The Radioprotective Activity of Resveratrol—Metabolomic Point of View
Abstract
1. The Cytoprotective Activity of Resveratrol
2. Resveratrol Is a Radioprotective Compound
3. Metabolomics and Radiation-Induced Changes in Cellular Metabolism
4. Resveratrol-Induced Changes in Cellular Metabolism
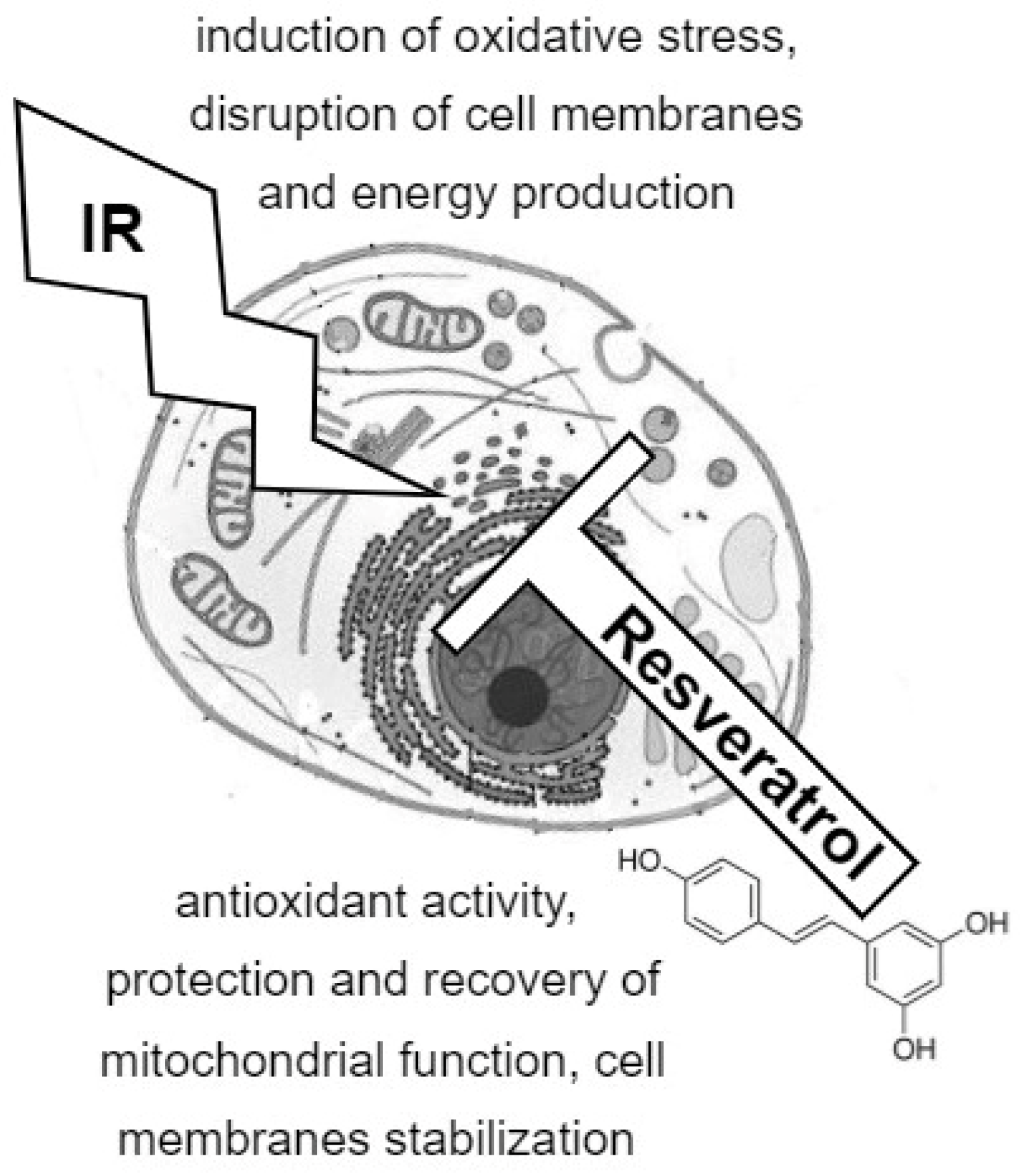
5. The Combined Effects of Ionizing Radiation and Resveratrol on Cellular Metabolism
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, P.-Y. Cardioprotection by Phytochemicals via Antiplatelet Effects and Metabolism Modulations. Cell Biochem. Biophys. 2015, 73, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Robb, E.L.; Stuart, J.A. Trans-Resveratrol as A Neuroprotectant. Molecules 2010, 15, 1196–1212. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Kopec, A.; Piatkowska, E.; Leszczynska, T.; Biezanowska-Kopec, R. Prozdrowotne wlasciwosci resweratrolu. Zywnosc Nauka Technol. Jakosc 2011, 5, 5–15. [Google Scholar]
- Leifert, W.R.; Abeywardena, M.Y. Cardioprotective Actions of Grape Polyphenols. Nutr. Res. 2008, 28, 729–737. [Google Scholar] [CrossRef]
- Rezk, Y.A.; Balulad, S.S.; Keller, R.S.; Bennett, J.A. Use of Resveratrol to Improve the Effectiveness of Cisplatin and Doxorubicin: Study in Human Gynecologic Cancer Cell Lines and in Rodent Heart. Am. J. Obstet. Gynecol. 2006, 194, e23–e26. [Google Scholar] [CrossRef]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef]
- Baarine, M.; Thandapilly, S.J.; Louis, X.L.; Mazué, F.; Yu, L.; Delmas, D.; Netticadan, T.; Lizard, G.; Latruffe, N. Pro-Apoptotic versus Anti-Apoptotic Properties of Dietary Resveratrol on Tumoral and Normal Cardiac Cells. Genes Nutr. 2011, 6, 161–169. [Google Scholar] [CrossRef]
- Movahed, A.; Yu, L.; Thandapilly, S.J.; Louis, X.L.; Netticadan, T. Resveratrol Protects Adult Cardiomyocytes against Oxidative Stress Mediated Cell Injury. Arch. Biochem. Biophys. 2012, 527, 74–80. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M.; Gajowik, A.; Radzikowska, J. The Effect of In Vivo Resveratrol Supplementation in Irradiated Mice on the Induction of Micronuclei in Peripheral Blood and Bone Marrow Reticulocytes. Mutagenesis 2016, 31, 393–399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Csiszar, A. Anti-Inflammatory Effects of Resveratrol: Possible Role in Prevention of Age-Related Cardiovascular Disease: Anti-Inflammatory Effects of Resveratrol in Aging. Ann. N. Y. Acad. Sci. 2011, 1215, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Massimi, M.; Tomassini, A.; Sciubba, F.; Sobolev, A.P.; Devirgiliis, L.C.; Miccheli, A. Effects of Resveratrol on HepG2 Cells as Revealed by 1H-NMR Based Metabolic Profiling. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK Signalling Pathway Coordinates Cell Growth, Autophagy and Metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, W.; Tao, J.; Xin, P.; Liu, M.; Li, J.; Wei, M. Resveratrol Protects Cardiomyocytes from Oxidative Stress through SIRT1 and Mitochondrial Biogenesis Signaling Pathways. Biochem. Biophys. Res. Commun. 2013, 438, 270–276. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, H.; Zhou, X.; Wang, H.; Yang, Y.; Zhang, J.; Wang, H. The Protective Effects of Resveratrol against Radiation-Induced Intestinal Injury. BMC Complement. Altern. Med. 2017, 17, 410. [Google Scholar] [CrossRef]
