acid composition of lung surfactant phospholipids in interstitial lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L1079–L1085. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Harlan, W.R., Jr.; Margraf, J.H.; Said, S.I. Pulmonary lipid composition of species with and without surfactant. Am. J. Physiol. 1966, 211, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, J.; Li, T.; Hu, H.; Li, X.; Xing, H.; Wang, J.; Yang, F.; Ma, Q.; Liu, B.; et al. Accurate Classification of Non-small Cell Lung Cancer (NSCLC) Pathology and Mapping of EGFR Mutation Spatial Distribution by Ambient Mass Spectrometry Imaging. Front. Oncol. 2019, 9, 804. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhao, J.; Xu, J.-F.; Zhang, X. Tuning the stability of organic radicals: From covalent approaches to non-covalent approaches. Chem. Sci. 2020, 11, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Tallman, K.A.; Roschek, B., Jr.; Porter, N.A. Factors influencing the autoxidation of fatty acids: Effect of olefin geometry of the nonconjugated diene. J. Am. Chem. Soc. 2004, 126, 9240–9247. [Google Scholar] [CrossRef]
- Xu, L.; Davis, T.A.; Porter, N.A. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc. 2009, 131, 13037–13044. [Google Scholar] [CrossRef]
- Porter, N.A.; Lehman, L.S.; Weber, B.A.; Smith, K.J. Unified mechanism for polyunsaturated fatty acid autoxidation. Competition of peroxy radical hydrogen atom abstraction, .beta.-scission, and cyclization. J. Am. Chem. Soc. 1981, 103, 6447–6455. [Google Scholar] [CrossRef]
- Yin, H.; Havrilla, C.M.; Gao, L.; Morrow, J.D.; Porter, N.A. Mechanisms for the formation of isoprostane endoperoxides from arachidonic acid. “Dioxetane” intermediate versus beta-fragmentation of peroxyl radicals. J. Biol. Chem. 2003, 278, 16720–16725. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, W.; Salomon, R.G. Fe2+ catalyzes vitamin E-induced fragmentation of hydroperoxy and hydroxy endoperoxides that generates gamma-hydroxy alkenals. J. Am. Chem. Soc. 2007, 129, 6088–6089. [Google Scholar] [CrossRef]
- Scibior, A.; Kurus, J. Vanadium and Oxidative Stress Markers—In Vivo Model: A Review. Curr. Med. Chem. 2019, 26, 5456–5500. [Google Scholar] [CrossRef]
- Jomova, K.; Baros, S.; Valko, M. Redox active metal-induced oxidative stress in biological systems. Transit. Met. Chem. 2012, 37, 127–134. [Google Scholar] [CrossRef]
- Aust, S.D.; Morehouse, L.A.; Thomas, C.E. Role of metals in oxygen radical reactions. J. Free Radic. Biol. Med. 1985, 1, 3–25. [Google Scholar] [CrossRef]
- Schaich, K.M. Metals and lipid oxidation. Contemporary issues. Lipids 1992, 27, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Posner, G.H.; O’Neill, P.M. Knowledge of the proposed chemical mechanism of action and cytochrome p450 metabolism of antimalarial trioxanes like artemisinin allows rational design of new antimalarial peroxides. Acc. Chem. Res. 2004, 37, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.A.; Zhang, G.; Decker, E.A. Biological Implications of Lipid Oxidation Products. J. Am. Oil Chem. Soc. 2017, 94, 339–351. [Google Scholar] [CrossRef]
- Imai, H.; Nakagawa, Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic. Biol. Med. 2003, 34, 145–169. [Google Scholar] [CrossRef]
- Toppo, S.; Flohe, L.; Ursini, F.; Vanin, S.; Maiorino, M. Catalytic mechanisms and specificities of glutathione peroxidases: Variations of a basic scheme. Biochim. Biophys. Acta 2009, 1790, 1486–1500. [Google Scholar] [CrossRef]
- Bui, P.H.; Hsu, E.L.; Hankinson, O. Fatty acid hydroperoxides support cytochrome P450 2S1-mediated bioactivation of benzo[a]pyrene-7,8-dihydrodiol. Mol. Pharmacol. 2009, 76, 1044–1052. [Google Scholar] [CrossRef]
- Frankel, E.N.; Neff, W.E. Formation of malonaldehyde from lipid oxidation products. Biochim. Biophys. Acta 1983, 754, 264–270. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Vil, V.A.; Demchuk, D.V.; Terent’ev, A.O. Rearrangements of organic peroxides and related processes. Beilstein J. Org. Chem. 2016, 12, 1647–1748. [Google Scholar] [CrossRef]
