Metabolomics Study Suggests the Mechanism of Different Types of Tieguanyin (Oolong) Tea in Alleviating Alzheimer’s Disease in APP/PS1 Transgenic Mice
Abstract
:1. Introduction
2. Results
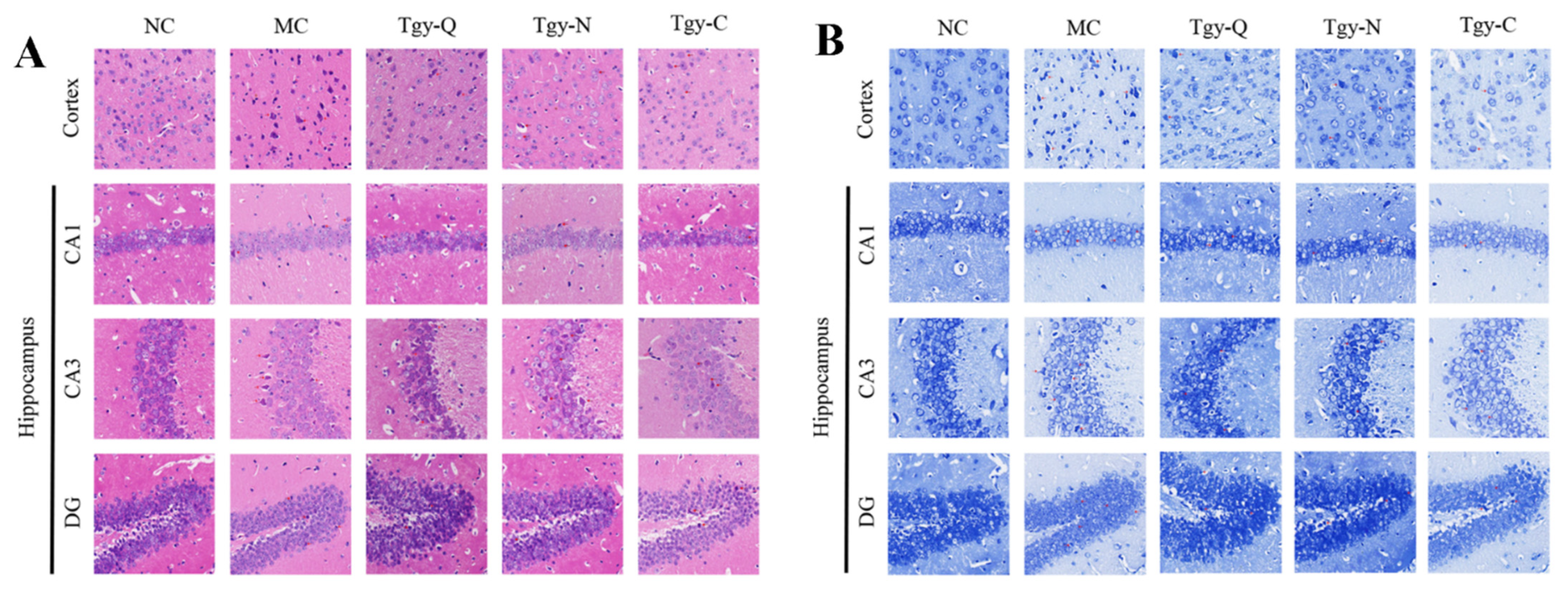
2.1. Tieguanyin Treatment Improved the Brain Pathological Conditions of AD Model Mouse
2.2. Quality Control for UHPLC-Q-TOF/MS Performance
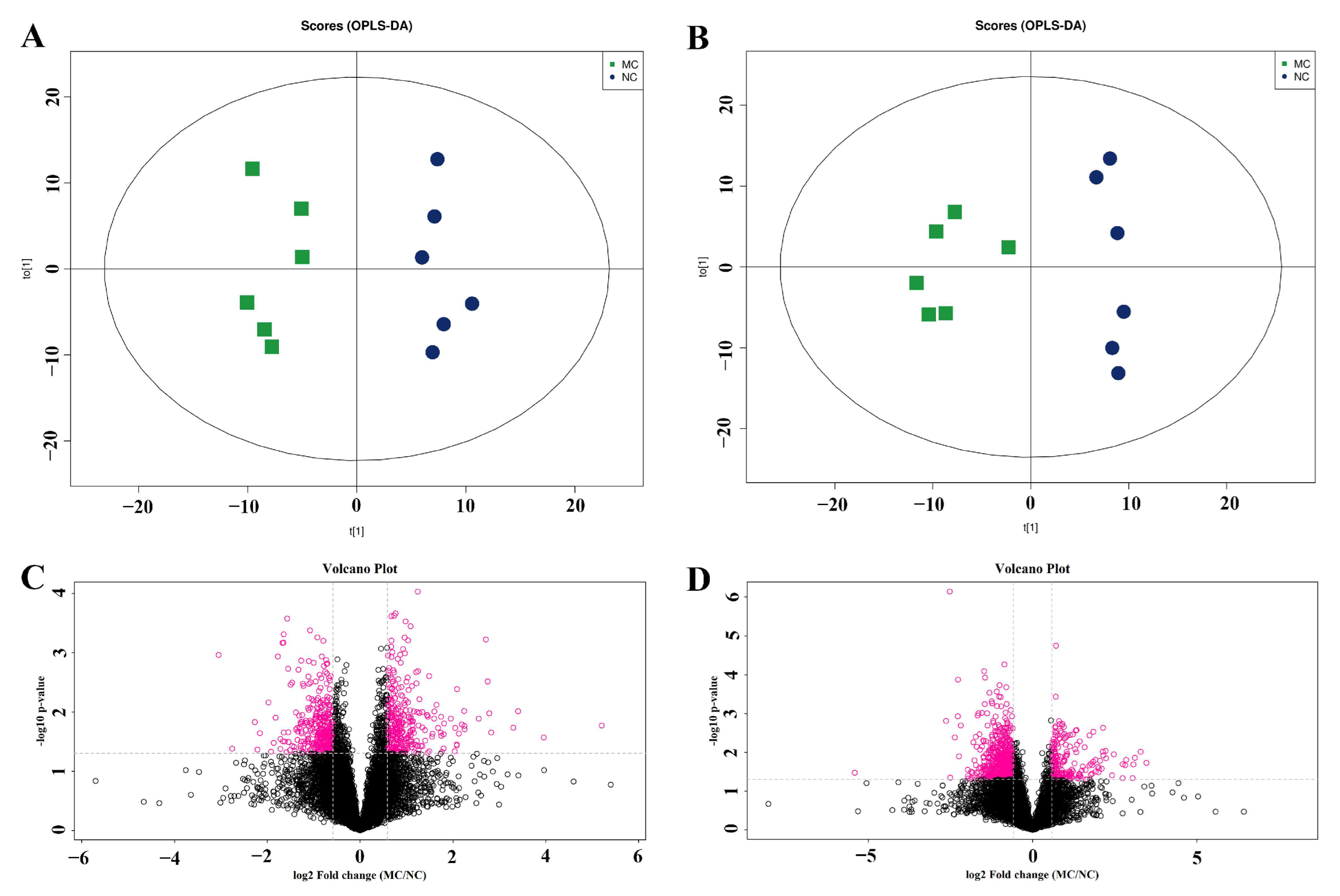
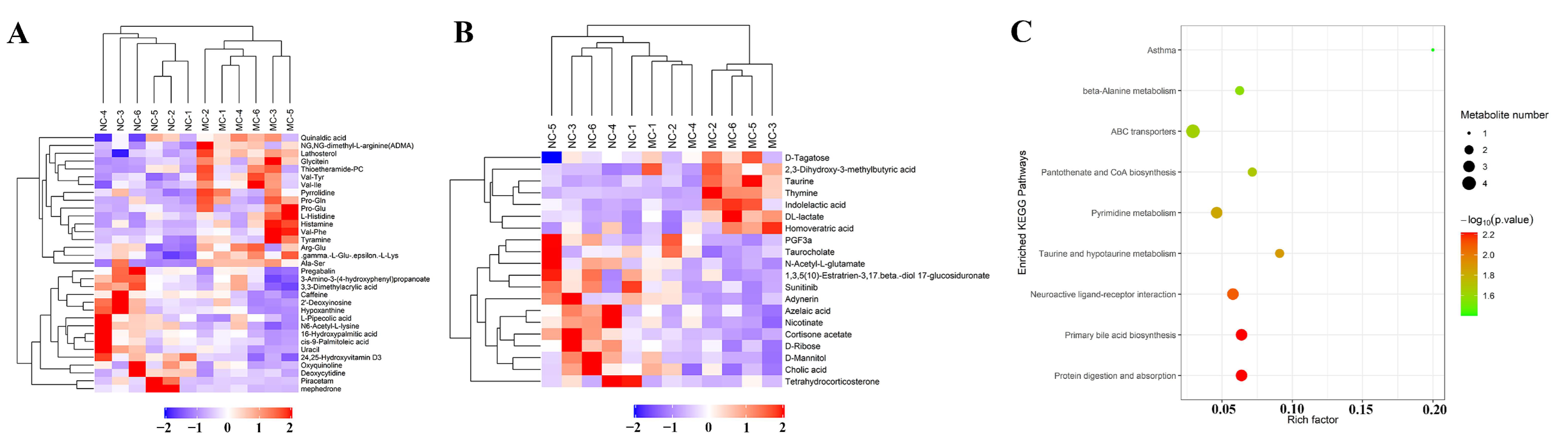
2.3. The Fecal Metabolome Was Obviously Changed in AD Mice
2.4. Tgy-C Changed the Profile of Fecal Metabolites in AD Mice
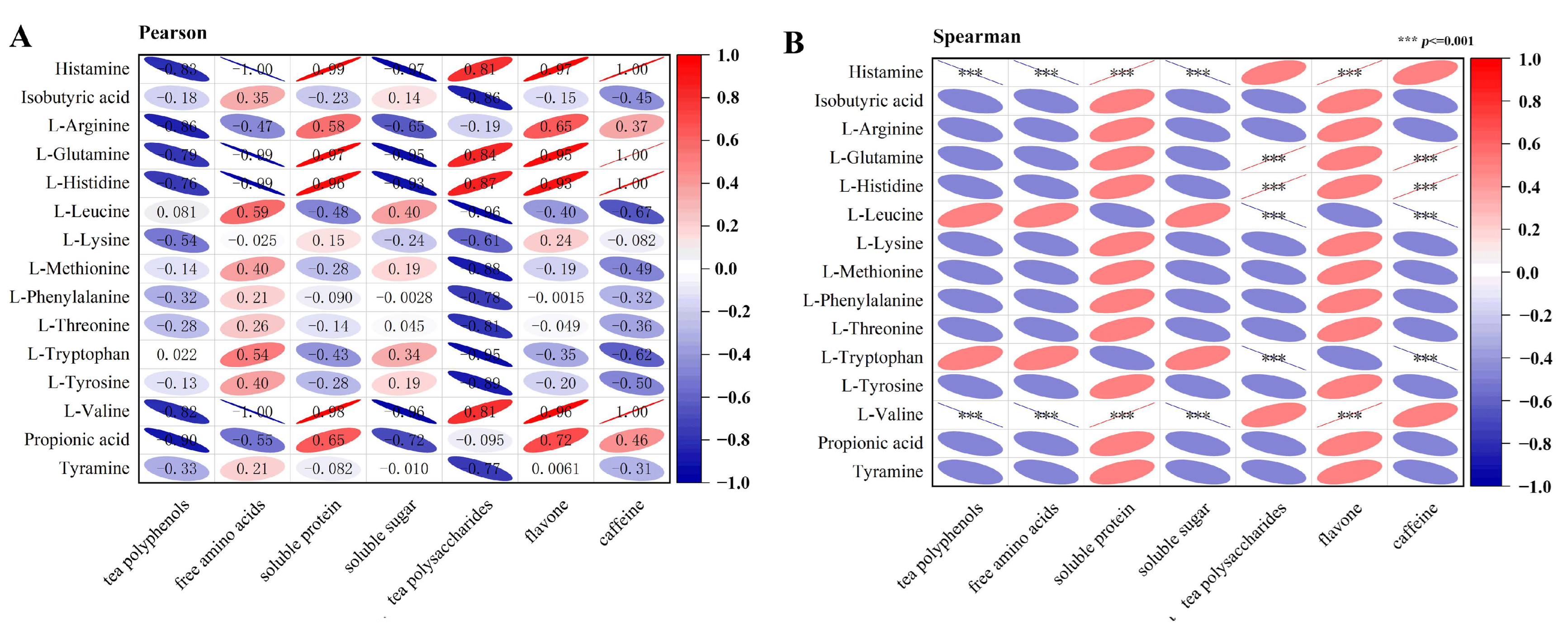
2.5. Different Types of Tieguanyin Exerted Distinct Effects on the Fecal Metabolites Which Correlated with the Contents of Functional Components in the Tea Extracts
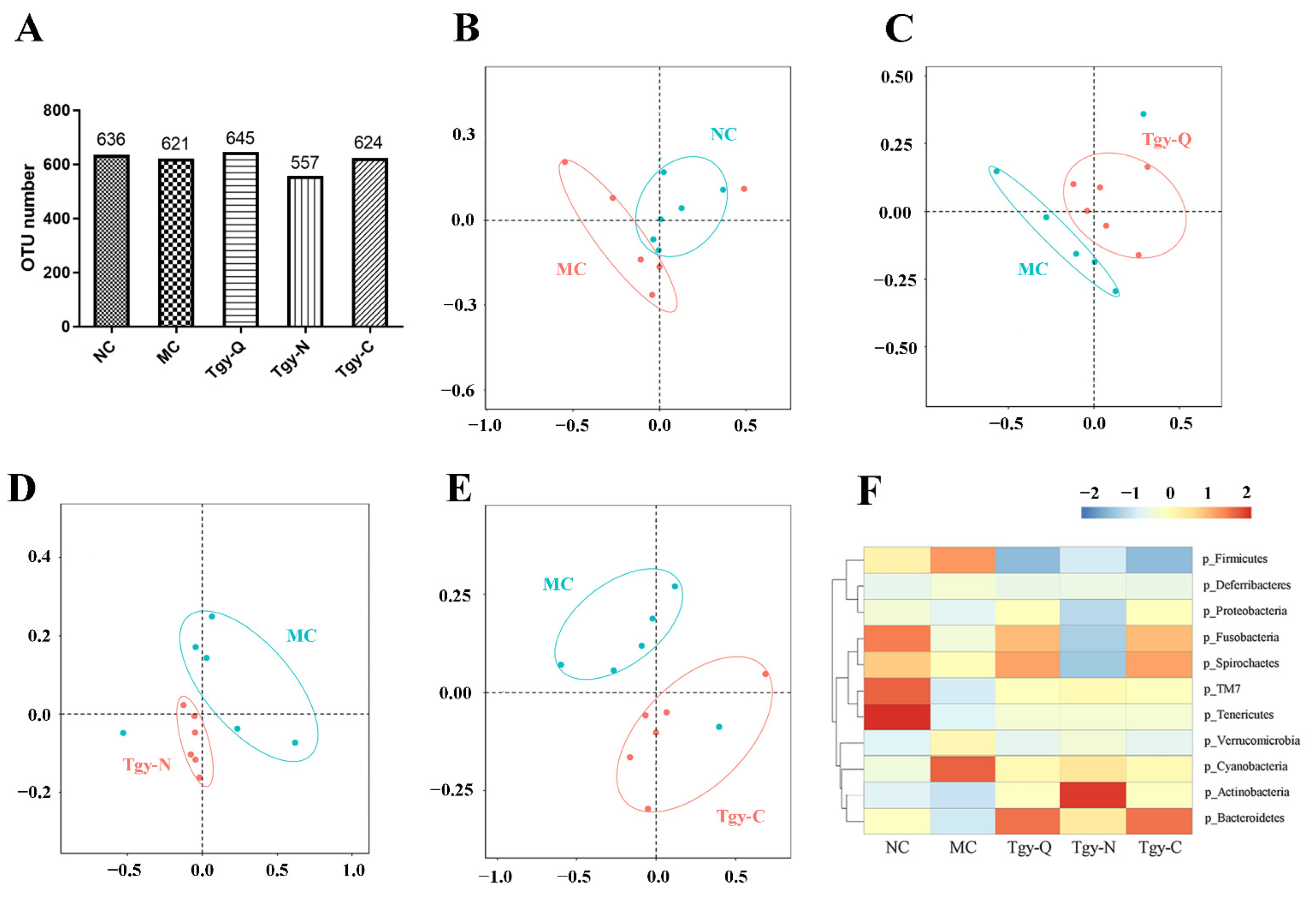
2.6. Different Effects of Tieguanyin on the Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Preparation Characterization of Tieguanyin Extracts
4.3. Animal Experiment
4.4. Paraffin Section, H&E and Nissl Staining
4.5. Metabolomics Analysis
4.6. 16S rDNA Sequencing and Bioinformatics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, Y.; Zhu, H.; Xiao, L.; Liu, C.; Liu, Y.L.; Gao, W. Identification of the function and mechanism of m6A reader IGF2BP2 in Alzheimer’s disease. Aging (Albany NY) 2021, 13, 24086–24100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Sun, L.H.; Lian, J.; Zhang, H.Y.; Yao, H.B. The Pathogenesis of Alzheimer and Pharmaceutical Research on Prevention. Adv. Educ. Res. 2013, 35, 172–176. [Google Scholar]
