Metabolomic Study of Dactylis glomerata Growing on Aeolian Archipelago (Italy)
Abstract
1. Introduction
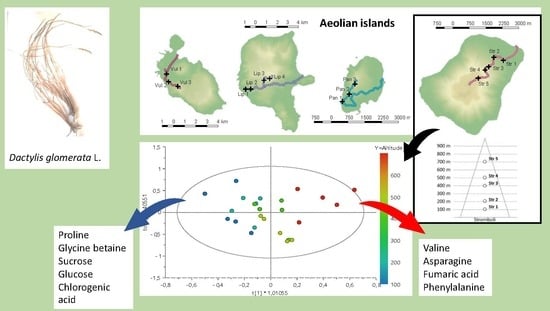
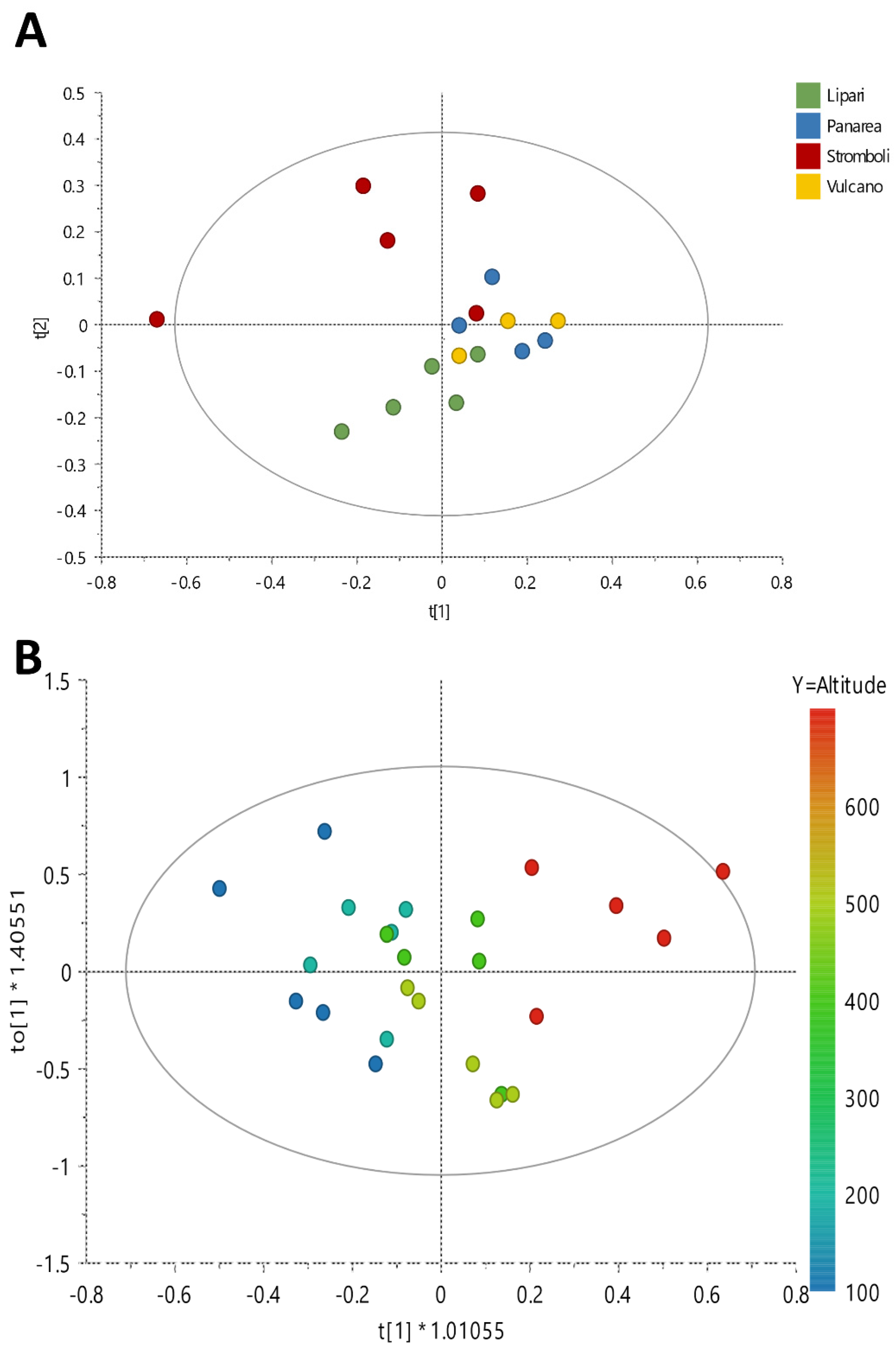
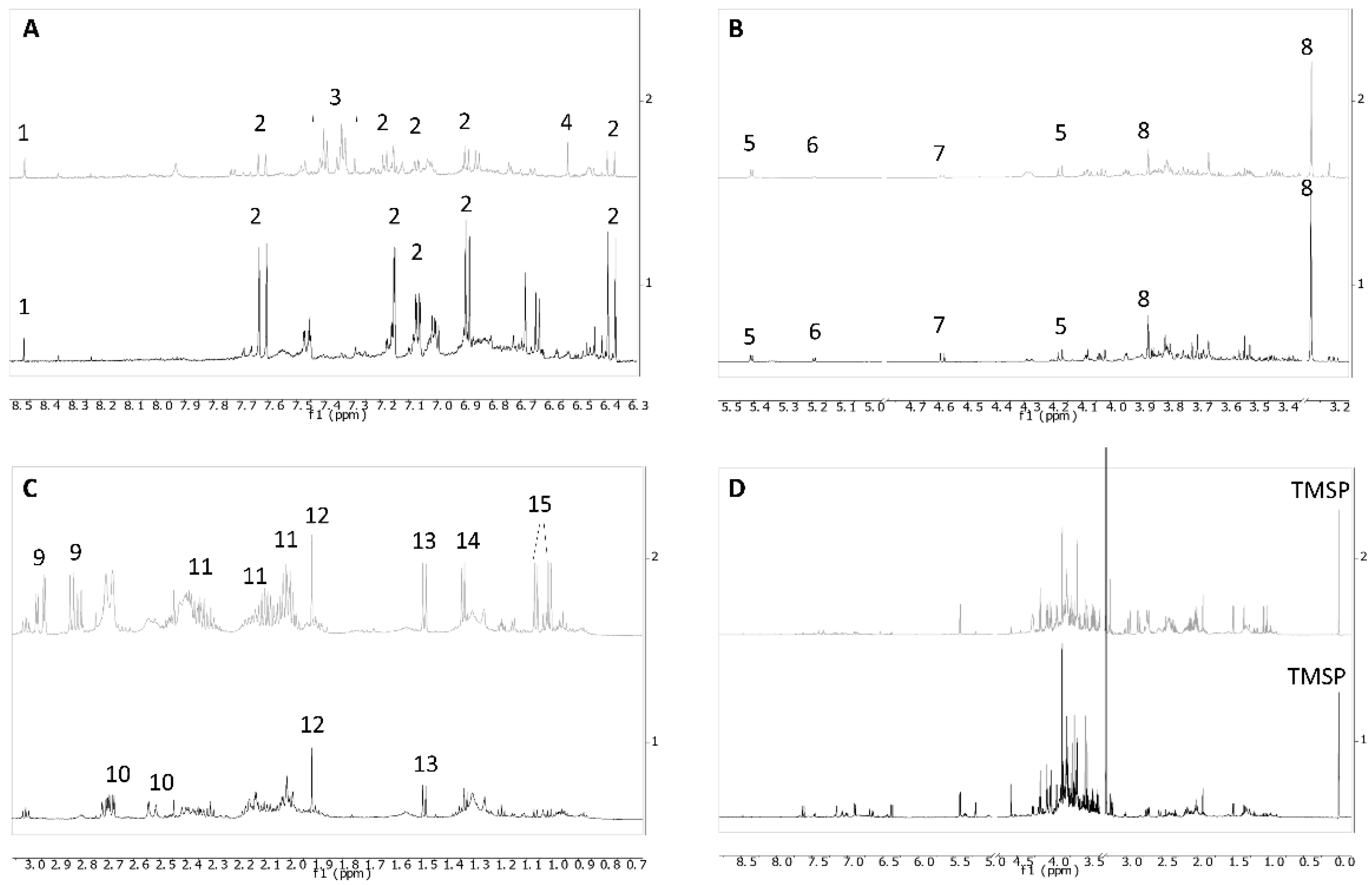
2. Results and Discussion
3. Materials and Methods
3.1. Plant Sampling and Pretreatment
3.2. Chemicals
3.3. Extract Preparation for NMR Analysis
3.4. Extract Preparation for Spectrophotometric Assays
3.5. NMR Spectra Measurement
3.6. NMR Processing and Multivariate Data Treatment
3.7. UHPLC−MS Analysis
3.8. Total Phenolic and Total Flavonoid Content
3.9. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, W.; Robins, J.G.; Bushman, B.S. A genetic linkage map of tetraploid orchardgrass (Dactylis glomerata L.) and quantitative trait loci for heading date. Genome 2012, 55, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Sanada, Y.; Tamura, K.; Yamada, T. Relationship between water-soluble carbohydrates in fall and spring and vigor of spring regrowth in orchard grass. Crop Sci. 2010, 50, 380–390. [Google Scholar] [CrossRef]
- Huang, L.K.; Yan, H.D.; Zhao, X.X.; Zhang, X.Q.; Wang, J.; Frazier, T.; Yin, G.; Huang, X.; Yan, D.F.; Zang, W.J.; et al. Identifying differentially expressed genes under heat stress and developing molecular markers in orchardgrass (Dactylis glomerata L.) through transcriptome analysis. Mol. Ecol. Resour. 2015, 15, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; Clark, S.G.; Reed, K.F.M.; Nie, Z.N.; Smith, K.F. Novel Festuca arundinacea Shreb. and Dactylis glomerata L. germplasm to improve adaptation for marginal environments. Aust. J. Exp. Agric. 2008, 48, 436–448. [Google Scholar] [CrossRef]
- Volaire, F.; Norton, M.R.; Norton, G.M.; Lelievre, F. Seasonal patterns of growth, dehydrins and water-soluble carbohydrates in genotypes of Dactylis glomerata varying in summer dormancy. Ann. Bot. 2005, 95, 981–990. [Google Scholar] [CrossRef][Green Version]
- Nie, Z.; Norton, M.R. Stress tolerance and persistence of perennial grasses: The role of the summer dormancy trait in temperate Australia. Crop Sci. 2009, 49, 2405–2411. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Liu, J.; Li, J.; Ding, C.; Qi, Z.; Shen, Q.; Guo, M. The effectiveness of selected vegetation communities in regulating runoff and soil loss from regraded gully banks in the Mollisol region of Northeast China. Land Degrad. Dev. 2021, 32, 2116–2129. [Google Scholar] [CrossRef]