- Petrovski, G.; Gurusamy, N.; Das, D.K. Resveratrol in Cardiovascular Health and Disease: Resveratrol in Heart Health. Ann. N. Y. Acad. Sci. 2011, 1215, 22–33. [Google Scholar] [CrossRef]
- Das, M.; Das, D.K. Resveratrol and Cardiovascular Health. Mol. Aspects Med. 2010, 31, 503–512. [Google Scholar] [CrossRef]
- Carsten, R.E.; Bachand, A.M.; Bailey, S.M.; Ullrich, R.L. Resveratrol Reduces Radiation-Induced Chromosome Aberration Frequencies in Mouse Bone Marrow Cells. Radiat. Res. 2008, 169, 633–638. [Google Scholar] [CrossRef]
- Stone, J.; Mitrofanis, J.; Johnstone, D.M.; Falsini, B.; Bisti, S.; Adam, P.; Nuevo, A.B.; George-Weinstein, M.; Mason, R.; Eells, J. Acquired Resilience: An Evolved System of Tissue Protection in Mammals. Dose-Response 2018, 16, 1559325818803428. [Google Scholar] [CrossRef]
- Sebastià, N.; Almonacid, M.; Villaescusa, J.I.; Cervera, J.; Such, E.; Silla, M.A.; Soriano, J.M.; Montoro, A. Radioprotective Activity and Cytogenetic Effect of Resveratrol in Human Lymphocytes: An In Vitro Evaluation. Food Chem. Toxicol. 2013, 51, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Basso, E.; Regazzo, G.; Fiore, M.; Palma, V.; Traversi, G.; Testa, A.; Degrassi, F.; Cozzi, R. Resveratrol Affects DNA Damage Induced by Ionizing Radiation in Human Lymphocytes in Vitro. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 806, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Przybyszewski, W.M.; Wideł, M.; Rzeszowska-Wolny, J. Kardiotoksyczne następstwa promieniowania jonizującego i antracyklin. Postepy Hig. Med. Dosw 2006, 60, 397–405. [Google Scholar]
- De Freitas, R.; Boligon, A.; Rovani, B.; Piana, M.; de Brum, T.; da Silva Jesus, R.; Rother, F.; Alves, N.; Teixeira da Rocha, J.; Athayde, M.; et al. Effect of Black Grape Juice against Heart Damage from Acute Gamma TBI in Rats. Molecules 2013, 18, 12154–12167. [Google Scholar] [CrossRef]
- Foray, N.; Bourguignon, M.; Hamada, N. Individual Response to Ionizing Radiation. Mutat. Res. Rev. Mutat. Res. 2016, 770, 369–386. [Google Scholar] [CrossRef]
- Berthel, E.; Foray, N.; Ferlazzo, M.L. The Nucleoshuttling of the ATM Protein: A Unified Model to Describe the Individual Response to High- and Low-Dose of Radiation? Cancers 2019, 11, 905. [Google Scholar] [CrossRef]
- Little, M.P.; Azizova, T.V.; Bazyka, D.; Bouffler, S.D.; Cardis, E.; Chekin, S.; Chumak, V.V.; Cucinotta, F.A.; de Vathaire, F.; Hall, P.; et al. Systematic Review and Meta-Analysis of Circulatory Disease from Exposure to Low-Level Ionizing Radiation and Estimates of Potential Population Mortality Risks. Environ. Health Perspect. 2012, 120, 1503–1511. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- Monceau, V.; Meziani, L.; Strup-Perrot, C.; Morel, E.; Schmidt, M.; Haagen, J.; Escoubet, B.; Dörr, W.; Vozenin, M.-C. Enhanced Sensitivity to Low Dose Irradiation of ApoE−/−Mice Mediated by Early Pro-Inflammatory Profile and Delayed Activation of the TGFβ1 Cascade Involved in Fibrogenesis. PLoS ONE 2013, 8, e57052. [Google Scholar] [CrossRef]