- Cruz-Rivera, Y.E.; Perez-Morales, J.; Santiago, Y.M.; Gonzalez, V.M.; Morales, L.; Cabrera-Rios, M.; Isaza, C.E. A Selection of Important Genes and Their Correlated Behavior in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 65, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Liu, J. Mechanism and Intervention Strategies of Oxidative Stress in the Pathogenesis of Alzheimer’s Disease. J. Sun Yat-Sen Univ. Med. Sci. 2020, 41, 661–668. [Google Scholar]
- Dong, Y.; Li, X.H.; Cheng, J.B.; Hou, L. Drug Development for Alzheimer’s Disease: Microglia Induced Neuroinflammation as a Target? Int. J. Mol. Sci. 2019, 20, 558. [Google Scholar] [CrossRef] [Green Version]
- Perry, G.; Taddeo, M.A.; Nunomura, A.; Zhu, X.W.; Zenteno-Savin, T.; Drew, K.L.; Shimohama, S.; Avila, J.; Castellani, R.J.; Smith, M.A. Comparative biology and pathology of oxidative stress in Alzheimer and other neurodegenerative diseases: Beyond damage and response. Comp. Biochem. Phys. C 2002, 133, 507–513. [Google Scholar] [CrossRef]
- Bostanciklioglu, M. An update on the interactions between Alzheimer’s disease, autophagy and inflammation. Gene 2019, 705, 157–166. [Google Scholar] [CrossRef]
- Ju, Y.J.; Tam, K.Y. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural. Regen. Res. 2022, 17, 543–549. [Google Scholar] [CrossRef]
- Singh, R.K. Recent Trends in the Management of Alzheimer’s Disease: Current Therapeutic Options and Drug Repurposing Approaches. Curr. Neuropharmacol. 2020, 18, 868–882. [Google Scholar] [CrossRef]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [Green Version]