- Li, S.; Nie, Z.; Zhang, D. Competition between cocksfoot (Dactylis glomerata L.) and companion species: Evidence for allelopathy. Field Crops Res. 2016, 196, 452–462. [Google Scholar] [CrossRef]
- Możdżeń, K.; Barabasz-Krasny, B.; Stachurska-Swakoń, A.; Zandi, P.; Puła, J.; Wang, Y.; Turisova, I. Allelopathic interaction between two common meadow plants: Dacytlis glomerata L. and Trifolium pratense L. Biologia 2020, 75, 653–663. [Google Scholar] [CrossRef]
- De las Heras, P.; Medina-Villar, S.; Pérez-Corona, M.E.; Vázquez-de-Aldana, B.R. Leaf litter age regulates the effect of native and exotic tree species on understory herbaceous vegetation of riparian forests. Basic Appl. Ecol. 2020, 48, 11–25. [Google Scholar] [CrossRef]
- Pruchniewicz, D.; Halarewicz, A. Allelopathic effects of wood small-reed (Calamagrostis epigejos) on germination and growth of selected grassland species. Pol. J. Ecol. 2019, 67, 122–136. [Google Scholar] [CrossRef]
- Parveen, I.; Winters, A.; Threadgill, M.D.; Hauck, B.; Morris, P. Extraction, structural characterisation and evaluation of hydroxycinnamate esters of orchard grass (Dactylis glomerata) as substrates for polyphenol oxidase. Phytochemistry 2008, 69, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Hauck, B.; Gallagher, J.A.; Morris, S.M.; Leemans, D.; Winters, A.L. Soluble phenolic compounds in fresh and ensiled orchard grass (Dactylis glomerata L.), a common species in permanent pastures with potential as a biomass feedstock. J. Agric. Food Chem. 2014, 62, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.; Lo Cascio, P. Contributi alla conoscenza botanica delle isole minori circumsiciliane. II. Note tassonomiche e geobotaniche sulla flora delle Isole Eolie. Nat. Sicil. 2002, 26, 131–145. [Google Scholar]
- Troia, A. Insular endemism in the Mediterranean vascular flora: The case of the Aeolian Islands (Sicily, Italy). Biodivers. J. 2012, 3, 369–374. [Google Scholar]
- Scossiroli, R.E.; Ronsisvalle, G.A. Origine e dispersione dei popolamenti animali e vegetali nel Mediterraneo centro-occidentale. Nat. Mont. 1991, 38, 45–52. [Google Scholar]