- Roy, J.; Galano, J.; Durand, T.; Le Guennec, J.; Chung-Yung Lee, J. Physiological Role of Reactive Oxygen Species as Promoters of Natural Defenses. FASEB J. 2017, 31, 3729–3745. [Google Scholar] [CrossRef]
- Gramatyka, M.; Sokół, M. Radiation Metabolomics in the Quest of Cardiotoxicity Biomarkers: The Review. Int. J. Radiat. Biol. 2020, 96, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Wang, Y.; Li, C.; Li, D.; Lu, L.; Wang, X.; et al. Resveratrol Ameliorates Ionizing Irradiation-Induced Long-Term Hematopoietic Stem Cell Injury in Mice. Free Radic. Biol. Med. 2013, 54, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Koohian, F.; Shanei, A.; Shahbazi-Gahrouei, D.; Hejazi, S.H.; Moradi, M.-T. The Radioprotective Effect of Resveratrol Against Genotoxicity Induced by γ-Irradiation in Mice Blood Lymphocytes. Dose-Response 2017, 15, 1559325817705699. [Google Scholar] [CrossRef] [PubMed]
- Denissova, N.G.; Nasello, C.M.; Yeung, P.L.; Tischfield, J.A.; Brenneman, M.A. Resveratrol Protects Mouse Embryonic Stem Cells from Ionizing Radiation by Accelerating Recovery from DNA Strand Breakage. Carcinogenesis 2012, 33, 149–155. [Google Scholar] [CrossRef]
- Simsek, Y.; Gurocak, S.; Turkoz, Y.; Akpolat, N.; Celik, O.; Ozer, A.; Yılmaz, E.; Turhan, U.; Ozyalin, F. Ameliorative Effects of Resveratrol on Acute Ovarian Toxicity Induced by Total Body Irradiation in Young Adult Rats. J. Pediatr. Adolesc. Gynecol. 2012, 25, 262–266. [Google Scholar] [CrossRef]
- Najafi, M.; Cheki, M.; Amini, P.; Javad, A.; Shabeeb, D.; Eleojo Musa, A. Evaluating the Protective Effect of Resveratrol, Q10, and Alpha-Lipoic Acid on Radiation-Induced Mice Spermatogenesis Injury: A Histopathological Study. Int. J. Reprod. Biomed. IJRM 2019, 17, 907–914. [Google Scholar] [CrossRef]
- Prager, I.; Patties, I.; Himmelbach, K.; Kendzia, E.; Merz, F.; Müller, K.; Kortmann, R.-D.; Glasow, A. Dose-Dependent Short- and Long-Term Effects of Ionizing Irradiation on Neural Stem Cells in Murine Hippocampal Tissue Cultures: Neuroprotective Potential of Resveratrol. Brain Behav. 2016, 6, e00548. [Google Scholar] [CrossRef]
- Little, M.P.; Lipshultz, S.E. Low Dose Radiation and Circulatory Diseases: A Brief Narrative Review. Cardio-Oncol. 2015, 1, 4. [Google Scholar] [CrossRef]
- Barba, I.; Garcia-Dorado, D. Metabolomics in Cardiovascular Disease: Towards Clinical Application. In Coronary Artery Disease-New Insights and Novel Approaches; Squeri, A., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0344-8. [Google Scholar]
- Xiao, X.; Hu, M.; Zhang, X.; Hu, J.Z. NMR-Based Metabolomics Analysis of Liver from C57BL/6 Mouse Exposed to Ionizing Radiation. Radiat. Res. 2017, 188, 44–55. [Google Scholar] [CrossRef]
- Jang, W.G.; Park, J.Y.; Lee, J.; Bang, E.; Kim, S.R.; Lee, E.K.; Yun, H.J.; Kang, C.-M.; Hwang, G.-S. Investigation of Relative Metabolic Changes in the Organs and Plasma of Rats Exposed to X-ray Radiation Using HR-MAS (1)H NMR and Solution (1)H NMR: Metabolic Changes in an Irradiated Rat Model. NMR Biomed. 2016, 29, 507–518. [Google Scholar] [CrossRef]