- Hazen, S.L. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 2008, 283, 15527–15531. [Google Scholar] [CrossRef]
- Loidl-Stahlhofen, A.; Hannemann, K.; Spiteller, G. Generation of α-hydroxyaldehydic compounds in the course of lipid peroxidation. Biochim. Biophys. Acta 1994, 1213, 140–148. [Google Scholar] [CrossRef]
- Hölzel, C.; Spiteller, G. Zellschädigung als Ursache für die Bildung von Hydroperoxiden ungesättigter Fettsäuren. Naturwissenschaften 1995, 82, 452–460. [Google Scholar] [CrossRef]
- Miakar, A.; Spiteller, G. Reinvestigation of lipid peroxidation of linolenic acid. Biochim. Biophys. Acta 1994, 1214, 209–220. [Google Scholar] [CrossRef]
- Kern, W.; Spiteller, G. Synthesis and properties of natural occurring α-hydroxyaldehydes. Tetrahedron 1996, 52, 4347–4362. [Google Scholar] [CrossRef]
- Muzio, G.; Ricci, M.; Traverso, N.; Monacelli, F.; Oraldi, M.; Maggiora, M.; Canuto, R.A. 4-Hydroxyhexenal and 4-hydroxynonenal are mediators of the anti-cachectic effect of n-3 and n-6 polyunsaturated fatty acids on human lung cancer cells. Free Radic. Biol. Med. 2016, 99, 63–70. [Google Scholar] [CrossRef]
- Vander Jagt, D.L.; Hunsaker, L.A.; Vander Jagt, T.J.; Gomez, M.S.; Gonzales, D.M.; Deck, L.M.; Royer, R.E. Inactivation of glutathione reductase by 4-hydroxynonenal and other endogenous aldehydes. Biochem. Pharma. 1997, 53, 1133–1140. [Google Scholar] [CrossRef]
- Uchida, K. 4-Hydroxy-2-nonenal: A product and mediator of oxidative stress. Prog. Lipid Res. 2003, 42, 318–343. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Ciamporcero, E.S.; Daga, M.; Ullio, C.; Arcaro, A.; Cetrangolo, G.P.; Ferretti, C.; Dianzani, C.; Lepore, A.; et al. Role of 4-hydroxynonenal-protein adducts in human diseases. Antioxid. Redox Signal. 2015, 22, 1681–1702. [Google Scholar] [CrossRef]
- Tamura, H.; Kitta, K.; Shibamoto, T. Formation of reactive aldehydes from fatty acids in a iron(2+)/hydrogen peroxide oxidation system. J. Agric. Food Chem. 1991, 39, 439–442. [Google Scholar] [CrossRef]
- Kawai, Y.; Takeda, S.; Terao, J. Lipidomic analysis for lipid peroxidation-derived aldehydes using gas chromatography-mass spectrometry. Chem. Re.s Toxicol. 2007, 20, 99–107. [Google Scholar] [CrossRef]
- Jones, A.W. Measuring and reporting the concentration of acetaldehyde in human breath. Alcohol Alcohol. 1995, 30, 271–285. [Google Scholar] [PubMed]
- Filipiak, W.; Ruzsanyi, V.; Mochalski, P.; Filipiak, A.; Bajtarevic, A.; Ager, C.; Denz, H.; Hilbe, W.; Jamnig, H.; Hackl, M.; et al. Dependence of exhaled breath composition on exogenous factors, smoking habits and exposure to air pollutants. J. Breath Res. 2012, 6, 36008. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-A.; Li, M.; Knipp, R.J.; Nantz, M.H.; Bousamra, M. Noninvasive detection of lung cancer using exhaled breath. Cancer Med. 2014, 3, 174–181. [Google Scholar] [CrossRef]
- Anderson, M.M.; Hazen, S.L.; Hsu, F.F.; Heinecke, J.W. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J. Clin. Investig. 1997, 99, 424–432. [Google Scholar] [CrossRef]
- Lorenzi, R.; Andrades, M.E.; Bortolin, R.C.; Nagai, R.; Dal-Pizzol, F.; Moreira, J.C.F. Circulating glycolaldehyde induces oxidative damage in the kidney of rats. Diabetes Res. Clin. Pract. 2010, 89, 262–267. [Google Scholar] [CrossRef]
- O’Neill, H.J.; Gordon, S.M.; O’Neill, M.H.; Gibbons, R.D.; Szidon, J.P. A computerized classification technique for screening for the presence of breath biomarkers in lung cancer. Clin. Chem. 1988, 34, 1613–1618. [Google Scholar] [CrossRef]
- Phillips, M.; Gleeson, K.; Hughes, J.M.B.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, X.; Li, N. Investigation of volatile biomarkers in lung cancer blood using solid-phase microextraction and capillary gas chromatography-mass spectrometry. J. Chromatogr. B 2004, 808, 269–277. [Google Scholar] [CrossRef]
- Chen, X.; Cao, M.; Hao, Y.; Li, Y.; Wang, P.; Ying, K.; Pan, H. A Non-invasive detection of lung cancer combined virtual gas sensors array with imaging recognition technique. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2005, 2005, 5873–5876. [Google Scholar] [CrossRef]