- Kuriyama, S.; Hozawa, A.; Ohmori, K.; Shimazu, T.; Matsui, T.; Ebihara, S.; Awata, S.; Nagatomi, R.; Arai, H.; Tsuji, I. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project 1. Am. J. Clin. Nutr. 2006, 83, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Kesse-Guyot, E.; Fezeu, L.; Andreeva, V.A.; Touvier, M.; Scalbert, A.; Hercberg, S.; Galan, P. Total and Specific Polyphenol Intakes in Midlife Are Associated with Cognitive Function Measured 13 Years Later. J. Nutr. 2012, 142, 76–83. [Google Scholar] [CrossRef]
- Feng, L.; Gwee, X.; Kua, E.H.; Ng, T.P. Cognitive Function and Tea Consumption in Community Dwelling Older Chinese in Singapore. J. Nutr. Health Aging 2010, 14, 433–438. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Cheng, H.G. Lower intake of vegetables and legumes associated with cognitive decline among illiterate elderly Chinese: A 3-year cohort study. J. Nutr. Health Aging 2012, 16, 549–552. [Google Scholar] [CrossRef]
- Schimidt, H.L.; Garcia, A.; Martins, A.; Mello-Carpes, P.B.; Carpes, F.P. Green tea supplementation produces better neuroprotective effects than red and black tea in Alzheimer-like rat model. Food Res. Int. 2017, 100, 442–448. [Google Scholar] [CrossRef]
- McDonald, J.D. Production of mouse models for the study of human inborn errors of metabolism. Mol. Genet. Metab. 2000, 71, 240–244. [Google Scholar] [CrossRef]
- Kang, H.Y.N.; Zhou, H.; Ye, Y.S.; Yang, J.F.; Liu, Z.H.; He, P.M.; Li, B.; Wu, Y.Y.; Wang, Y.M.; Tu, Y.Y. Tieguanyin Oolong Tea Extracts Alleviate Behavioral Abnormalities by Modulating Neuroinflammation in APP/PS1 Mouse Model of Alzheimer’s Disease. Foods 2022, 11, 81. [Google Scholar] [CrossRef]