- Gramatyka, M.; Skorupa, A.; Sokół, M. Nuclear Magnetic Resonance Spectroscopy Reveals Metabolic Changes in Living Cardiomyocytes after Low Doses of Ionizing Radiation. Acta Biochim. Pol. 2018, 65, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Gramatyka, M.; Boguszewicz, Ł.; Ciszek, M.; Gabryś, D.; Kulik, R.; Sokół, M. Metabolic Changes in Mice Cardiac Tissue after Low-Dose Irradiation Revealed by 1H NMR Spectroscopy. J. Radiat. Res. 2020, 61, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Gramatyka, M.; Widłak, P.; Gabryś, D.; Kulik, R.; Sokół, M. Resveratrol Administration Prevents Radiation-Related Changes in Metabolic Profiles of Hearts 20 Weeks after Irradiation of Mice with a Single 2 Gy Dose. Acta Biochim. Pol. 2020, 67, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Sersa, I.; Kranjc, S.; Sersa, G.; Nemec-Svete, A.; Lozar, B.; Sepe, A.; Vidmar, J.; Sentjurc, M. Study of Radiation Induced Changes of Phosphorus Metabolism in Mice by 31P NMR Spectroscopy. Radiol. Oncol. 2010, 44, 174–179. [Google Scholar] [CrossRef]
- Chen, C.; Brenner, D.J.; Brown, T.R. Identification of Urinary Biomarkers from X-Irradiated Mice Using NMR Spectroscopy. Radiat. Res. 2011, 175, 622–630. [Google Scholar] [CrossRef]
- Kwon, Y.-K.; Ha, I.J.; Bae, H.-W.; Jang, W.G.; Yun, H.J.; Kim, S.R.; Lee, E.K.; Kang, C.-M.; Hwang, G.-S. Dose-Dependent Metabolic Alterations in Human Cells Exposed to Gamma Irradiation. PLoS ONE 2014, 9, e113573. [Google Scholar] [CrossRef]
- Tsuyama, N.; Mizuno, H.; Katafuchi, A.; Abe, Y.; Kurosu, Y.; Yoshida, M.; Kamiya, K.; Sakai, A. Identification of Low-Dose Responsive Metabolites in X-Irradiated Human B Lymphoblastoid Cells and Fibroblasts. J. Radiat. Res. 2015, 56, 46–58. [Google Scholar] [CrossRef]
- Goudarzi, M.; Mak, T.D.; Chen, C.; Smilenov, L.B.; Brenner, D.J.; Fornace, A.J. The Effect of Low Dose Rate on Metabolomic Response to Radiation in Mice. Radiat. Environ. Biophys. 2014, 53, 645–657. [Google Scholar] [CrossRef][Green Version]
- Cheema, A.K.; Pathak, R.; Zandkarimi, F.; Kaur, P.; Alkhalil, L.; Singh, R.; Zhong, X.; Ghosh, S.; Aykin-Burns, N.; Hauer-Jensen, M. Liver Metabolomics Reveals Increased Oxidative Stress and Fibrogenic Potential in Gfrp Transgenic Mice in Response to Ionizing Radiation. J. Proteome Res. 2014, 13, 3065–3074. [Google Scholar] [CrossRef]
- Cheema, A.K.; Suman, S.; Kaur, P.; Singh, R.; Fornace, A.J.; Datta, K. Long-Term Differential Changes in Mouse Intestinal Metabolomics after γ and Heavy Ion Radiation Exposure. PLoS ONE 2014, 9, e87079. [Google Scholar] [CrossRef]
- Golla, S.; Golla, J.P.; Krausz, K.W.; Manna, S.K.; Simillion, C.; Beyoğlu, D.; Idle, J.R.; Gonzalez, F.J. Metabolomic Analysis of Mice Exposed to Gamma Radiation Reveals a Systemic Understanding of Total-Body Exposure. Radiat. Res. 2017, 187, 612–629. [Google Scholar] [CrossRef] [PubMed]