- Chen, X.; Xu, F.; Wang, Y.; Pan, Y.; Lu, D.; Wang, P.; Ying, K.; Chen, E.; Zhang, W. A study of the volatile organic compounds exhaled by lung cancer cells in vitro for breath diagnosis. Cancer 2007, 110, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, E.M.; Lucena, A.F.; Duro da Costa, J.; Chaves das Neves, H. Organic metabolites in exhaled human breath--a multivariate approach for identification of biomarkers in lung disorders. J. Chromatogr. A 2009, 1216, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Ligor, M.; Ligor, T.; Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Denz, H.; Fiegl, M.; Hilbe, W.; Weiss, W.; et al. Determination of volatile organic compounds in exhaled breath of patients with lung cancer using solid phase microextraction and gas chromatography mass spectrometry. Clin. Chem. Lab. Med. 2009, 47, 550–560. [Google Scholar] [CrossRef]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef]
- Kischkel, S.; Miekisch, W.; Sawacki, A.; Straker, E.M.; Trefz, P.; Amann, A.; Schubert, J.K. Breath biomarkers for lung cancer detection and assessment of smoking related effects--confounding variables, influence of normalization and statistical algorithms. Clin. Chim. Acta 2010, 411, 1637–1644. [Google Scholar] [CrossRef]
- Rudnicka, J.; Kowalkowski, T.; Ligor, T.; Buszewski, B. Determination of volatile organic compounds as biomarkers of lung cancer by SPME-GC-TOF/MS and chemometrics. J. Chromatogr. B 2011, 879, 3360–3366. [Google Scholar] [CrossRef]
- Ulanowska, A.; Kowalkowski, T.; Trawinska, E.; Buszewski, B. The application of statistical methods using VOCs to identify patients with lung cancer. J. Breath Res. 2011, 5, 46008. [Google Scholar] [CrossRef]
- Buszewski, B.; Ulanowska, A.; Kowalkowski, T.; Cieslinski, K. Investigation of lung cancer biomarkers by hyphenated separation techniques and chemometrics. Clin. Chem. Lab. Med. 2011, 50, 573–581. [Google Scholar] [CrossRef]
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef]
- Peled, N.; Hakim, M.; Bunn, P.A., Jr.; Miller, Y.E.; Kennedy, T.C.; Mattei, J.; Mitchell, J.D.; Hirsch, F.R.; Haick, H. Non-invasive breath analysis of pulmonary nodules. J. Thorac. Oncol. 2012, 7, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Bousamra, M., 2nd; Schumer, E.; Li, M.; Knipp, R.J.; Nantz, M.H.; van Berkel, V.; Fu, X.A. Quantitative analysis of exhaled carbonyl compounds distinguishes benign from malignant pulmonary disease. J. Thorac. Cardiovasc. Surg. 2014, 148, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, W.; Filipiak, A.; Sponring, A.; Schmid, T.; Zelger, B.; Ager, C.; Klodzinska, E.; Denz, H.; Pizzini, A.; Lucciarini, P.; et al. Comparative analyses of volatile organic compounds (VOCs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. J. Breath Res. 2014, 8, 27111. [Google Scholar] [CrossRef]
- Handa, H.; Usuba, A.; Maddula, S.; Baumbach, J.I.; Mineshita, M.; Miyazawa, T. Exhaled breath analysis for lung cancer detection using ion mobility spectrometry. PLoS ONE 2014, 9, e114555. [Google Scholar] [CrossRef]
- Rudnicka, J.; Walczak, M.; Kowalkowski, T.; Jezierski, T.; Buszewski, B. Determination of volatile organic compounds as potential markers of lung cancer by gas chromatography–mass spectrometry versus trained dogs. Sens. Actuators B Chem. 2014, 202, 615–621. [Google Scholar] [CrossRef]
- Corradi, M.; Poli, D.; Banda, I.; Bonini, S.; Mozzoni, P.; Pinelli, S.; Alinovi, R.; Andreoli, R.; Ampollini, L.; Casalini, A.; et al. Exhaled breath analysis in suspected cases of non-small-cell lung cancer: A cross-sectional study. J. Breath Res. 2015, 9, 27101. [Google Scholar] [CrossRef]
- Li, M.; Yang, D.; Brock, G.; Knipp, R.J.; Bousamra, M.; Nantz, M.H.; Fu, X.A. Breath carbonyl compounds as biomarkers of lung cancer. Lung Cancer 2015, 90, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Ligor, T.; Pater, Ł.; Buszewski, B. Application of an artificial neural network model for selection of potential lung cancer biomarkers. J. Breath Res. 2015, 9, 27106. [Google Scholar] [CrossRef] [PubMed]
