Serum Metabolomics Based on GC-MS Reveals the Antipyretic Mechanism of Ellagic Acid in a Rat Model
Abstract
:1. Introduction
2. Results
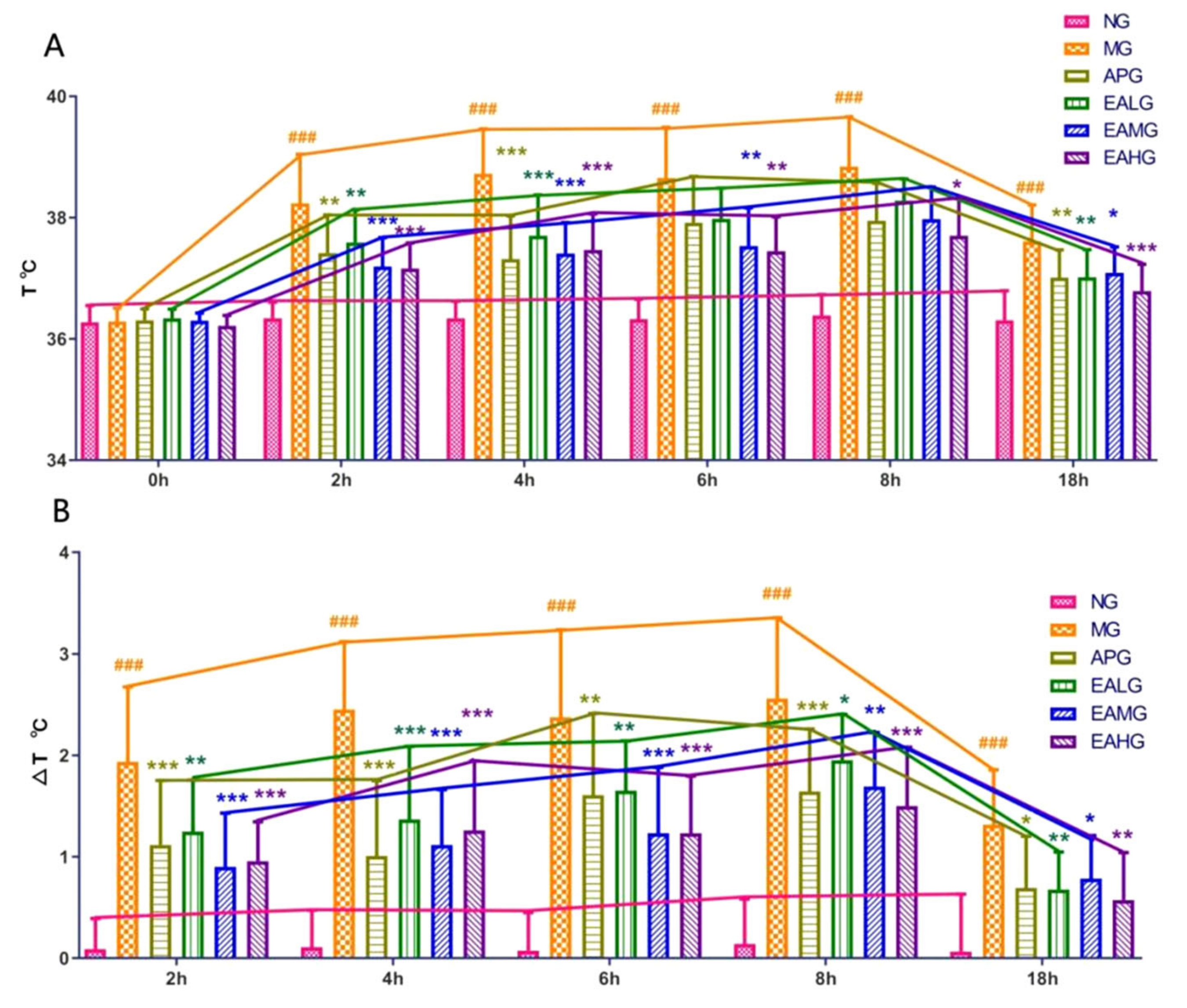
2.1. Antipyretic Effects of Ellagic Acid
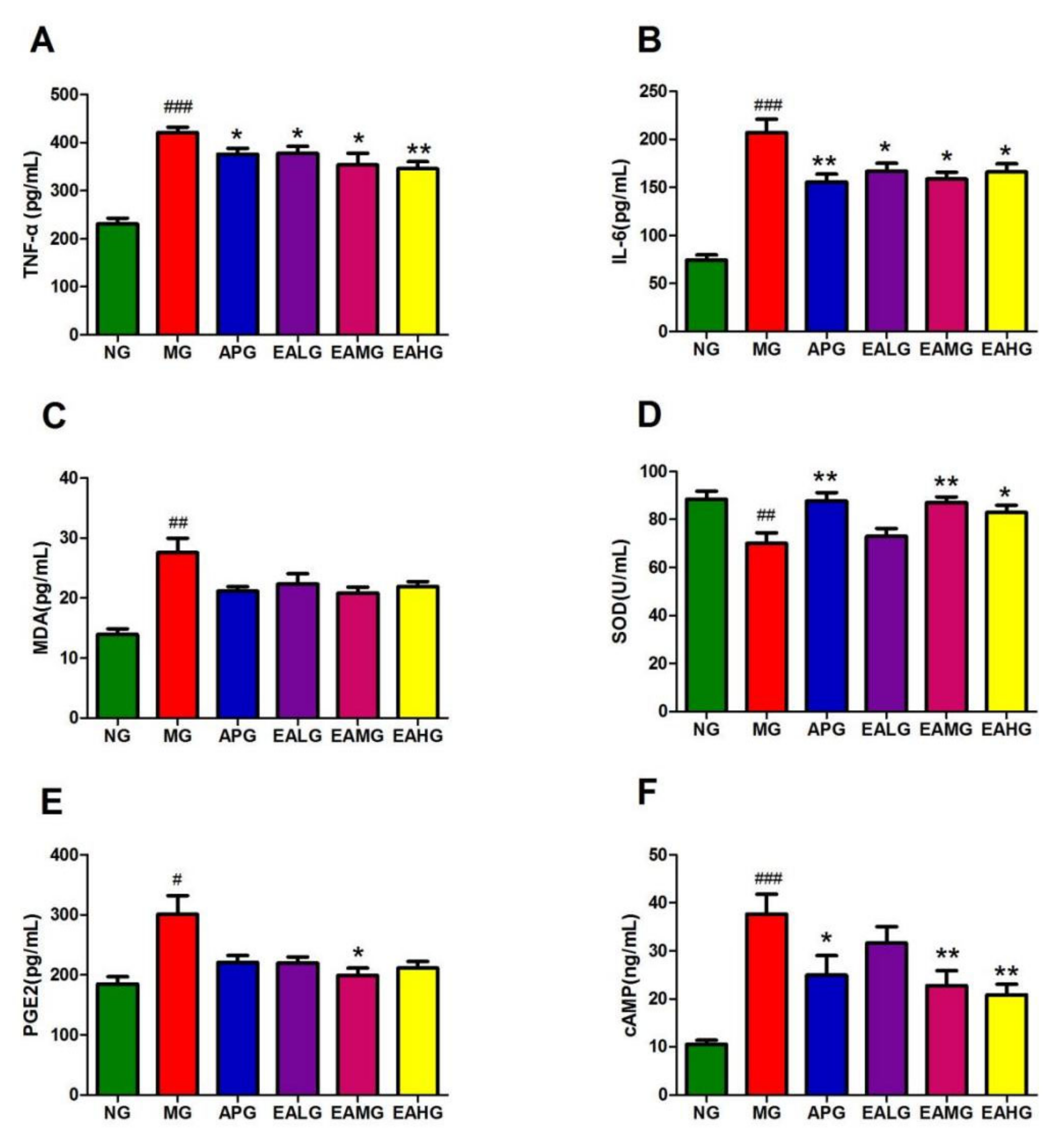
2.2. ELISA Results
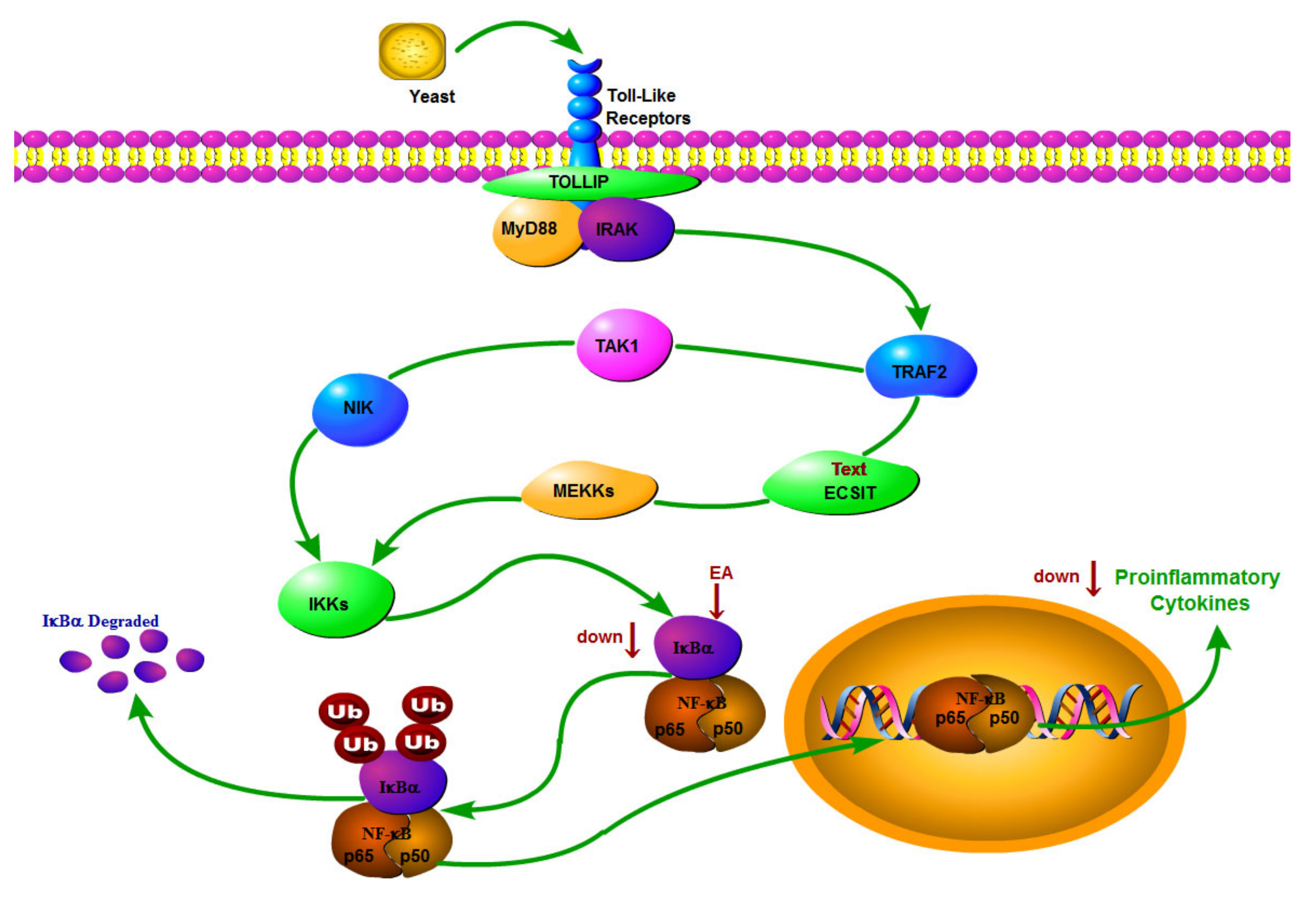
2.3. Expression of P-NF-κB P65 and IKB-α in Hypothalamus
2.4. Serum Metabolomics Profile and Multivariate Data Analysis
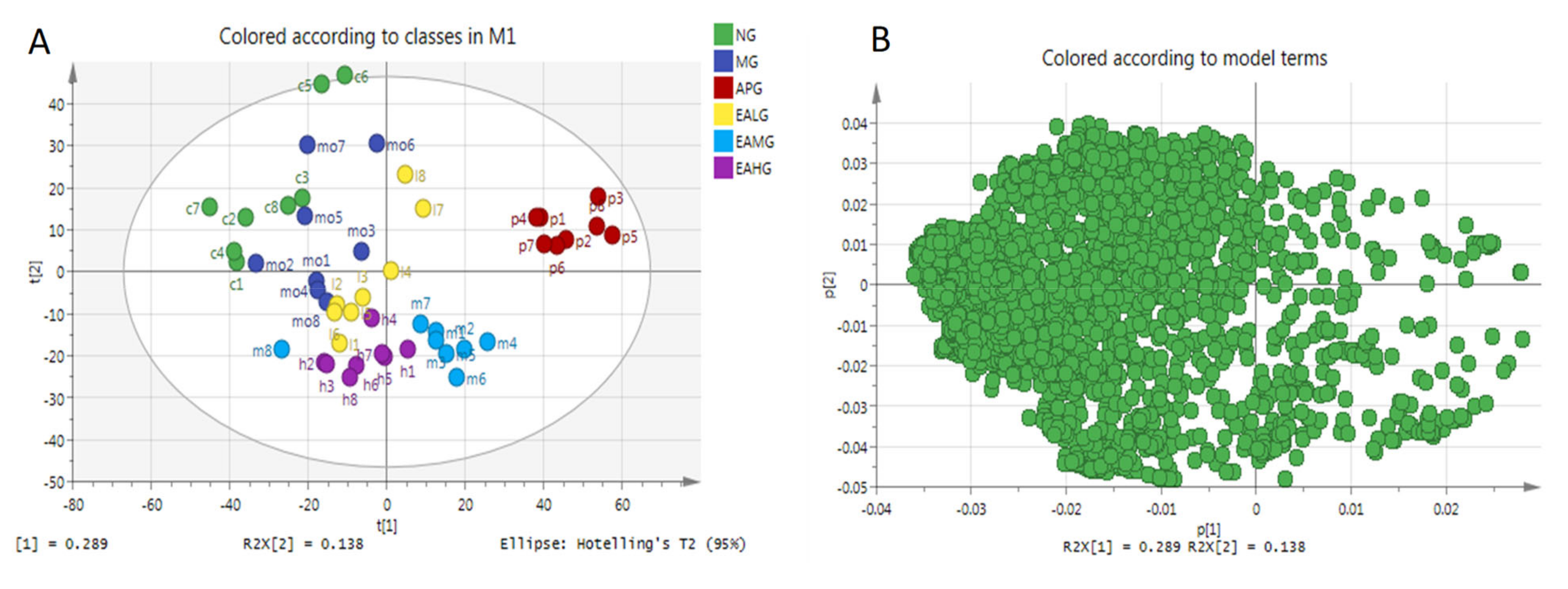
2.4.1. Principal Component Analysis
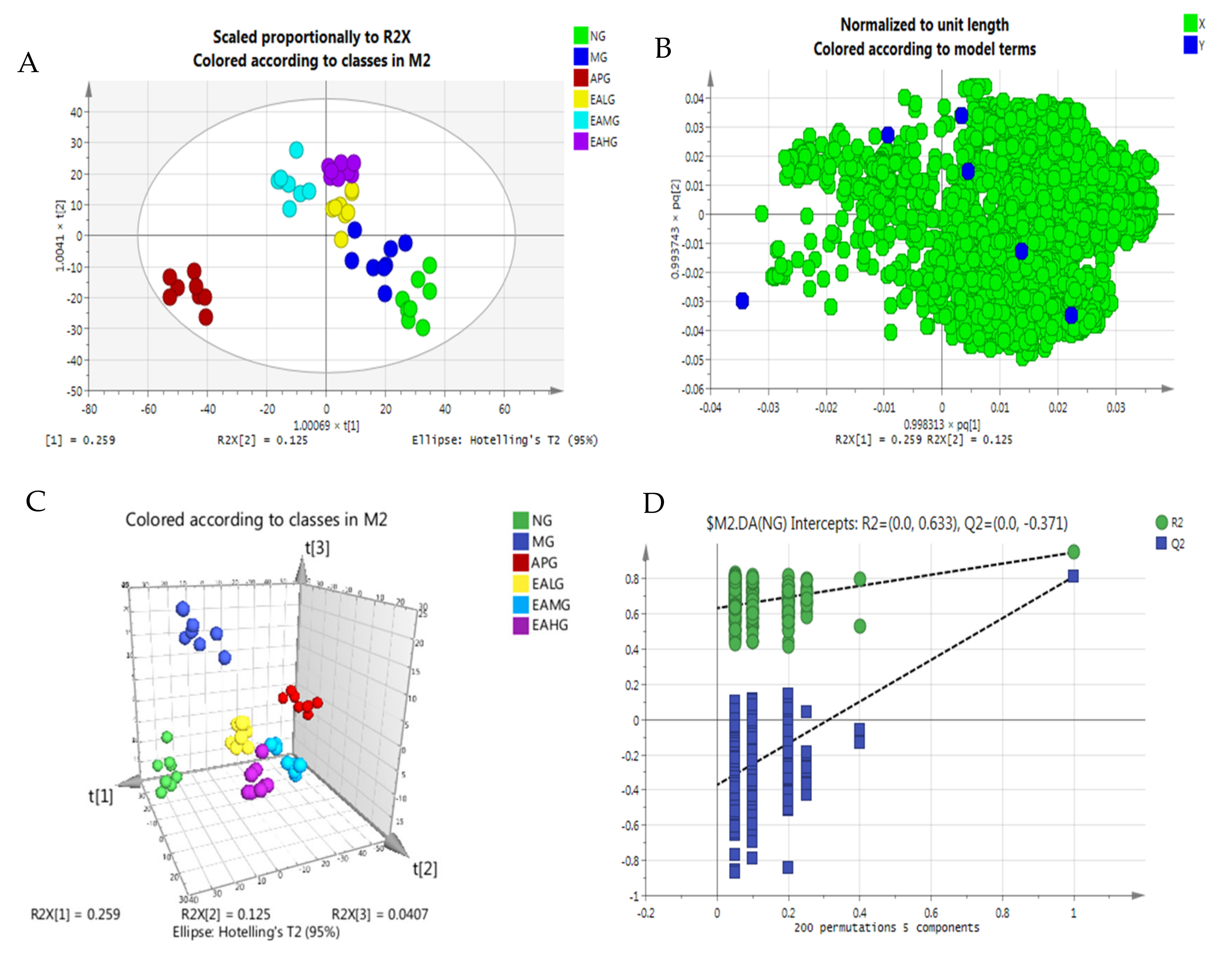
2.4.2. Orthogonal Partial Least Squares-Discriminant Analysis
2.4.3. Identification of Potential Biomarkers and the Changing Trends among Six Groups
2.4.4. Metabolic Pathway Analysis of the Potential Biomarkers
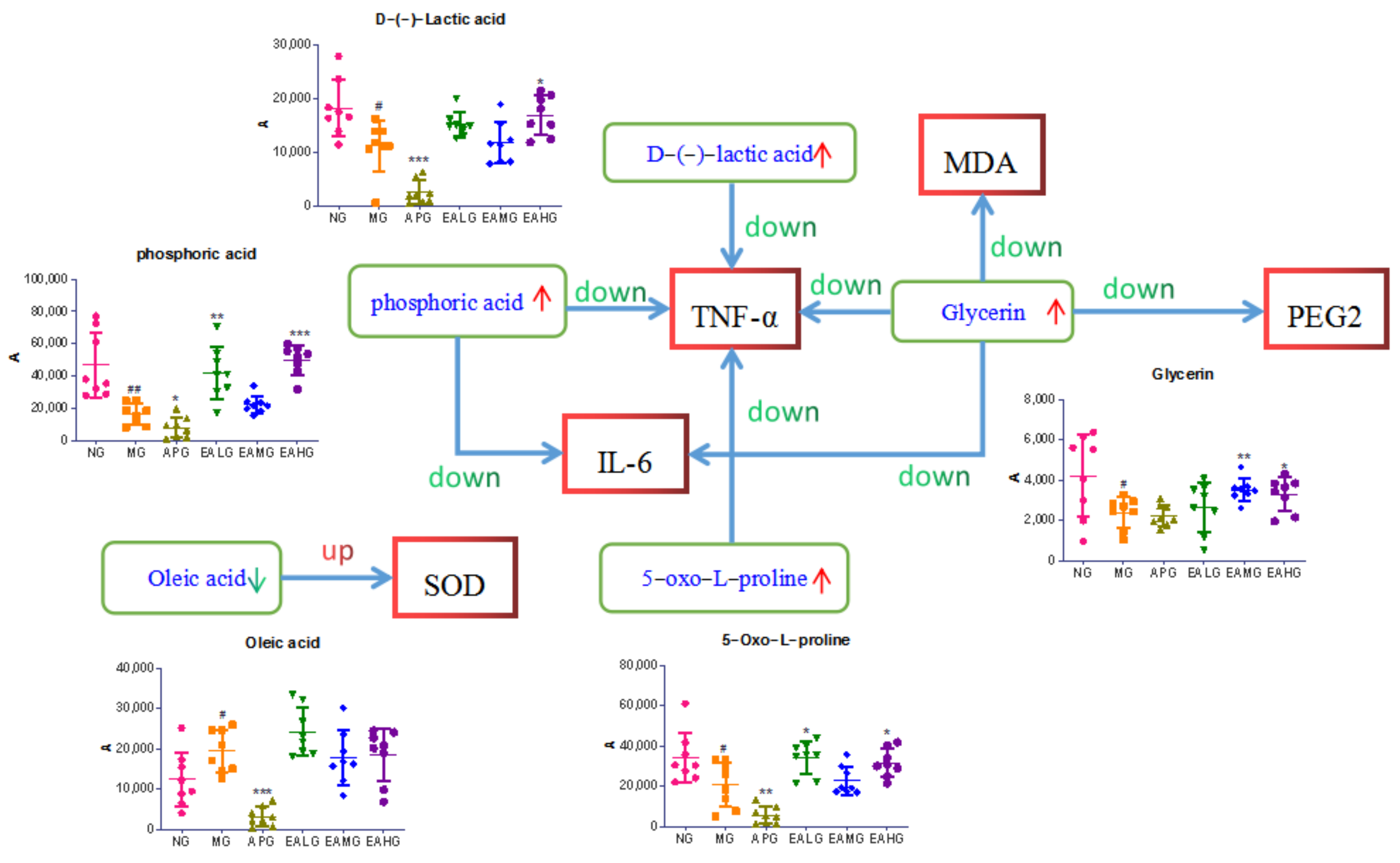
2.5. Correlation Analysis between Biomarkers and Pharmacodynamic Indicators
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Animals and Fever Model Processes
4.3. Samples Collection and Preparation
4.3.1. Serum Samples
4.3.2. Cerebrospinal Fluid
4.3.3. Hypothalamic Tissue Samples
4.4. Hypothalamic Western Blot
4.5. The Serum Sample Processing and GC-MS Analysis
4.5.1. The Serum Sample Processing
4.5.2. GC-MS Analysis Conditions
4.6. Data Processing and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lammert, E. Metabolism of Human Diseases; Organ Physiology and Pathophysiology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 313–317. [Google Scholar]