- Pannkuk, E.; Laiakis, E.; Girgis, M.; Dowd, S.; Dhungana, S.; Nishita, D.; Bujold, K.; Bakke, J.; Gahagen, J.; Authier, S.; et al. Temporal Effects on Radiation Responses in Nonhuman Primates: Identification of Biofluid Small Molecule Signatures by Gas Chromatography–Mass Spectrometry Metabolomics. Metabolites 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-R.; Tsai, Y.-F.; Lau, Y.-T.; Yu, H.-P. Plasma Metabolite Profiles Following Trauma-Hemorrhage: Effect of Posttreatment With Resveratrol. Shock 2015, 43, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Torres Santiago, G.; Serrano Contreras, J.I.; Meléndez Camargo, M.E.; Zepeda Vallejo, L.G. NMR-Based Metabonomic Approach Reveals Changes in the Urinary and Fecal Metabolome Caused by Resveratrol. J. Pharm. Biomed. Anal. 2019, 162, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ye, G.; Zhang, X.; Liu, X.; Tu, Y.; Ye, Z.; Liu, J.; Guo, Q.; Wang, Z.; Wang, L.; et al. Metabolomics Reveals Protection of Resveratrol in Diet-Induced Metabolic Risk Factors in Abdominal Muscle. Cell. Physiol. Biochem. 2018, 45, 1136–1148. [Google Scholar] [CrossRef]
- Korsholm, A.; Kjær, T.; Ornstrup, M.; Pedersen, S. Comprehensive Metabolomic Analysis in Blood, Urine, Fat, and Muscle in Men with Metabolic Syndrome: A Randomized, Placebo-Controlled Clinical Trial on the Effects of Resveratrol after Four Months’ Treatment. Int. J. Mol. Sci. 2017, 18, 554. [Google Scholar] [CrossRef]
- Morvan, D.; Demidem, A. NMR Metabolomics of Fibroblasts with Inherited Mitochondrial Complex I Mutation Reveals Treatment-Reversible Lipid and Amino Acid Metabolism Alterations. Metabolomics 2018, 14, 55. [Google Scholar] [CrossRef]
- Jäger, W.; Gruber, A.; Giessrigl, B.; Krupitza, G.; Szekeres, T.; Sonntag, D. Metabolomic Analysis of Resveratrol-Induced Effects in the Human Breast Cancer Cell Lines MCF-7 and MDA-MB-231. OMICS J. Integr. Biol. 2011, 15, 9–14. [Google Scholar] [CrossRef]

| Research Model | Resveratrol Dose | Radiation Dose | The Observed Effect of Resveratrol | Reference |
|---|---|---|---|---|
| bone marrow cells from CBA/CaJ irradiated mice | 100 mg/kg/day from 2 days before the irradiation until the end of the experiment | 3 Gy, γ radiation | 2.8-fold reduction of total chromosome aberrations, including gaps, dicentrics, and Robertsonian translocations for the resveratrol + radiation group compared to the radiation group | [19] |
| peripheral blood cells and bone marrow cells from irradiated C57BL/6-Ly-5.1 mice | 20 mg/kg/day from 7 days before to 30 days after irradiation | 6.0 Gy or 7.2 Gy, 137 Cs irradiator, TBI | increased survival after TBI, decreased acute and long-term bone marrow damage, reduced oxidative stress after exposure to 7.2 Gy in the resveratrol group | [32] |
| blood and bone marrow from irradiated Swiss mice | 7 mg/kg/day or 28 mg/kg/day for 2 weeks | 5 Gy and 10 Gy total doses in 0.5 Gy and 1 Gy fractions, X-radiation, TBI | reduction in the number of micronuclei in reticulocytes in the resveratrol + radiation group when compared to the radiation group | [11] |
| peripheral blood lymphocyte from irradiated NMRI mice | 50 mg/kg or 100 mg/kg 2 h before irradiation | 2 Gy, γ radiation | reduction of radiation-induced DNA damage (assessed by comet assay) in the resveratrol group | [33] |