- Schumer, E.M.; Trivedi, J.R.; van Berkel, V.; Black, M.C.; Li, M.; Fu, X.-A.; Bousamra, M. High sensitivity for lung cancer detection using analysis of exhaled carbonyl compounds. J. Thorac. Cardiovasc. Surg. 2015, 150, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, T.; Alkoby-Meshulam, L.; Herbig, J.; Cancilla, J.C.; Torrecilla, J.S.; Gai Mor, N.; Bar, J.; Ilouze, M.; Haick, H.; Peled, N. Cancerous glucose metabolism in lung cancer-evidence from exhaled breath analysis. J. Breath Res. 2016, 10, 26012. [Google Scholar] [CrossRef]
- Schallschmidt, K.; Becker, R.; Jung, C.; Bremser, W.; Walles, T.; Neudecker, J.; Leschber, G.; Frese, S.; Nehls, I. Comparison of volatile organic compounds from lung cancer patients and healthy controls-challenges and limitations of an observational study. J. Breath Res. 2016, 10, 46007. [Google Scholar] [CrossRef] [PubMed]
- Schumer, E.M.; Black, M.C.; Bousamra, M., II; Trivedi, J.R.; Li, M.; Fu, X.-A.; van Berkel, V. Normalization of Exhaled Carbonyl Compounds After Lung Cancer Resection. Ann. Thorac. Surg. 2016, 102, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Shehada, N.; Cancilla, J.C.; Torrecilla, J.S.; Pariente, E.S.; Bronstrup, G.; Christiansen, S.; Johnson, D.W.; Leja, M.; Davies, M.P.; Liran, O.; et al. Silicon Nanowire Sensors Enable Diagnosis of Patients via Exhaled Breath. ACS Nano 2016, 10, 7047–7057. [Google Scholar] [CrossRef] [PubMed]
- Callol-Sanchez, L.; Munoz-Lucas, M.A.; Gomez-Martin, O.; Maldonado-Sanz, J.A.; Civera-Tejuca, C.; Gutierrez-Ortega, C.; Rodriguez-Trigo, G.; Jareno-Esteban, J. Observation of nonanoic acid and aldehydes in exhaled breath of patients with lung cancer. J. Breath Re.s 2017, 11, 26004. [Google Scholar] [CrossRef]
- Jouyban, A.; Djozan, D.; Mohammadandashti, P.; Alizadeh-Nabil, A.; Ghorbanpour, H.; Khoubnasabjafari, M.; Mohammadzadeh, M. Co-liquefaction with acetone and GC analysis of volatile compounds in exhaled breath as lung cancer biomarkers. Bioimpacts 2017, 7, 99–108. [Google Scholar] [CrossRef][Green Version]
- Sakumura, Y.; Koyama, Y.; Tokutake, H.; Hida, T.; Sato, K.; Itoh, T.; Akamatsu, T.; Shin, W. Diagnosis by Volatile Organic Compounds in Exhaled Breath from Lung Cancer Patients Using Support Vector Machine Algorithm. Sensors 2017, 17, 287. [Google Scholar] [CrossRef]
- Wang, M.; Sheng, J.; Wu, Q.; Zou, Y.; Hu, Y.; Ying, K.; Wan, H.; Wang, P. Confounding effect of benign pulmonary diseases in selecting volatile organic compounds as markers of lung cancer. J. Breath Res. 2018, 12, 46013. [Google Scholar] [CrossRef]
- Rudnicka, J.; Kowalkowski, T.; Buszewski, B. Searching for selected VOCs in human breath samples as potential markers of lung cancer. Lung Cancer 2019, 135, 123–129. [Google Scholar] [CrossRef]
- Koureas, M.; Kirgou, P.; Amoutzias, G.; Hadjichristodoulou, C.; Gourgoulianis, K.; Tsakalof, A. Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons. Metabolites 2020, 10, 317. [Google Scholar] [CrossRef]
- Muñoz-Lucas, M.Á.; Jareño-Esteban, J.; Gutiérrez-Ortega, C.; López-Guijarro, P.; Collado-Yurrita, L.; Quintana-Díaz, M.; Callol-Sánchez, L. Influence of Chronic Obstructive Pulmonary Disease on Volatile Organic Compounds in Patients with Non-Small Cell Lung Cancer. Arch. Bronconeumol. 2020, 56, 801–805. [Google Scholar] [CrossRef]
- Chen, X.; Muhammad, K.G.; Madeeha, C.; Fu, W.; Xu, L.; Hu, Y.; Liu, J.; Ying, K.; Chen, L.; Yurievna, G.O. Calculated indices of volatile organic compounds (VOCs) in exhalation for lung cancer screening and early detection. Lung Cancer 2021, 154, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Gashimova, E.; Osipova, A.; Temerdashev, A.; Porkhanov, V.; Polyakov, I.; Perunov, D.; Dmitrieva, E. Exhaled breath analysis using GC-MS and an electronic nose for lung cancer diagnostics. Anal. Methods 2021, 13, 4793–4804. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Zhan, L.; Meng, L.; Huang, X.; Wang, T.; Li, Y.; Nie, Z. Point-of-Care Test Paper for Exhaled Breath Aldehyde Analysis via Mass Spectrometry. Anal. Chem. 2021, 93, 9158–9165. [Google Scholar] [CrossRef]