- Glenn, J.M.; Madero, E.N.; Bott, N.T. Dietary Protein and Amino Acid Intake: Links to the Maintenance of Cognitive Health. Nutrients 2019, 11, 1315. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.S.; Zhang, T.; Park, S. Protective effects of Forsythiae fructus and Cassiae semen water extract against memory deficits through the gut-microbiome-brain axis in an Alzheimer’s disease model. Pharm. Biol. 2022, 60, 212–224. [Google Scholar] [CrossRef]
- Vuong, Q.V. Epidemiological evidence linking tea consumption to human health: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Matsuoka, N.; Yamada, H.; Furushima, D.; Kawakami, K. Effects of Tea Catechins on Alzheimer’s Disease: Recent Updates and Perspectives. Molecules 2018, 23, 2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unno, K.; Hoshino, M. Brain senescence and neuroprotective dietary components. Cent. Nerv. Syst. Agents Med. Chem. 2007, 7, 109–114. [Google Scholar] [CrossRef]
- Bao, J.; Liu, W.; Zhou, H.Y.; Gui, Y.R.; Yang, Y.H.; Wu, M.J.; Xiao, Y.F.; Shang, J.T.; Long, G.F.; Shu, X.J. Epigallocatechin-3-gallate Alleviates Cognitive Deficits in APP/PS1 Mice. Curr. Med. Sci. 2020, 40, 18–27. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Nan, Y.; Wang, X.; Chen, Y.; Wang, S. (-)-Epigallocatechin-3-gallate ameliorates memory impairment and rescues the abnormal synaptic protein levels in the frontal cortex and hippocampus in a mouse model of Alzheimer’s disease. Neuroreport 2017, 28, 590–597. [Google Scholar] [CrossRef]
