Reprogramming of Fatty Acid Metabolism in Gynaecological Cancers: Is There a Role for Oestradiol?
Abstract
1. Introduction
2. Methods
3. Altered Fatty Acid Metabolism in Gynaecological Cancers
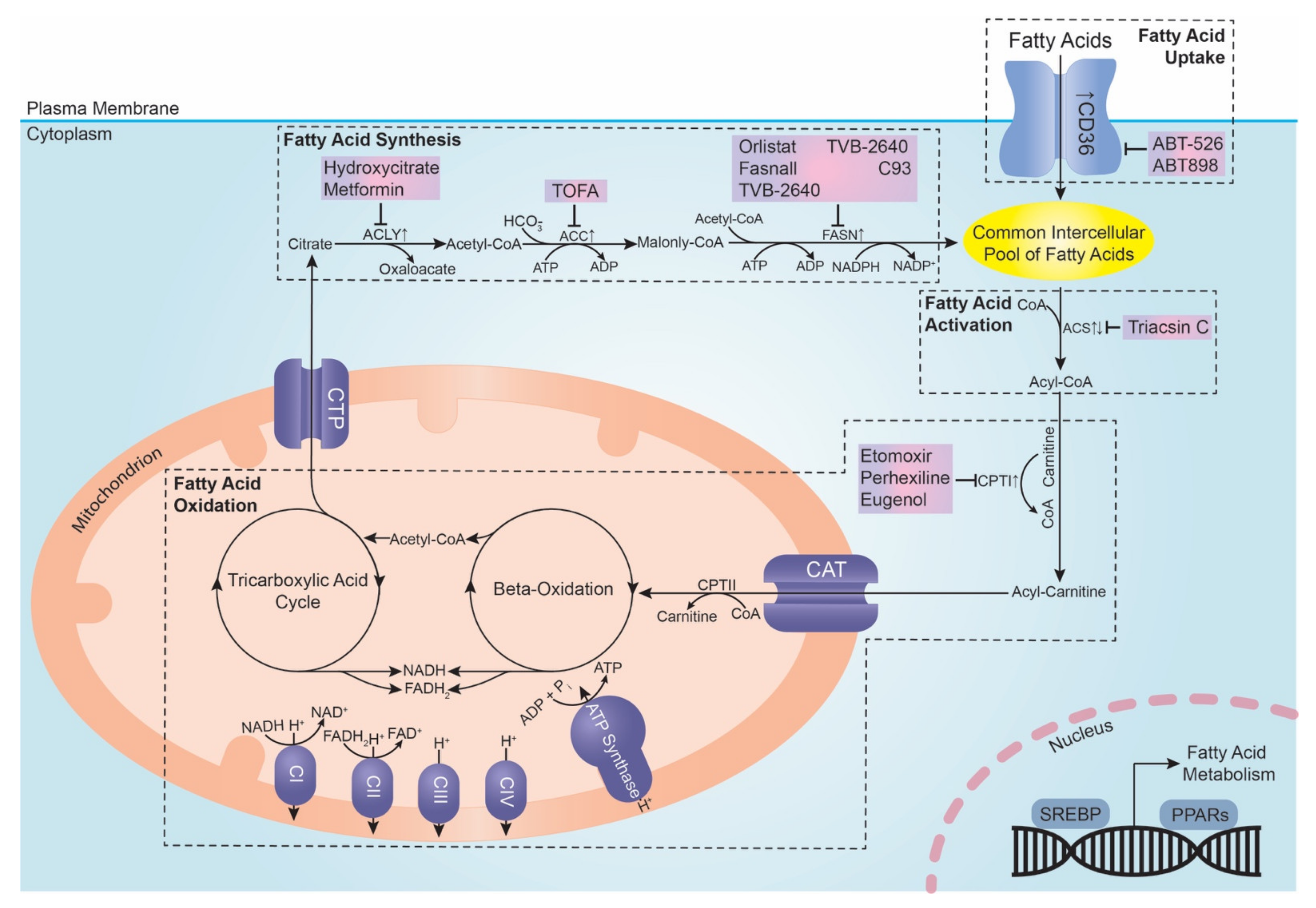
3.1. Fatty Acid Uptake
CD36
3.2. De Novo Fatty Acid Synthesis
3.2.1. ATP Citrate Lyase
3.2.2. Acetyl-Coenzyme A Carboxylase
3.2.3. Fatty Acid Synthetase
3.3. Fatty Acid Activation
Acyl-CoA Synthetase
3.4. Fatty Acid Oxidation
Carnitine Palmitoyltransferase I
4. Oestradiol Regulation of Fatty Acid Metabolism in Gynaecological Cancers
4.1. Oestrogen Receptor Pathway
4.2. Insulin-like Growth 1 Receptor Pathway
5. Additional Roles of Oestradiol on Fatty Acid Metabolism in Gynaecological Cancer in the Context of Obesity
5.1. Inflammatory Pathway
5.2. Oxidative Stress Pathway
6. Current Update on Therapeutic Strategies Targeting Fatty Acid Metabolism
6.1. CD36 Inhibition
6.2. ATP-Citrate Lyase (ACLY) Inhibition
6.3. Acetyl-CoA Carboxylase (ACC) Inhibition
6.4. Fatty Acid Synthase (FASN) Inhibition
6.5. Acyl-CoA Synthetase (ACS) Inhibition
6.6. Carnitine Palmitoyltransferase (CPTI) Inhibition
6.7. Omega-3 Fatty Acids Supplementation
7. Future Directions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Deng, Y.; Zhou, L.; Tian, T.; Yang, S.; Wu, Y.; Zheng, Y.; Zhai, Z.; Hao, Q.; Song, D.; et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: Results from the Global Burden of Disease Study 2017. J. Hematol. Oncol. 2019, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International Patterns and Trends in Endometrial Cancer Incidence, 1978-2013. J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef]
- Kumar, V.; Santhosh Kumar, T.R.; Kartha, C.C. Mitochondrial membrane transporters and metabolic switch in heart failure. Heart Fail. Rev. 2019, 24, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, C.; Wei, Y. The metabolic switch and its regulation in cancer cells. Sci. China Life Sci. 2010, 53, 942–958. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Tvrzicka, E.; Kremmyda, L.S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—A review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2011, 155, 117–130. [Google Scholar] [CrossRef]
- Georgiadi, A.; Kersten, S. Mechanisms of Gene Regulation by Fatty Acids. Adv. Nutr. 2012, 3, 127–134. [Google Scholar] [CrossRef]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V., Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013, 18, 153–161. [Google Scholar] [CrossRef]
- Chen, M.; Huang, J. The expanded role of fatty acid metabolism in cancer: New aspects and targets. Precis. Clin. Med. 2019, 2, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Folkerd, E.; Dowsett, M. Sex hormones and breast cancer risk and prognosis. Breast 2013, 22, S38–S43. [Google Scholar] [CrossRef] [PubMed]
- Endogenous, H.; Breast Cancer Collaborative, G.; Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.W.; Helzlsouer, K.J.; Alberg, A.J.; Rollison, D.E.; Dorgan, J.F.; et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: Reanalysis of 13 studies. Br. J. Cancer 2011, 105, 709–722. [Google Scholar] [CrossRef]
- Beral, V.; Bull, D.; Green, J.; Reeves, G. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet 2007, 369, 1703–1710. [Google Scholar] [CrossRef]