- Long, Y.; Wang, C.; Wang, T.; Li, W.; Dai, W.; Xie, S.; Tian, Y.; Liu, M.; Liu, Y.; Peng, X.; et al. High performance exhaled breath biomarkers for diagnosis of lung cancer and potential biomarkers for classification of lung cancer. J. Breath Res. 2021, 15, 16017. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, Y.; Jiang, Z.; Zhou, Y.; Chen, Y.; Hu, Y.; Jiang, G.; Xie, D. Breath profile as composite biomarkers for lung cancer diagnosis. Lung Cancer 2021, 154, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Larracy, R.; Phinyomark, A.; Scheme, E. Infrared cavity ring-down spectroscopy for detecting non-small cell lung cancer in exhaled breath. J. Breath Res. 2022, 16, 26008. [Google Scholar] [CrossRef] [PubMed]
- Soufi, G.; Bagheri, H.; Yeganeh Rad, L.; Minaeian, S. Perylene diimide-POSS network for semi selective solid-phase microextraction of lung cancer biomarkers in exhaled breath. Anal. Chim. Acta 2022, 1198, 339550. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Y.; Jiang, Z.; Chen, Y.; Zhou, Y.; Wang, Z.; Wang, Y.; Jiang, G.; Tan, Z.; Hu, F. Exhaled metabolic markers and relevant dysregulated pathways of lung cancer: A pilot study. Ann. Med. 2022, 54, 790–802. [Google Scholar] [CrossRef]
- Schmidt, K.; Podmore, I. Current Challenges in Volatile Organic Compounds Analysis as Potential Biomarkers of Cancer. J. Biomark. 2015, 2015, 981458. [Google Scholar] [CrossRef]
- Brunton, N.P.; Cronin, D.A.; Monahan, F.J.; Durcan, R. A comparison of solid-phase microextraction (SPME) fibres for measurement of hexanal and pentanal in cooked turkey. Food Chem. 2000, 68, 339–345. [Google Scholar] [CrossRef]
- Rösch, C.; Kohajda, T.; Röder, S.; Bergen, M.v.; Schlink, U. Relationship between sources and patterns of VOCs in indoor air. Atmos. Pollut. Res. 2014, 5, 129–137. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Nantz, M.H.; Fu, X.-A. Analysis of Carbonyl Compounds in Ambient Air by a Microreactor Approach. ACS Omega 2018, 3, 6764–6769. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K.; Shibamoto, T. Determination of toxic carbonyl compounds in cigarette smoke. Environ. Toxicol. 2006, 21, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Headspace volatile aldehydes as indicators of lipid oxidation in foods. Adv. Exp. Med. Biol. 2001, 488, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, R.; Nilsson, C.; Andersson, B. Emissions of aldehydes and ketones from a two-stroke engine using ethanol and ethanol-blended gasoline as fuel. Environ. Sci. Technol. 2002, 36, 1656–1664. [Google Scholar] [CrossRef]
- Kumar, S.; Nayek, M.; Kumar, A.; Tandon, A.; Mondal, P.; Vijay, P.; Bhangale, U.D.; Tyagi, D. Aldehyde, Ketone and Methane Emissions from Motor Vehicle Exhaust: A Critical Review. Am. Chem. Sci. J. 2011, 1, 1–27. [Google Scholar] [CrossRef]
- Cheah, N.P.; Borst, S.; Hendrickx, L.; Cremers, H.; Jansen, E.; Opperhuizen, A.; Talhout, R. Effect of Adding Sugar to Burley Tobacco on the Emission of Aldehydes in Mainstream Tobacco Smoke. Tob. Regul. Sci. 2018, 4, 61–72. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, H.; Yang, N.; Shao, L.; He, P. Gaseous pollutants emitted from a mechanical biological treatment plant for municipal solid waste: Odor assessment and photochemical reactivity. J. Air Waste Manag. Assoc. 2013, 63, 1287–1297. [Google Scholar] [CrossRef]
- Daisey, J.M.; Hodgson, A.T.; Fisk, W.J.; Mendell, M.J.; Ten Brinke, J. Volatile organic compounds in twelve California office buildings: Classes, concentrations and sources. Atmos. Environ. 1994, 28, 3557–3562. [Google Scholar] [CrossRef]
- Stevens, J.F.; Maier, C.S. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008, 52, 7–25. [Google Scholar] [CrossRef]
- Wilson, V.L.; Foiles, P.G.; Chung, F.L.; Povey, A.C.; Frank, A.A.; Harris, C.C. Detection of acrolein and crotonaldehyde DNA adducts in cultured human cells and canine peripheral blood lymphocytes by 32P-postlabeling and nucleotide chromatography. Carcinog. 1991, 12, 1483–1490. [Google Scholar] [CrossRef]