- Jahangir, M.; Kim, H.K.; Choi, Y.H. Metabolomic response of Brassica rapa submitted to pre-harvest bacterial contamination. Food Chem. 2008, 107, 362–368. [Google Scholar] [CrossRef]
- Goðevac, D.; Anðelković, B.; Vajs, V.; Tešević, V. The effects of altitude on the chemical composition of Populus type propolis. Planta Med. 2016, 82 (Suppl. 1), S1–S381. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef]
- Ali, K.; Iqbal, M.; Yuliana, N.D.; Lee, Y.J.; Park, S.; Han, S.; Lee, J.W.; Lee, H.S.; Verpoorte, R.; Choi, Y.H. Identification of bioactive metabolites against adenosine A1 receptor using NMR-based metabolomics. Metabolomics 2013, 9, 778–785. [Google Scholar] [CrossRef]
- Mandrone, M.; Coqueiro, A.; Poli, F.; Antognoni, F.; Choi, Y.H. Identification of a collagenase-inhibiting flavonoid from Alchemilla vulgaris using NMR-based metabolomics. Planta Med. 2018, 84, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Salomé-Abarca, L.F.; Mandrone, M.; Sanna, C.; Poli, F.; van der Hondel, C.A.; Klinkhamer, P.G.; Choi, Y.H. Metabolic variation in Cistus monspeliensis L. ecotypes correlated to their plant-fungal interactions. Phytochemistry 2020, 176, 112402. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Bruno, M.; Guarino, R.; Senatore, F.; Ilardi, V. Contribution to a taxonomic revision of the Sicilian taxa of Helichrysum by PCA analysis of their essential oil compositions. Chem. Biodivers. 2016, 13, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Shu, H.; Zhang, S.; Luo, B.; Gu, R.; Zhang, R.; Ji, Y.; Li, F.; Long, C. From Folk Taxonomy to Species Confirmation of Acorus (Acoraceae): Evidences Based on Phylogenetic and Metabolomic Analyses. Front. Plant Sci. 2020, 11, 965. [Google Scholar] [CrossRef]
- Callao, M.P.; Ruisánchez, I. An overview of multivariate qualitative methods for food fraud detection. Food Control 2018, 86, 283–293. [Google Scholar] [CrossRef]
- Sherman, E.; Coe, M.; Grose, C.; Martin, D.; Greenwood, D.R. A metabolomics approach to assessing the relative contributions of the volatile and non-volatile composition to expert quality ratings of pinot noir wine quality. J. Agric. Food Chem. 2020, 68, 13380–13396. [Google Scholar] [CrossRef]
- Mandrone, M.; Marincich, L.; Chiocchio, I.; Petroli, A.; Gođevac, D.; Maresca, I.; Poli, F. NMR-based metabolomics for frauds detection and quality control of oregano samples. Food Control 2021, 127, 108141. [Google Scholar] [CrossRef]