- Jo, M.; Park, M.H.; Choi, D.Y.; Yuk, D.Y.; Lee, Y.M.; Lee, J.M.; Jeong, J.H.; Oh, K.W.; Lee, M.S.; Han, S.B.; et al. Neuroprotective Effect of L-Theanine on a Beta-Induced Neurotoxicity through Anti-Oxidative Mechanisms in SK-N-SH and SK-N-MC Cells. Biomol. Ther. 2011, 19, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.I.; Lee, Y.K.; Park, S.G.; Choi, I.S.; Ban, J.O.; Park, H.K.; Nam, S.Y.; Yun, Y.W.; Han, S.B.; Oh, K.W.; et al. l-Theanine, an amino acid in green tea, attenuates beta-amyloid-induced cognitive dysfunction and neurotoxicity: Reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-kappaB pathways. Free Radic. Biol. Med. 2009, 47, 1601–1610. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Z.; Cai, S.; Liu, Z. Inhibitory Effects and Mechanism of Tieguanyin Tea Extracts on LPS-induced Inflammatory Response in RAW264.7 Cells. Sci. Technol. Food Ind. 2018, 39, 294–299. [Google Scholar]
- Cheng, Y.J.; Lin, C.H.; Lane, H.Y. d-Amino Acids and pLG72 in Alzheimer’s Disease and Schizophrenia. Int. J. Mol. Sci. 2021, 22, 10917. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Liu, P.P.; Shi, J.; Gao, Y.; Wang, Q.S.; Yin, J.F. Quality development and main chemical components of Tieguanyin oolong teas processed from different parts of fresh shoots. Food Chem. 2018, 249, 176–183. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Laurentin, A.; Edwards, C.A. A microtiter modification of the anthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Anal. Biochem. 2003, 315, 143–145. [Google Scholar] [CrossRef]