- Brito, H.O.; Barbosa, F.L.; Reis, R.C.; Fraga, D.; Borges, B.S.; Franco, C.R.; Zampronio, A.R. Evidence of substance P autocrine circuitry that involves TNFalpha, IL-6, and PGE2 in endogenous pyrogen-induced fever. J. Neuroimmunol. 2016, 293, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Cytokines as endogenous pyrogens. Infect. Dis. 1999, 179 (Suppl. S2), S294–S304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Qin, X.; Cao, Y.; Yang, Y.; Zhao, S. Effects of naloxone on the contents of cAMP in hypothalamus and AVP in ventral septal area in fever rats. J. Appl. Physiol. 2009, 25, 408–410. [Google Scholar]
- Hu, C.F.; Wang, H.D.; Wang, D.A.; Wang, Y.P.; Li, C.J. Studies on the mechanism of alpha-MSH in reducing IL-1 beta-induced fever. Acta Physiol. Sinica 1998, 50, 490–494. [Google Scholar]
- Wang, K.S.; Li, J.; Wang, Z.; Mi, C.; Ma, J.; Piao, L.X.; Xu, G.H.; Li, X.; Jin, X. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharm. Immunot. 2017, 39, 28–36. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Jin, A. Rapamycin Inhibits Nf-ΚB Activation by Autophagy to Reduce Catabolism in Human Chondrocytes. J. Invest. Surg. 2019, 33, 861–873. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D. NF-kappaB signaling pathway and its therapeutic implications in human diseases. Int. Rev. Immunol. 2008, 27, 293–319. [Google Scholar] [CrossRef]