| human peripheralblood lymphocytes | 2.2, 22 or 220 µM 1 h before irradiation | 2 Gy γ radiation | reduction in chromosome aberrations after irradiation with maximal protection observed for 2.2 µM dose; however, resveratrol induced chromosomal aberrations in the absence of irradiation | [21] |
| human peripheral blood lymphocytes | 20 µM or 40 µM 3 h before irradiation | 0.5 Gy or 1 Gy, X-radiation | in the 40 µM resveratrol group increased level of dicentric chromosomes induced by radiation; resveratrol alone did not induce DNA or chromosome damage | [22] |
| mouse embryonic stem cells | 10 µM 48 h before irradiation | 5 Gy, X-radiation | improvement of the viability of irradiated cells and acceleration of DNA damage repair | [34] |
| ovaries from irradiated Wistar rats | 10 mg/kg or 100 mg/kg 24 h before irradiation | 720 cGy, photon, TBI | increased follicle count in ovaries after irradiation and increase of antioxidant enzymes activity in the resveratrol group | [35] |
| testes from irradiated NMRI mice | 100 mg/kg/day for two days before irradiation | 2 Gy, γ radiation, TBI | reduction of spermatogenic arrest, thickening of the basal lamina, decreased sperm density and vacuolation in the resveratrol group; resveratrol increased atrophy of seminiferous tubules | [36] |
| small intestines of irradiated C57BL/6 N mice | 40 mg/kg/day1 day before and 5 days after irradiation | 7 Gy, 137 Cs irradiator, partial-body irradiation | normalization of the intestinal cell morphology in irradiated mice (enhanced regeneration of intestinal crypt cells, increased villi length, shorter basal lamina length) | [16] |
| organotypic entorhinal–hippocampal slice cultures generated from nestin-CFPnuc C57BL/J6 mice | 15 µM 2 h before irradiation until 48 h after irradiation | 4.5, 8, 12, or 16 Gy, X-radiation | increased number of nestin-positive neural progenitor cells in the resveratrol + radiation group when compared to the radiation group | [37] |
| Experimental Model | Radiation Dose and Experimental Design | Affected Metabolic Pathway | Reference |
|---|---|---|---|
| murine liver | 3 Gy and 7.8 Gy, proton and gamma, 4 and 11 days | GSH metabolism; Ala/Asp/Glu metabolism; Gly/Ser/Thr metabolism; TCA cycle; Glycerophospholipid metabolism; Pyruvate metabolism | [40] |
| rat jejunum, spleen, liver and plasma | 2 Gy and 6 Gy X-ray, 1, 2, and 3 days | Gln/Glu metabolism; Phe/Tyr/Trp biosynthesis; Taurine and hypotaurine metabolism; Ala/Asp/Glu metabolism; GSH metabolism; Phe metabolism; Gly/Ser/Thr metabolism; Glyoxylate and dicarboxylate metabolism; Arg biosynthesis; TCA cycle; Arg/Pro metabolism; Glycerophospholipid metabolism; Primary bile acid biosynthesis | [41] |
| cardiomyocytes | 2 Gy, photons, 2 days | Taurine and hypotaurine metabolism; Gln/Glu metabolism; GSH metabolism; Gly/Ser/Thr metabolism; Ala/Asp/Glu metabolism; Glyoxylate and dicarboxylate metabolism; Arg biosynthesis; Glycerophospholipid metabolism | [42] |