- Jabeen, S.; Alam, S.; Saleem, M.; Ahmad, W.; Bibi, R.; Hamid, F.S.; Shah, H.U. Withering timings affect the total free amino acids and mineral contents of tea leaves during black tea manufacturing. Arab. J. Chem. 2019, 12, 2411–2417. [Google Scholar] [CrossRef] [Green Version]
- Tai, Y.; Wei, C.; Yang, H.; Zhang, L.; Chen, Q.; Deng, W.; Wei, S.; Zhang, J.; Fang, C.; Ho, C.; et al. Transcriptomic and phytochemical analysis of the biosynthesis of characteristic constituents in tea (Camellia sinensis) compared with oil tea (Camellia oleifera). BMC Plant Biol. 2015, 15, 190. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Mao, F.; Cai, X.; Wang, Y. Composition and bioactivity of polysaccharides from tea seeds obtained by water extraction. Int. J. Biol. Macromol. 2011, 49, 587–590. [Google Scholar] [CrossRef]
- Li, F.; Jiang, Y.; Bai, Y.; Wang, X.; Bu, Q.; Gao, T.; Zhang, Z.; Xue, W. Progress of APP/PS1 double transgenic mice model for Alzheimer’s disease. Chin. J. Geriatr. 2015, 34, 109–112. [Google Scholar]





| Description | VIP | Fold Change | p-Value |
|---|---|---|---|
| L-Threonine | 1.1 | 0.55 | 5.9 × 10−6 |
| L-Leucine | 4.4 | 0.56 | 5.3 × 10−4 |
| L-Methionine | 2.8 | 0.46 | 1.4 × 10−3 |
| L-Phenylalanine | 5.5 | 0.56 | 1.9 × 10−3 |
| Tyramine | 9.1 | 0.56 | 2.2 × 10−3 |
| L-Tryptophan | 1.7 | 0.57 | 5.0 × 10−2 |
| L-Tyrosine | 2.3 | 1.38 | 9.9 × 10−2 |
| Isobutyric acid | 6.2 | 0.33 | 8.1 × 10−6 |
| L-Alanine | 2.5 | 0.48 | 1.5 × 10−3 |
| L-Lysine | 2.6 | 0.28 | 9.6 × 10−3 |
| L-Glutamate | 3.1 | 0.55 | 2.3 × 10−2 |
| Propionic acid | 1.3 | 0.60 | 4.9 × 10−2 |
| Metabolites | Tgy-N vs. MC | Tgy-N vs. Tgy-C | ||
|---|---|---|---|---|
| VIP | Fold Change | p-Value | Fold Change | |