- Mandrone, M.; Chiocchio, I.; Barbanti, L.; Tomasi, P.; Tacchini, M.; Poli, F. Metabolomic Study of Sorghum (Sorghum bicolor) to Interpret Plant Behavior under Variable Field Conditions in View of Smart Agriculture Applications. J. Agric. Food Chem. 2021, 69, 1132–1145. [Google Scholar] [CrossRef]
- Mandrone, M.; Antognoni, F.; Aloisi, I.; Potente, G.; Poli, F.; Cai, G.; Faleri, C.; Parrotta, L.; Del Duca, S. Compatible and incompatible pollen-styles interaction in Pyrus communis L. show different transglutaminase features, polyamine pattern and metabolomics profiles. Front. Plant Sci. 2019, 10, 741. [Google Scholar] [CrossRef]
- Allard, P.M.; Genta-Jouve, G.; Wolfender, J.L. Deep metabolome annotation in natural products research: Towards a virtuous cycle in metabolite identification. Curr. Opin. Chem. Biol. 2017, 36, 40–49. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Gul, F.; Ahmad, P.; Akram, N.A. Environmental stresses and metabolomics—Deciphering the role of stress responsive metabolites. In Book Plant Metabolites and Regulation under Environmental Stress, 1st ed.; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 53–67. [Google Scholar] [CrossRef]
- Hanson, A.D.; Hitz, W.D. Metabolic responses of mesophytes to plant water deficits. Food Control 1982, 33, 163–203. [Google Scholar] [CrossRef]
- Ashraf, M.F.M.R.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Dutta, T.; Neelapu, N.R.R.; Wani, S.H.; Challa, S. Chapter 11—Compatible solute engineering of crop plants for improved tolerance toward abiotic stresses. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 221–254. [Google Scholar] [CrossRef]
- Nedjimi, B. Is salinity tolerance related to osmolytes accumulation in Lygeum spartum L. seedlings? J. Saudi Soc. Agric. Sci. 2011, 10, 81–87. [Google Scholar] [CrossRef]
- Cicala, A. Nozioni Informative di Meteorologia con Riferimenti Alle Isole Eolie; La Grafica Editoriale: Messina, Italy, 1997. [Google Scholar]
- Zaia, R.; Pasta, S.; Di Rita, F.; Laudicina, V.A.; Cascio, P.L.; Magri, D.; Troia, A.; Guarino, R. Staying alive on an active volcano: 80 years population dynamics of Cytisus aeolicus (Fabaceae) from Stromboli (Aeolian Islands, Italy). Ecol. Processes 2020, 9, 64. [Google Scholar] [CrossRef]