- Mankan, A.K.; Lawless, M.W.; Gray, S.G.; Kelleher, D.; McManus, R. NF-kappaB regulation: The nuclear response. J. Cell. Mol. Med. 2009, 13, 631–643. [Google Scholar] [CrossRef]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kumar, M.N.V.R. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. 2006, 40, 206–210. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Kulkarni, V.H.; Chakraborty, M.; Habbu, P.V.; Ray, A. Ellagic acid restored lead-induced nephrotoxicity by anti-inflammatory, anti-apoptotic and free radical scavenging activities. Heliyon 2021, 7, e05921. [Google Scholar] [CrossRef]
- Garcia, G.; Pais, T.F.; Pinto, P.; Dobson, G.; Santos, C.N. Bioaccessible Raspberry Extracts Enriched in Ellagitannins and Ellagic Acid Derivatives Have Anti-Neuroinflammatory Properties. Antioxidants 2020, 9, 790. [Google Scholar] [CrossRef]
- Marín, M.; Giner, R.M.; Ríos, J.L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef]
- Akkoyun, H.T.; Karadeniz, A. Investigation of the protective effect of ellagic acid for preventing kidney injury in rats exposed to nicotine during the fetal period. Biotech. Histochem. 2016, 91, 108–115. [Google Scholar] [CrossRef]
- Tomita, M.; Kami, K. Cancer. Systems biology, metabolomics, and cancer metabolism. Science 2012, 336, 990–991. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Wilson, I.D. Understanding ‘global’ systems biology: Metabonomics and the continuum of metabolism. Nat. Rev. 2003, 2, 668–676. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Yan, G.; Wang, P.; Wang, X. Mass spectrometry-based metabolomics:applications to biomarker and metabolic pathway research. Biomed. Chromatogr. 