| L-Threonine | 1.2 | 0.50 | 3.5 × 10−4 | 0.92 |
| L-Phenylalanine | 6.7 | 0.49 | 2.4 × 10−3 | 0.89 |
| Tyramine | 11 | 0.49 | 2.6 × 10−3 | 1.01 |
| L-Methionine | 2.8 | 0.45 | 3.0 × 10−3 | 1.00 |
| L-Leucine | 4.3 | 0.59 | 3.2 × 10−3 | 0.95 |
| Histamine | 1.5 | 0.41 | 1.8 × 10−2 | 0.88 |
| L-Tyrosine | 3.1 | 0.55 | 2.0 × 10−2 | 0.88 |
| L-Glutamate | 2.1 | 0.55 | 3.1 × 10−2 | 0.95 |
| L-Histidine | 1.8 | 0.52 | 4.5 × 10−2 | 0.73 |
| L-Arginine | 14 | 0.44 | 8.6 × 10−2 | 0.64 |
| L-Tryptophan | 2.1 | 0.58 | 9.4 × 10−2 | 1.01 |
| Isobutyric acid | 5.9 | 0.25 | 1.6 × 10−7 | 0.75 |
| L-Alanine | 2.4 | 0.45 | 2.7 × 10−4 | 0.63 |
| Propionic acid | 1.6 | 0.40 | 2.2 × 10−3 | 0.67 |
| L-Valine | 1.3 | 0.29 | 2.3 × 10−3 | 0.51 |
| L-Lysine | 2.5 | 0.22 | 6.1 × 10−3 | 0.80 |
| L-Glutamine | 1.6 | 0.44 | 9.5 × 10−2 | 0.72 |
| Metabolites | Tgy-Q vs. MC | Tgy-Q vs. Tgy-C | ||
|---|---|---|---|---|
| VIP | Fold Change | p-Value | Fold Change | |
| Histamine | 1.1 | 0.51 | 2.5 × 10−3 | 0.73 |
| L-Arginine | 5.5 | 0.39 | 3.1 × 10−2 | 1.05 |
| L-Histidine | 1.8 | 0.50 | 4.8 × 10−2 | 0.93 |
| L-Tryptophan | 1.7 | 0.62 | 9.0 × 10−2 | 1.08 |
| L-Valine | 1.4 | 0.30 | 1.3 × 10−3 | 0.53 |
| L-Lysine | 2.4 | 0.36 | 2.1 × 10−2 | 1.12 |
| Propionic acid | 1.5 | 0.60 | 2.5 × 10−2 | 1.01 |
| L-Threonine | 1.4 | 0.62 | 5.9 × 10−2 | 1.34 |
| L-Glutamine | 1.6 | 0.44 | 6.9 × 10−2 | 0.71 |
| Comparisons | POS | NEG | Total |
|---|---|---|---|
| MC vs. NC | 33 (17↑, 16↓) | 20 (7↑, 13↓) | 53 (24↑, 29↓) |
| Tgy-C vs. MC | 101 (11↑, 90↓) | 54 (12↑, 42↓) | 155 (23↑, 132↓) |