- Siegel, D.A.; Fedewa, S.A.; Henley, S.J.; Pollack, L.A.; Jemal, A. Proportion of Never Smokers Among Men and Women With Lung Cancer in 7 US States. JAMA Oncol. 2021, 7, 302–304. [Google Scholar] [CrossRef]
- Long, E.K.; Picklo Sr, M.J. Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: Make some room HNE. Free Radic. Biol. Med. 2010, 49, 1–8. [Google Scholar] [CrossRef]
- Corradi, M.; Folesani, G.; Andreoli, R.; Manini, P.; Bodini, A.; Piacentini, G.; Carraro, S.; Zanconato, S.; Baraldi, E. Aldehydes and glutathione in exhaled breath condensate of children with asthma exacerbation. Am. J. Respir. Crit. Care Med. 2003, 167, 395–399. [Google Scholar] [CrossRef]
- Corradi, M.; Rubinstein, I.; Andreoli, R.; Manini, P.; Caglieri, A.; Poli, D.; Alinovi, R.; Mutti, A. Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003, 167, 1380–1386. [Google Scholar] [CrossRef]
- Antus, B.; Harnasi, G.; Drozdovszky, O.; Barta, I. Monitoring oxidative stress during chronic obstructive pulmonary disease exacerbations using malondialdehyde. Respirology 2014, 19, 74–79. [Google Scholar] [CrossRef]
- Corradi, M.; Pignatti, P.; Manini, P.; Andreoli, R.; Goldoni, M.; Poppa, M.; Moscato, G.; Balbi, B.; Mutti, A. Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. Eur. Respir. J. 2004, 24, 1011–1017. [Google Scholar] [CrossRef]
- Bartoli, M.L.; Novelli, F.; Costa, F.; Malagrino, L.; Melosini, L.; Bacci, E.; Cianchetti, S.; Dente, F.L.; Di Franco, A.; Vagaggini, B.; et al. Malondialdehyde in exhaled breath condensate as a marker of oxidative stress in different pulmonary diseases. Mediat. Inflamm. 2011, 2011, 891752. [Google Scholar] [CrossRef]
- Casimirri, E.; Stendardo, M.; Bonci, M.; Andreoli, R.; Bottazzi, B.; Leone, R.; Schito, M.; Vaccari, A.; Papi, A.; Contoli, M.; et al. Biomarkers of oxidative-stress and inflammation in exhaled breath condensate from hospital cleaners. Biomarkers 2016, 21, 115–122. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, Y.C.; Shin, J.H.; Lee, J.H.; Lee, Y.; Park, S.Y.; Baek, J.E.; Park, J.D.; Ahn, K.; Yu, I.J. Health surveillance study of workers who manufacture multi-walled carbon nanotubes. Nanotoxicology 2015, 9, 802–811. [Google Scholar] [CrossRef]
- Sakhvidi, M.J.; Biabani Ardekani, J.; Firoozichahak, A.; Zavarreza, J.; Hajaghazade, M.; Mostaghaci, M.; Mehrparvar, A.; Barkhordari, A. Exhaled breath malondialdehyde, spirometric results and dust exposure assessment in ceramics production workers. Int. J. Occup. Med. Environ. Health 2015, 28, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Kacer, P.; Komarc, M.; Fenclova, Z.; Vlckova, S.; Zikova, N.; Schwarz, J.; Makes, O.; Navratil, T.; et al. Markers of lipid oxidative damage among office workers exposed intermittently to air pollutants including nanoTiO2 particles. Rev. Environ. Health 2017, 32, 193–200. [Google Scholar] [CrossRef]
- Gong, J.; Zhu, T.; Kipen, H.; Wang, G.; Hu, M.; Ohman-Strickland, P.; Lu, S.-E.; Zhang, L.; Wang, Y.; Zhu, P.; et al. Malondialdehyde in exhaled breath condensate and urine as a biomarker of air pollution induced oxidative stress. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 322–327. [Google Scholar] [CrossRef]
- Larstad, M.; Ljungkvist, G.; Olin, A.C.; Toren, K. Determination of malondialdehyde in breath condensate by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B 2002, 766, 107–114. [Google Scholar] [CrossRef]
- Manini, P.; Andreoli, R.; Sforza, S.; Dall’Asta, C.; Galaverna, G.; Mutti, A.; Niessen, W.M. Evaluation of Alternate Isotope-Coded Derivatization Assay (AIDA) in the LC-MS/MS analysis of aldehydes in exhaled breath condensate. J. Chromatogr. B 2010, 878, 2616–2622. [Google Scholar] [CrossRef]
- Kartavenka, K.; Panuwet, P.; Greenwald, R.; Ehret, K.M.; D’Souza, P.E.; Barr, D.B.; Ryan, P.B. Quantification of malondialdehyde in exhaled breath condensate using pseudo two-dimensional ultra-performance liquid chromatography coupled with single quadrupole mass spectrometry. J. Chromatogr. B 2019, 1105, 210–216. [Google Scholar] [CrossRef]