2016, 30, 7–12. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, Z.; Guo, M.; Zhang, Q.; Wang, Q.; Lu, Z.; Zhao, H.; Liu, Y.; Fu, S.; Wang, M.; et al. Plasma metabolomics combined with lipidomics profiling reveals the potential antipyretic mechanisms of qingkailing injection in a rat model. Chem. Biol. Interact. 2016, 254, 24–33. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Q.; Zhao, H.; Zhou, X.; Sun, H.; Nan, Y.; Zou, S.; Wah Ma, C.; Wang, X. Phenotypic characterization of nanshi oral liquid alters metabolic signatures during disease prevention. Sci. Rep. 2016, 6, 19333. [Google Scholar] [CrossRef]
- Qian, W.; Shan, J.; Shen, C.; Yang, R.; Xie, T.; Di, L. Brain Metabolomics Reveal the Antipyretic Effects of Jinxin Oral Liquid in Young Rats by Using Gas Chromatography–Mass Spectrometry. Metabolites 2019, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Park, B.S.; Song, N.; Tu, T.H.; Lee, S.; Kim, J.K.; Kim, J.G. Metabolic profiling in the hypothalamus of aged mice. Biochem. Biophys. Res. Commun. 2022, 599, 134–141. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampronio, A.R.; Hoadley, M.E.; Luheshi, G.; Rothwell, N.J.; Souza, G.E.; Hopkins, S.J. Interleukin (IL)-6 release and fever induced by a pre-formed pyrogenic factor (PFPF) derived from LPS-stimulated macrophages. Eur. Cytokine Network 2000, 11, 589–596. [Google Scholar]
- Mitchell, J.P.; Ruaidhrí, J.; Carmody. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar] [PubMed]