- Revil, A.; Finizola, A.; Ricci, T.; Delcher, E.; Peltier, A.; Barde-Cabusson, S.; Avard, G.; Bailly, T.; Bennati, L.; Byrdina, S. Hydrogeology of Stromboli volcano, Aeolian Islands (Italy) from the interpretation of resistivity tomograms, self-potential, soil temperature and soil CO2 concentration measurements. Geophys. J. Int. 2011, 186, 1078–1094. [Google Scholar] [CrossRef]
- Carapezza, M.L.; Ricci, T.; Ranaldi, M.; Tarchini, L. Active degassing structures of Stromboli and variations in diffuse CO2 output related to the volcanic activity. J. Volc. Geotherm. Res. 2009, 182, 231–245. [Google Scholar] [CrossRef]
- Brusca, L.; Inguaggiato, S.; Longo, M.; Madonia, P.; Maugeri, R. The 2002–2003 eruption of Stromboli (Italy): Evaluation of the volcanic activity by means of continuous monitoring of soil temperature, CO2 flux, and meteorological parameters. Geochem. Geophys. Geosyst. 2004, 5, Q12001. [Google Scholar] [CrossRef]
- Liotta, M.; Brusca, L.; Grassa, F.; Inguaggiato, S.; Longo, M.; Madonia, P. Geochemistry of rainfall at Stromboli volcano (Aeolian Islands): Isotopic composition and plume-rain interaction. Geochem. Geophys. Geosyst. 2006, 7, Q07006. [Google Scholar] [CrossRef]
- Zeng, J.; Quan, X.; He, X.; Cai, S.; Ye, Z.; Chena, G.; Zhang, G. Root and leaf metabolite profiles analysis reveals the adaptive strategies to low potassium stress in barley. BMC Plant Biol. 2018, 18, 187. [Google Scholar] [CrossRef]
- Ellam, R.M.; Hawkesworth, C.J.; Menzies, M.A.; Rogers, N.W. The volcanism of southern Italy: Role of subduction and the relationship between potassic and sodic alkaline magmatism. J. Geophys. Res. 1989, 94, 4589–4601. [Google Scholar] [CrossRef]
- Francalanci, L.; Manetti, P.; Peccerillo, A. Volcanological and magmatological evolution of Stromboli volcano (Aeolian Islands): The roles of fractional crystallization, magma mixing, crustal contamination, and source heterogeneity. Bull. Volcanol. 1989, 51, 355–378. [Google Scholar] [CrossRef]
- Hongo, A.; Oinuma, H. Effect of artificial treading on morphology and ethylene production in orchardgrass (Dactylis glomerata L.). Grassl. Sci. 1988, 44, 198–202. [Google Scholar] [CrossRef]