- Jafari, M.; Solhi, E.; Tagi, S.; Hasanzadeh, M.; Jouyban-Gharamaleki, V.; Jouyban, A.; Shadjou, N. Non-invasive quantification of malondialdehyde biomarker in human exhaled breath condensate using self-assembled organic-inorganic nanohybrid: A new platform for early diagnosis of lung disease. J. Pharm. Biomed. Anal. 2019, 164, 249–257. [Google Scholar] [CrossRef]
- Andreoli, R.; Manini, P.; Corradi, M.; Mutti, A.; Niessen, W.M.A. Determination of patterns of biologically relevant aldehydes in exhaled breath condensate of healthy subjects by liquid chromatography/atmospheric chemical ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 637–645. [Google Scholar] [CrossRef]
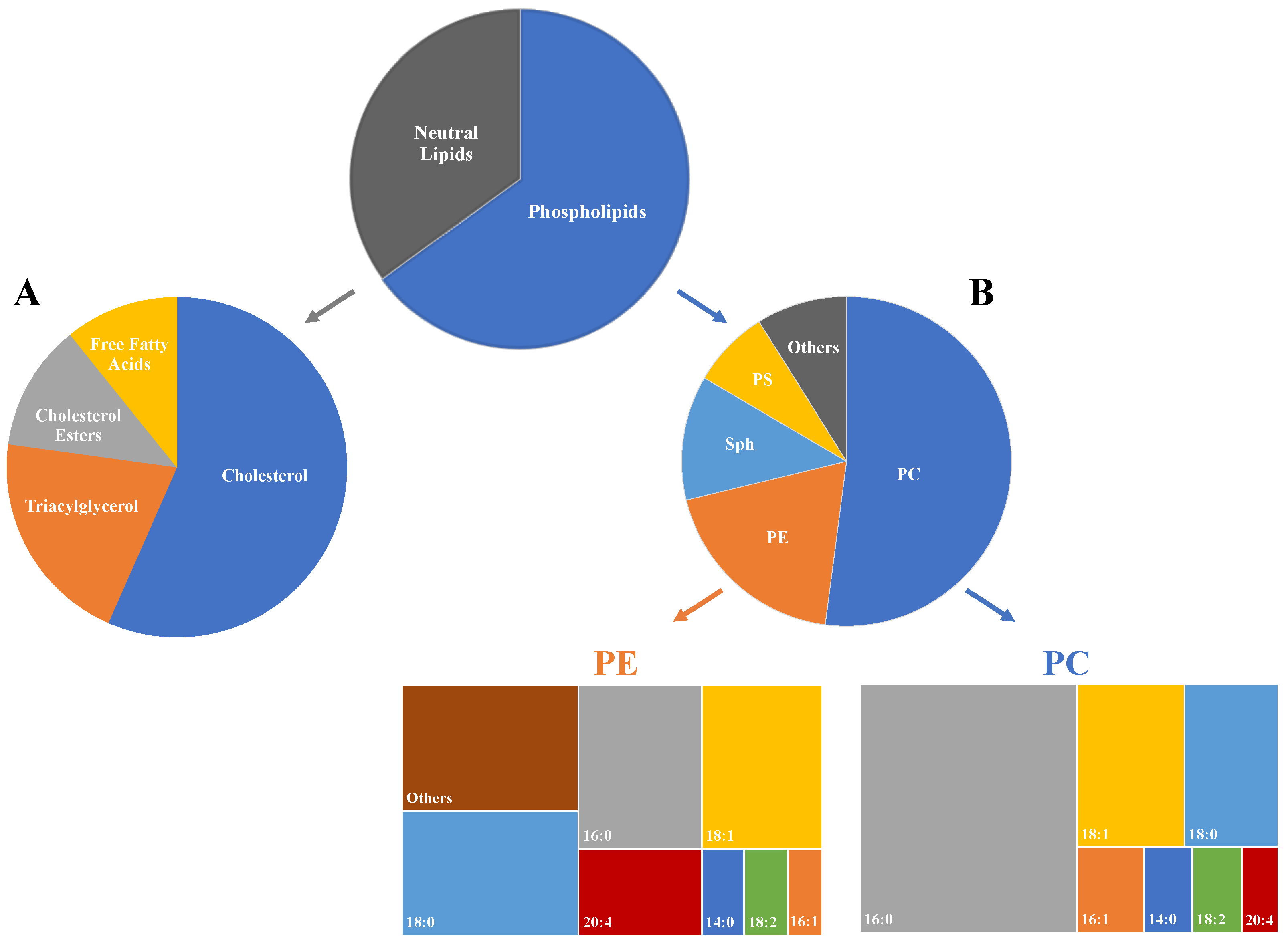

| Saturated FA | Monounsaturated FA (MUFA) | Polyunsaturated FA (PUFA) | |||
|---|---|---|---|---|---|
| 12:0 a | Lauric acid | 16:1 | Palmitoleic acid | 18:2 | Linoleic acid |
| 14:0 | Myristic acid | 18:1 | Oleic acid | 18:3 | Linolenic acid |
| 16:0 | Palmitic acid | 20:1 | Eicosenoic acid | 20:2 | Eicosadienoic acid |
| 18:0 | Stearic acid | 20:3 | Eicosatrienoic acid | ||
| 20:4 | Arachidonic acid | ||||
| 22:6 | Docosahexaenoic acid | ||||
| Fatty Acid Sidechain | Aldehydes Predicted as LPO Products | ||
|---|---|---|---|
| Saturated | Unsaturated | Hydroxy | |
ω-3 n = 5: α-linolenic acid (Δ9,12,15) n = 7: eicosatrienoic acid (Δ11,14,17) | propanal butanal | 2-pentenal 2-hexenal | 2-hydroxybutanal |
| 4-hydroxyhexenal (4-HHE) | |||
ω-6 n = 5: linoleic acid (Δ9,12) n = 7: eicosadienoic acid (Δ11,14) | pentanal hexanal heptanal | 2-octenal 2-nonenal | 2-hydroxyheptanal |
| 4-hydroxynonenal (4-HNE) | |||
ω-7 n = 5: palmitoleic (Δ9) | hexanal heptanal octanal | 2-octenal | 2-hydroxyheptanal |
ω-9 n = 5: oleic acid (Δ9) n = 7: eicosenoic acid (Δ11) | octanal nonanal decanal | 2-decenal | 2-hydroxynonanal |
| Year | Study b | Patients c | Stage d | Breath Collection | Preconcentration Method | Analytical Instrument | Saturated Aldehydes | Unsaturated Aldehydes |
|---|---|---|---|---|---|---|---|---|
| 1988 | O’Neill [59] | 8 | NR | Teflon bag | Tenax TA | GC-MS | propanal, octanal, nonanal | |
| 1999 | Phillips [60] | 108 | I–IV | 10 L collection apparatus | activated carbon | GC-MS | hexanal, heptanal | |
| 2004 | Deng [61] | 10 | I | sampling bulb | CAR/PDMS | GC-MS | hexanal, heptanal | |
| 2005 | Chen [62] | 24 | NR | Tedlar bag | SPME (unspecified) | GC-SAW sensor | hexanal, heptanal | |
| 2007 | Chen [63] | 29 | NR | Tedlar bag | PDMS | GC-FID | hexanal, heptanal | |
| 2009 | Bajtarevic [64] | 285 e | NR | Tedlar bag | CAR/PDMS | PTR-MS/ GC-MS | pentanal | |
| 2009 | Gaspar [65] | 18 | IV | Tedlar bag | PDMS | GC-MS | hexanal, heptanal | |
| 2009 | Ligor [66] | 65 | NR | Tedlar bag | CAR/PDMS | GC-MS | pentanal | |
| 2010 | Fuchs [67] | 12 | III–IV | Tedlar bag | PDMS/DVB (PFBHA derivatization) | GC-MS | propanal, butanal, pentanal, hexanal, heptanal, octanal, nonanal, decanal | |
| 2010 | Kischkel [68] | 31 | II–IV | Tedlar bag | CAR/PDMS | GC-MS | propanal, butanal, pentanal, hexanal, heptanal, octanal | 2-butenal |
| 2010 | Poli [8] | 40 | I–III | Bio-VOC tube | PDMS/DVB (PFBHA derivatization) | GC-MS | propanal, butanal, pentanal, hexanal, heptanal, octanal, nonanal | |
| 2011 | Rudnicka [69] | 23 | NR | Tedlar bag | CAR/PDMS | GC-MS | propanal, butanal, pentanal | |
| 2011 | Ulanowska [70] | 137 | NR | Tedlar bag | CAR/PDMS | GC-MS | propanal, pentanal, hexanal | |
| 2011 | Buszewski [71] | 115 | NR | Tedlar bag | CAR/PDMS | GC-MS | propanal, pentanal, hexanal | |
| 2012 | Buszewski [72] | 29 | NR | Tedlar bag | CAR/PDMS | GC-MS | propanal, butanal | |
| 2012 | Peled [73] | 53 | I–IV | Mylar bag | Tenax PA | GC-MS | decanal | |
| 2014 | Bousamra [74] | 107 | I–IV | Tedlar bag | Si microreactor (ATM derivatization) | FT-ICR-MS | 4-HHE | |
| 2014 | Filipiak [75] | 36 | NR | Tedlar bag | Tenax TA/CAR | GC-MS | butanal, pentanal, hexanal, nonanal, decanal | |