| murine hearts | 2 Gy, photons, 2 days, 20 weeks | Gln/Glu metabolism; Phe/Tyr/Trp biosynthesis; Ala/Asp/Glu metabolism; Gly/Ser/Thr metabolism; Glyoxylate and dicarboxylate metabolism; Arg biosynthesis; GSH metabolism; Inositol phosphate metabolism; Tyr metabolism | [43] |
| murine hearts | 2 Gy, photons, 20 weeks | Glycerophospholipid metabolism; Lipids metabolism | [44] |
| whole mice, 31P NMR MRI | 7 Gy, X-ray, 0–14 days | Arg/Pro metabolism; Gly/Ser/Thr metabolism | [45] |
| murine urine | 8 Gy, X-ray, 7 days | Taurine and hypotaurine metabolism; TCA cycle; Ala/Asp/Glu metabolism; Butanoate metabolism; Gly/Ser/Thr metabolism; Gln/Glu metabolism; Phe metabolism; Arg biosynthesis; Propanoate metabolism; Glyoxylate and dicarboxylate metabolism; Glycerophospholipid metabolism; Arg/Pro metabolism; Primary bile acid biosynthesis | [46] |
| fibroblasts | 1 Gy and 5 Gy, gamma, 1, 2, and 3 days | Lipids metabolism; Phe/Tyr/Trp biosynthesis; Phe metabolism; GSH metabolism; Arg/Pro metabolism; Glycerophospholipid metabolism; Arg biosynthesis; Aminoacyl-tRNA biosynthesis; Ubiquinone and another terpenoid-quinone biosynthesis; Pantothenate and CoA biosynthesis; Ether lipid metabolism; Gly/Ser/Thr metabolism; Cys/Met metabolism; Trp metabolism; Tyr metabolism | [47] |
| fibroblasts, B lymphoblastoid cells | 0.02 Gy, 0.1 Gy, and 1 Gy, X-ray, 1 and 10 h. | Purine metabolism; Cys/Met metabolism | [48] |
| murine urine | 1.1 Gy and 4.4 Gy, X-ray, 2 days | intermediates in the Trp metabolism and Ile catabolism; TCA cycle; Pyruvate metabolism; Glycolysis/Gluconeogenesis; Ala/Asp/Glu metabolism; Glyoxylate and dicarboxylate metabolism; Cys/Met metabolism | [49] |
| murine liver | 8.5 Gy, gamma, 1 and 4 days | Lipids metabolism; GSH metabolism; Porphyrin and chlorophyll metabolism; Pyrimidine metabolism; Glycerophospholipid metabolism; Primary bile acid biosynthesis; Purine metabolism | [50] |
| murine intestines | 2 Gy or 1.6 Gy, gamma or heavy-ion, 2 months | Phe/Tyr/Trp biosynthesis; Phe metabolism; Ala/Asp/Glu metabolism; beta-Ala metabolism; His metabolism; Pyruvate metabolism; GSH metabolism; Trp metabolism; Pyrimidine metabolism; Glycolysis/Gluconeogenesis; Pantothenate and CoA biosynthesis; TCA cycle | [51] |
| bone marrow, ileum, liver, muscle, lung, serum, urine of mice | 6 Gy, gamma, 12 h | Gln/Glu metabolism; Taurine and hypotaurine metabolism; Phe/Tyr/Trp biosynthesis; GSH metabolism; Ala/Asp/Glu metabolism; Phe metabolism; Purine metabolism; Arg biosynthesis; Pyrimidine metabolism; Arg/Pro metabolism; Glycerophospholipid metabolism; Amino sugar and nucleotide sugar metabolism; Primary bile acid biosynthesis; Pentose and glucuronate interconversions | [52] |
| urine and serum of rhesus monkeys | 4 Gy, gamma, up to 60 days | Phe/Tyr/Trp biosynthesis; TCA cycle; Phe metabolism; Glyoxylate and dicarboxylate metabolism | [53] |
| Experimental Model | Resveratrol Dose and Experimental Design | Affected Metabolic Pathway | Reference |
|---|---|---|---|