| Tgy-Q vs. MC | 81 (28↑, 53↓) | 42 (17↑, 25↓) | 123 (45↑, 78↓) |
| Tgy-N vs. MC | 111 (26↑, 85↓) | 60 (12↑, 48↓) | 171 (38↑, 133↓) |
| Tgy-C vs. Tgy-Q | 36 (4↑, 32↓) | 10 (3↑, 7↓) | 46 (7↑, 39↓) |
| Tgy-C vs. Tgy-N | 22 (5↑, 17↓) | 12 (7↑, 5↓) | 34 (12↑, 22↓) |
| Tgy-N vs. Tgy-Q | 20 (4↑, 16↓) | 9 (3↑, 6↓) | 29 (7↑, 22↓) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Y.; Kang, H.; Kim, E.; Yang, J.; He, P.; Wu, Y.; Li, B.; Liu, X.; Liu, J. Metabolomics Study Suggests the Mechanism of Different Types of Tieguanyin (Oolong) Tea in Alleviating Alzheimer’s Disease in APP/PS1 Transgenic Mice. Metabolites 2022, 12, 466. https://doi.org/10.3390/metabo12050466
Tu Y, Kang H, Kim E, Yang J, He P, Wu Y, Li B, Liu X, Liu J. Metabolomics Study Suggests the Mechanism of Different Types of Tieguanyin (Oolong) Tea in Alleviating Alzheimer’s Disease in APP/PS1 Transgenic Mice. Metabolites. 2022; 12(5):466. https://doi.org/10.3390/metabo12050466
Chicago/Turabian StyleTu, Youying, Hyunuk Kang, Eunhye Kim, Jiangfan Yang, Puming He, Yuanyuan Wu, Bo Li, Xiaobo Liu, and Junsheng Liu. 2022. "Metabolomics Study Suggests the Mechanism of Different Types of Tieguanyin (Oolong) Tea in Alleviating Alzheimer’s Disease in APP/PS1 Transgenic Mice" Metabolites 12, no. 5: 466. https://doi.org/10.3390/metabo12050466
APA StyleTu, Y., Kang, H., Kim, E., Yang, J., He, P., Wu, Y., Li, B., Liu, X., & Liu, J. (2022). Metabolomics Study Suggests the Mechanism of Different Types of Tieguanyin (Oolong) Tea in Alleviating Alzheimer’s Disease in APP/PS1 Transgenic Mice. Metabolites, 12(5), 466. https://doi.org/10.3390/metabo12050466