- Bellezza, I.; Mierla, A.G.; Grottelli, S.; Marcotullio, M.C.; Messina, F.; Roscini, L.; Cardinali, G.; Curini, M.; Minelli, A. Furanodien-6-one from Commiphora erythraea inhibitsthe NF-κB signaling and attenuates LPS-induced neuroinflammation. Mol. Immunol. 2013, 54, 347–354. [Google Scholar] [CrossRef]
- Mc Guire, C.; Prinz, M.; Beyaert, R.; van Loo, G. Nuclear factor kappa B (NF-κB) in multiple sclerosis pathology. Trends Mol. Med. 2013, 19, 604–613. [Google Scholar] [CrossRef]
- Shao, D.Z.; Lee, J.J.; Huang, W.T.; Liao, J.F.; Lin, M.T. Inhibition of nuclear factor-kappa B prevents staphylococcal enterotoxin A-induced fever. Mol. Cell. Biochem. 2004, 262, 177–185. [Google Scholar] [CrossRef]
- Chen, X.; Yao, Z.; Peng, X.; Wu, L.; Wu, H.; Ou, Y.; Lai, J. Eupafolin alleviates cerebral ischemia/reperfusion injury in rats via blocking the TLR4/NFκB signaling pathway. Mol. Med. Rep. 2020, 22, 5135–5144. [Google Scholar] [CrossRef]
- Ates, T.; Gezercan, Y.; Menekse, G.; Turkoz, Y.; Parlakpinar, H.; Okten, A.I.; Akyuva, Y.; Onal, S.C. The Effects of Stereotactic Cerebroventricular Administration of Albumin, Mannitol, Hypertonic Sodium Chloride, Glycerin and Dextran in Rats with Experimental Brain Edema. Turk. Neurosurg. 2016, 27, 917–923. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nakamura, K.; Morrison, S.F. Different populations of prostaglandin EP3 receptor expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience 2009, 2, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Osaka, T. Heat loss responses and blockade of prostaglandin E2-induced thermogenesis elicited by α1-adrenergic activation in the rostromedial preoptic area. Neuroscience 2009, 162, 1420–1428. [Google Scholar] [CrossRef]