- Peiser, G.D.; Wang, T.T.; Hoffman, N.E.; Yang, S.F.; Liu, H.W.; Walsh, C.T. Formation of cyanide from carbon 1 of 1-aminocyclopropane-1-carboxylic acid during its conversion to ethylene. Proc. Natl. Acad. Sci. USA 1984, 81, 3059–3063. [Google Scholar] [CrossRef] [PubMed]
- Jenrich, R.; Trompetter, I.; Bak, S.; Olsen, C.E.; Møller, B.L.; Piotrowski, M. Evolution of heteromeric nitrilase complexes in Poaceae with new functions in nitrile metabolism. Proc. Natl. Acad. Sci. USA 2007, 104, 18848–18853. [Google Scholar] [CrossRef]
- Allard, P.; Aiuppa, A.; Loyer, H.; Carrot, F.; Gaudry, A.; Pinte, G.; Michel, A.; Dongarrà, G. Acid gas and metal emission rates during long-lived basalt degassing at Stromboli Volcano. Geophys. Res. Lett. 2000, 27, 1207–1210. [Google Scholar] [CrossRef]
- Zidorn, C. Altitudinal variation of secondary metabolites in flowering heads of the Asteraceae: Trends and causes. Phytochem. Rev. 2010, 9, 197–203. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Lonati, M.; Cioni, P.L.; Pistelli, L.; Najar, B.; Scariot, V. Latitude and altitude influence secondary metabolite production in peripheral alpine populations of the Mediterranean species Lavandula angustifolia Mill. Front. Plant Sci. 2018, 9, 983. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef]
- Mandrone, M.; Scognamiglio, M.; Fiorentino, A.; Sanna, C.; Cornioli, L.; Antognoni, F.; Bonvicini, F.; Poli, F. Phytochemical profile and α-glucosidase inhibitory activity of Sardinian Hypericum scruglii and Hypericum hircinum. Fitoterapia 2017, 120, 184–193. [Google Scholar] [CrossRef]
- Malzert-Fréon, A.; Hennequin, D.; Rault, S. Partial least squares analysis and mixture design for the study of the influence of composition variables on lipidic nanoparticle characteristics. J. Pharm. Sci. 2010, 99, 4603–4615. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Mandrone, M.; Lorenzi, B.; Venditti, A.; Guarcini, L.; Bianco, A.; Sanna, C.; Ballero, M.; Poli, F.; Antognoni, F. Antioxidant and anti-collagenase activity of Hypericum hircinum L. Ind. Crops Prod. 2015, 76, 402–408. [Google Scholar] [CrossRef]
- Carpaneto, G.M. Dati faunistici e considerazioni zoogeografiche sugli Scarabeoidei delle Isole Eolie (Coleoptera, Scarabaeoidea). Animalia 1985, 12, 87–99. [Google Scholar]

| Total Phenolic Content (µg GAE/mg DW) | Total Flavonoid Content (µg RE/mg DW) | Antioxidant Activity (µg TE/mg DW) | |