| plasma from Sprague –Dawley rats subjected to trauma-hemorrhagic shock | 30 mg/kg administered 30 min after hemorrhage | Synthesis and degradation of ketone bodies; His metabolism; Butanoate metabolism; TCA cycle; Glyoxylate and dicarboxylate metabolism; Lys degradation; Aminoacyl-tRNA biosynthesis; Biotin metabolism; beta-Ala metabolism; Pyruvate metabolism; Ala/Asp/Glu metabolism; Val/Leu/Ile degradation; Glycolysis/Gluconeogenesis; Tyr metabolism | [54] |
| urine and feces of Wistar rats | 50 mg/kg or 250 mg/kg after 12 h of food deprivation | Taurine and hypotaurine metabolism; Pyruvate metabolism; TCA cycle; Gly/Ser/Thr metabolism; Ala/Asp/Glu metabolism; Glycolysis/Gluconeogenesis | [55] |
| abdominal muscle tissue from ApoE-/- mice fed with a high fat diet | 10 mg/kg/day for 24 weeks | Pentose phosphate pathway; pentose and glucuronate interconversions; galactose metabolism; fructose and mannose metabolism; Ala/Asp/Glu metabolism; Glyoxylate and dicarboxylate metabolism | [56] |
| blood, urine, adipose tissue, and skeletal muscle from men with metabolic syndrome | 150 mg/day or 1 g/day for 4 months | Linoleic acid metabolism; Ubiquinone and another terpenoid-quinone biosynthesis; His metabolism, Tyr metabolism; Trp metabolism; Biosynthesis of unsaturated fatty acids; Phe/Tyr/Trp biosynthesis | [57] |
| fibroblasts with mitochondrial Complex 1 disorder | 50 µM for 24 h | Lipids transformations; Gln/Glu metabolism; Taurine and hypotaurine metabolism; Ala/Asp/Glu metabolism; Gly/Ser/Thr metabolism; Glyoxylate and dicarboxylate metabolism; Arg biosynthesis; GSH metabolism; Inositol phosphate metabolism; Arg/Pro metabolism; Aminoacyl-tRNA biosynthesis; Val/Leu/Ile biosynthesis; Val/Leu/Ile degradation; Nitrogen metabolism | [58] |
| MCF-7 and MDA-MB-231 breast cancer cells | 100 µM for 72 h | Phe metabolism; Arg biosynthesis; Ala/Asp/Glu metabolism; Gln/Glu metabolism; Arg/Pro metabolism; Taurine and hypotaurine metabolism; Phe metabolism; Trp metabolism; Arachidonic acid metabolism; His metabolism; Gly/Ser/Thr metabolism; GSH metabolism; Glyoxylate and dicarboxylate metabolism; Cys/Met metabolism; Tyr metabolism; Aminoacyl-tRNA biosynthesis; Val/Leu/Ile biosynthesis; beta-Ala metabolism; Lipids transformations | [59] |
| hearts from irradiated C57Bl/6NCrl mice | 5 mg/kg/day or 25 mg/kg/day from 4 weeks before until 2 weeks after irradiation | Lipids transformations; Gly/Ser/Thr metabolism; Taurine and hypotaurine metabolism; Glyoxylate and dicarboxylate metabolism; Glycerophospholipid metabolism; GSH metabolism; Primary bile acid biosynthesis | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gramatyka, M. The Radioprotective Activity of Resveratrol—Metabolomic Point of View. Metabolites 2022, 12, 478. https://doi.org/10.3390/metabo12060478
Gramatyka M. The Radioprotective Activity of Resveratrol—Metabolomic Point of View. Metabolites. 2022; 12(6):478. https://doi.org/10.3390/metabo12060478
Chicago/Turabian StyleGramatyka, Michalina. 2022. "The Radioprotective Activity of Resveratrol—Metabolomic Point of View" Metabolites 12, no. 6: 478. https://doi.org/10.3390/metabo12060478
APA StyleGramatyka, M. (2022). The Radioprotective Activity of Resveratrol—Metabolomic Point of View. Metabolites, 12(6), 478. https://doi.org/10.3390/metabo12060478