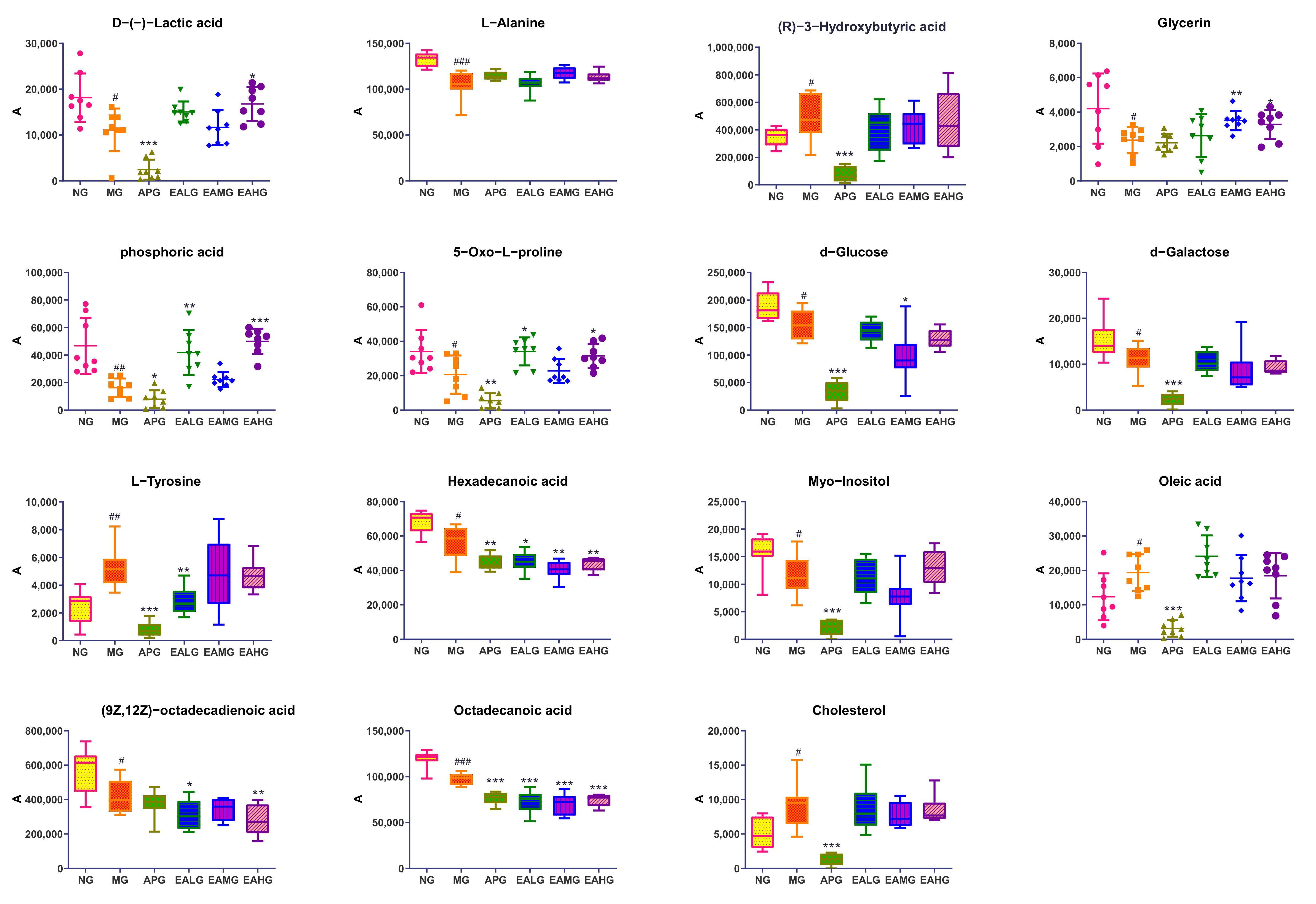

| NO. | HMDB | tR/min | Metabolite | Formula | m/z | KEGG | p | Match Score % | NG vs. MG | MG vs. APG | MG vs. EALG | MG vs. EAMG | MG vs. EAHG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HMDB0001311 | 5.909 | D-(−)-Lactic acid | C3H6O3 | 45, 29, 27 | C00256 | 0.013 | 92.64 | ↓ | ↓ | ↑ | ↑ | ↑ |
| 2 | HMDB0000161 | 6.457 | L-Alanine | C3H7NO2 | 44, 42, 28 | C00041 | 0.001 | 81.59 | ↓ | ↑ | ↑ | ↑ | ↑ |
| 3 | HMDB0000011 | 7.447 | (R)-3-Hydroxybutyric acid | C4H8O3 | 45, 43, 60 | C01089 | 0.042 | 92.19 | ↑ | ↓ | ↓ | ↓ | ↓ |
| 4 | HMDB0000131 | 8.993 | Glycerin | C3H8O3 | 61, 43, 44 | C00116 | 0.042 | 91.77 | ↓ | ↓ | ↑ | ↑ | ↑ |
| 5 | HMDB0002142 | 10.318 | phosphoric acid | H3O4P | 299, 73, 314 | C00009 | 0.003 | 90.64 | ↓ | ↓ | ↑ | ↑ | ↑ |
| 6 | HMDB0000267 | 14.767 | 5-Oxo-L-proline | C5H7NO3 | 84, 28, 41 | C01879 | 0.040 | 85.41 | ↓ | ↓ | ↑ | ↑ | ↑ |
| 7 | HMDB0000122 | 17.901 | d-Glucose | C6H13NO6 | 75, 71, 43 | C00221 | 0.030 | 92.37 | ↓ | ↓ | ↓ | ↓ | ↓ |
| 8 | HMDB0000143 | 18.162 | d-Galactose | C6H13NO6 | 75, 71, 43 | C00984 | 0.041 | 89.42 | ↓ | ↓ | ↓ | ↓ | ↓ |
| 9 | HMDB0000158 | 19.198 | L-Tyrosine | C9H11NO2 | 107, 77, 91 | C00082 | 0.044 | 81.41 | ↑ | ↓ | ↓ | ↓ | ↓ |