| 2014 | Fu [56] | 97 | I–IV | Tedlar bag | Si microreactor (ATM derivatization) | FT-ICR-MS | pentanal, hexanal, octanal, nonanal | 4-HHE, 4-HNE |
| 2014 | Handa [76] | 50 | I–IV | — | expiration into spirometer | IMS | hexanal, heptanal, nonanal | |
| 2014 | Rudnicka [77] | 108 | I–IV | Tedlar bag | CAR/PDMS | GC-MS | propanal, pentanal, hexanal | |
| 2015 | Corradi [78] | 71 | I–IV | Bio-VOC tube | CAR/PDMS or PDMS/DVB (PFBHA derivatization) | GC-MS | propanal, butanal, pentanal, hexanal, heptanal, octanal, nonanal | 2-hexenal, 2-heptenal, 2-nonenal |
| 2015 | Li [79] | 85 | I–IV | Tedlar bag | Si microreactor (ATM derivatization) | FT-ICR-MS | pentanal | MDA, 4-HHE, 4-HNE |
| 2015 | Ligor [80] | 123 | III–IV | Tedlar bag | CAR/PDMS | GC-MS | propanal | |
| 2015 | Schumer [81] | 156 | 0–IV | Tedlar bag | Si microreactor (ATM derivatization) | FT-ICR-MS | 4-HHE | |
| 2016 | Feinberg [82] | 22 | III–IV | QuinTron bag | aliquot f | PTR-MS | butanal, pentanal, hexanal | |
| 2016 | Schallschmidt [83] | 37 | NR | gas bulb and fleece tube | CAR/PDMS | GC-MS | propanal, butanal, pentanal, hexanal, heptanal, octanal, nonanal, decanal | |
| 2016 | Schumer [84] | 31 | 0–IV | Tedlar bag | Si microreactor (ATM derivatization) | FT-ICR-MS | 4-HHE | |
| 2016 | Shehada [85] | 149 | I–IV | Tedlar bag | Tenax TA | Si nanowire sensor | propanal, pentanal | |
| 2017 | Callol-Sanchez [86] | 81 | I–IV | Bio-VOC tube | Tenax TA/graphitized carbon black/carbonized mol. sieve | GC-MS | hexanal, heptanal, octanal, nonanal | |
| 2017 | Jouyban [87] | 7 | IV | 1 L glass sphere | breath condensate | GC-FID | hexanal, heptanal, octanal, decanal | 2-decenal |
| 2017 | Sakumura [88] | 107 | I–IV | analytical barrier bag | breath condensate | GC-MS | nonanal | |
| 2018 | Wang [89] | 233 g | NR | Tedlar bag | PDMS/Tenax TA | GC-MS | octanal, nonanal, decanal | |
| 2019 | Rudnicka [90] | 108 | I–IV | Tedlar bag | CAR/PDMS | GC-MS | propanal, pentanal, hexanal | |
| 2020 | Koureas [91] | 51 | NR | Tedlar bag | CAR/PDMS | GC-MS | hexanal, octanal, nonanal | |
| 2020 | Munoz-Lucas [92] | 107 | NR | Bio-VOC tube | Tenax TA/graphitized carbon black/carbonized mol. sieve | GC-MS | hexanal, heptanal, nonanal | |
| 2021 | Chen [93] | 160 | I–IV | Tedlar bag | Tenax TA | GC-MS | hexanal, heptanal | |
| 2021 | Gashimova [94] | 40 | I–IV | Tedlar bag | Tenax TA | e-nose sensor and GC-MS | butanal, pentanal, hexanal, heptanal, octanal, nonanal, decanal | |
| 2021 | Li [95] | 6 | NR | Tedlar bag | AgNP-coated chromatography paper | GC-MS | propanal, butanal, pentanal, hexanal, heptanal, octanal, nonanal, decanal | |
| 2021 | Long [96] | 116 | I–IV | Tedlar bag | DVB/CAR/PDMS | GC-MS | nonanal, decanal | |
| 2021 | Zou [97] | 60 | I–IV | Tedlar bar | Tenax TA | GC-MS | octanal | |
| 2022 | Larracy [98] | 100 | NR | — | Tenax TA | CRDS | hexanal | |
| 2022 | Soufi [99] | 5 | NR | Tedlar bag | POSS naphthalene diimine | GC-MS | pentanal, octanal, nonanal | |
| 2022 | Zou [100] | 60 | I–IV | Tedlar bag | Tenax TA | GC-MS | hexanal, octanal, nonanal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutaria, S.R.; Gori, S.S.; Morris, J.D.; Xie, Z.; Fu, X.-A.; Nantz, M.H. Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review. Metabolites 2022, 12, 561. https://doi.org/10.3390/metabo12060561
Sutaria SR, Gori SS, Morris JD, Xie Z, Fu X-A, Nantz MH. Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review. Metabolites. 2022; 12(6):561. https://doi.org/10.3390/metabo12060561
Chicago/Turabian StyleSutaria, Saurin R., Sadakatali S. Gori, James D. Morris, Zhenzhen Xie, Xiao-An Fu, and Michael H. Nantz. 2022. "Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review" Metabolites 12, no. 6: 561. https://doi.org/10.3390/metabo12060561
APA StyleSutaria, S. R., Gori, S. S., Morris, J. D., Xie, Z., Fu, X.-A., & Nantz, M. H. (2022). Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review. Metabolites, 12(6), 561. https://doi.org/10.3390/metabo12060561