|---|---|---|---|
| 100 m a.s.l. | 7.25 ± 0.32 | 8.34 ± 0.18 | 17.05 ± 1.66 |
| 5.82 ± 0.1 | 6.13 ± 0.42 | 10.76 ± 0.72 | |
| 11.6 ± 0.4 | 15.8 ± 0.2 | 12.25 ± 0.25 | |
| 15.77 ± 0.34 | 17 ± 1.13 | 21.79 ± 2.77 | |
| 20.92 ± 0.14 | 27.48 ± 0.63 | 17.95 ± 0.68 | |
| 200 m a.s.l. | 15.66 ± 0.63 | 19.09 ± 0.43 | 28.64 ± 3.14 |
| 11.47 ± 0.23 | 12.37 ± 0.29 | 23.34 ± 1.55 | |
| 19.38 ± 0.4 | 33.72 ± 0.4 | 27.23 ± 0.64 | |
| 10.42 ± 0.03 | 14.11 ± 0.27 | 21.57 ± 1.3 | |
| 12.3 ± 0.09 | 17.39 ± 0.38 | 31.81 ± 0.41 | |
| 400 m a.s.l. | 7.98 ± 0.11 | 12.08 ± 0.27 | 16.12 ± 2.94 |
| 29.33 ± 1.27 | 15.09 ± 0.76 | 19.38 ± 1.7 | |
| 22.27 ± 0.56 | 10.26 ± 0.15 | 9.52 ± 1.14 | |
| 9.09 ± 0.19 | 10.95 ± 0.2 | 13.22 ± 1.03 | |
| 10.75 ± 0.12 | 15.95 ± 0.02 | 20.71 ± 1.68 | |
| 500 m a.s.l. | 9.57 ± 0.03 | 11.47 ± 0.05 | 7.21 ± 1.06 |
| 9.7 ± 0.19 | 13.03 ± 0.13 | 13.83 ± 0.09 | |
| 8.67 ± 0.18 | 12.4 ± 0.59 | 7.66 ± 1.16 | |
| 6.12 ± 0.95 | 6.82 ± 0.45 | 9.28 ± 0.21 | |
| 7.24 ± 0.63 | 9.16 ± 0.05 | 9.3 ± 0.55 | |
| 700 m a.s.l. | 1.61 ± 0.33 | 7.63 ± 0.05 | 8.31 ± 1.21 |
| 9.67 ± 1.61 | 8.46 ± 1.11 | 12.04 ± 2.76 | |
| 6.27 ± 1.4 | 7.62 ± 0.29 | 8.22 ± 0.2 | |
| 4.97 ± 0.29 | 7.25 ± 0.33 | 6.37 ± 0.63 | |
| 3.65 ± 0.85 | 3.76 ± 0 | 2.76 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandrone, M.; Marincich, L.; Chiocchio, I.; Zannini, P.; Guarino, R.; Poli, F. Metabolomic Study of Dactylis glomerata Growing on Aeolian Archipelago (Italy). Metabolites 2022, 12, 533. https://doi.org/10.3390/metabo12060533
Mandrone M, Marincich L, Chiocchio I, Zannini P, Guarino R, Poli F. Metabolomic Study of Dactylis glomerata Growing on Aeolian Archipelago (Italy). Metabolites. 2022; 12(6):533. https://doi.org/10.3390/metabo12060533
Chicago/Turabian StyleMandrone, Manuela, Lorenzo Marincich, Ilaria Chiocchio, Piero Zannini, Riccardo Guarino, and Ferruccio Poli. 2022. "Metabolomic Study of Dactylis glomerata Growing on Aeolian Archipelago (Italy)" Metabolites 12, no. 6: 533. https://doi.org/10.3390/metabo12060533
APA StyleMandrone, M., Marincich, L., Chiocchio, I., Zannini, P., Guarino, R., & Poli, F. (2022). Metabolomic Study of Dactylis glomerata Growing on Aeolian Archipelago (Italy). Metabolites, 12(6), 533. https://doi.org/10.3390/metabo12060533