| 10 | HMDB0000220 | 19.809 | Hexadecanoic acid | C16H32O2 | 43, 73, 60 | C00249 | 0.015 | 92.26 | ↓ | ↓ | ↓ | ↓ | ↓ |
| 11 | HMDB0000211 | 20.221 | Myo-Inositol | C6H12O6 | 73, 60, 102 | C00137 | 0.001 | 90.22 | ↓ | ↑ | ↓ | ↓ | ↑ |
| 12 | HMDB0000207 | 21.760 | Oleic acid | C18H34O2 | 41, 55, 43 | C00712 | 0.043 | 91.83 | ↑ | ↓ | ↓ | ↓ | ↓ |
| 13 | HMDB0000673 | 21.848 | (9Z, 12Z)-octadecadienoic acid | C18H32O2 | 67, 81, 95 | C01595 | 0.020 | 81.35 | ↓ | ↓ | ↑ | ↓ | ↓ |
| 14 | HMDB0000827 | 21.948 | Octadecanoic acid | C18H36O2 | 43, 73, 60 | C01530 | 0.000 | 92.59 | ↓ | ↓ | ↓ | ↓ | ↓ |
| 15 | HMDB0000067 | 31.801 | Cholesterol | C27H46O | 43, 55, 386 | C00187 | 0.011 | 84.56 | ↑ | ↓ | ↓ | ↓ | ↓ |
| Metabolite | The Correlation Coefficient: r | |||||
|---|---|---|---|---|---|---|
| TNF-α | IL-6 | MDA | SOD | PGE2 | cAMP | |
| D-(−)-Lactic acid | −0.430 ** | −0.146 | −0.177 | −0.143 | −0.043 | −0.114 |
| L-Alanine | −0.430 ** | −0.607 ** | −0.490 ** | +0.035 | −0.234 | −0.421 ** |
| (R)-3-Hydroxybutyric acid | −0.081 | +0.122 | +0.145 | −0.262 | +0.290 * | +0.133 |
| Glycerin | −0.389 ** | −0.348 * | −0.427 ** | +0.141 | −0.312 * | −0.213 |
| phosphoric acid | −0.494 ** | −0.285 * | −0.222 | −0.013 | −0.104 | −0.269 |
| 5-Oxo-L-proline | −0.431 ** | −0.181 | −0.118 | −0.088 | +0.086 | −0.129 |
| d-Glucose | −0.241 | −0.251 | −0.027 | −0.212 | +0.046 | −0.073 |
| d-Galactose | −0.162 | −0.328 * | −0.084 | −0.078 | −0.079 | −0.145 |
| L-Tyrosine | +0.124 | +0.277 | +0.216 | −0.181 | +0.220 | +0.159 |
| Hexadecanoic acid | −0.289 * | −0.391 ** | −0.282 | −0.037 | +0.072 | −0.197 |
| Myo-Inositol | −0.396 ** | −0.157 | −0.182 | −0.092 | +0.102 | −0.019 |
| Oleic acid | +0.156 | +0.197 | +0.201 | −0.321 * | +0.240 | +0.189 |
| (9Z, 12Z)-octadecadienoic acid | −0.243 | −0.411 ** | −0.192 | +0.085 | +0.004 | −0.258 |
| Octadecanoic acid | −0.331 * | −0.377 ** | −0.22 | −0.009 | +0.006 | −0.131 |
| Cholesterol | +0.097 | +0.340 * | +0.209 | −0.298 * | +0.255 | +0.196 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, F.; Xu, L.; Zhu, H.; Li, Y.; Nong, L.; Chen, Y.; Zeng, Y.; Cen, S. Serum Metabolomics Based on GC-MS Reveals the Antipyretic Mechanism of Ellagic Acid in a Rat Model. Metabolites 2022, 12, 479. https://doi.org/10.3390/metabo12060479
Xie F, Xu L, Zhu H, Li Y, Nong L, Chen Y, Zeng Y, Cen S. Serum Metabolomics Based on GC-MS Reveals the Antipyretic Mechanism of Ellagic Acid in a Rat Model. Metabolites. 2022; 12(6):479. https://doi.org/10.3390/metabo12060479
Chicago/Turabian StyleXie, Fengfeng, Liba Xu, Hua Zhu, Yinlan Li, Lizhen Nong, Yaling Chen, Yanfang Zeng, and Sijie Cen. 2022. "Serum Metabolomics Based on GC-MS Reveals the Antipyretic Mechanism of Ellagic Acid in a Rat Model" Metabolites 12, no. 6: 479. https://doi.org/10.3390/metabo12060479
APA StyleXie, F., Xu, L., Zhu, H., Li, Y., Nong, L., Chen, Y., Zeng, Y., & Cen, S. (2022). Serum Metabolomics Based on GC-MS Reveals the Antipyretic Mechanism of Ellagic Acid in a Rat Model. Metabolites, 12(6), 479. https://doi.org/10.3390/metabo12060479