- Lukanova, A.; Kaaks, R. Endogenous Hormones and Ovarian Cancer: Epidemiology and Current Hypotheses. Cancer Epidemiol. Biomark. Prev. 2005, 14, 98. [Google Scholar]
- Allen, N.E.; Key, T.J.; Dossus, L.; Rinaldi, S.; Cust, A.; Lukanova, A.; Peeters, P.H.; Onland-Moret, N.C.; Lahmann, P.H.; Berrino, F.; et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr. Relat. Cancer 2008, 15, 485–497. [Google Scholar] [CrossRef]
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, Endogenous Hormones, and Endometrial Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1531. [Google Scholar]
- Hunter, D.J.; Spiegelman, D.; Adami, H.O.; van den Brandt, P.A.; Folsom, A.R.; Goldbohm, R.A.; Graham, S.; Howe, G.R.; Kushi, L.H.; Marshall, J.R.; et al. Non-dietary factors as risk factors for breast cancer, and as effect modifiers of the association of fat intake and risk of breast cancer. Cancer Causes Control 1997, 8, 49–56. [Google Scholar] [CrossRef]
- Gong, T.-T.; Wu, Q.-J.; Vogtmann, E.; Lin, B.; Wang, Y.-L. Age at menarche and risk of ovarian cancer: A meta-analysis of epidemiological studies. Int. J. Cancer 2013, 132, 2894–2900. [Google Scholar] [CrossRef]
- La Vecchia, C.; Franceschi, S.; Decarli, A.; Gallus, G.; Tognoni, G. Risk factors for endometrial cancer at different ages. J. Natl. Cancer Inst. 1984, 73, 667–671. [Google Scholar] [PubMed]
- Gong, T.-T.; Wang, Y.-L.; Ma, X.-X. Age at menarche and endometrial cancer risk: A dose-response meta-analysis of prospective studies. Sci. Rep. 2015, 5, 14051. [Google Scholar] [CrossRef] [PubMed]
- Breast cancer and breastfeeding: Collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50 302 women with breast cancer and 96 973 women without the disease. Lancet 2002, 360, 187–195. [CrossRef]
- Babic, A.; Sasamoto, N.; Rosner, B.A.; Tworoger, S.S.; Jordan, S.J.; Risch, H.A.; Harris, H.R.; Rossing, M.A.; Doherty, J.A.; Fortner, R.T.; et al. Association Between Breastfeeding and Ovarian Cancer Risk. JAMA Oncol. 2020, 6, e200421. [Google Scholar] [CrossRef]
- Jordan, S.J.; Na, R.; Johnatty, S.E.; Wise, L.A.; Adami, H.O.; Brinton, L.A.; Chen, C.; Cook, L.S.; Dal Maso, L.; De Vivo, I.; et al. Breastfeeding and Endometrial Cancer Risk: An Analysis From the Epidemiology of Endometrial Cancer Consortium. Obs. Gynecol. 2017, 129, 1059–1067. [Google Scholar] [CrossRef]
- Dara Hope, C.; Derek, L. Obesity, type 2 diabetes, and cancer: The insulin and IGF connection. Endocr.-Relat. Cancer 2012, 19, F27–F45. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.; Dorgan, J.F.; Longcope, C.; Stanczyk, F.Z.; Stephenson, H.E., Jr.; Falk, R.T.; Miller, R.; et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J. Natl. Cancer Inst. 2003, 95, 1218–1226. [Google Scholar] [CrossRef]
- Feng, Y.-H. The association between obesity and gynecological cancer. Gynecol. Minim. Invasive Ther. 2015, 4, 102–105. [Google Scholar] [CrossRef]
- Ratnayake, W.M.; Galli, C. Fat and fatty acid terminology, methods of analysis and fat digestion and metabolism: A background review paper. Ann. Nutr Metab 2009, 55, 8–43. [Google Scholar] [CrossRef]
- Gimeno, R.E. Fatty acid transport proteins. Curr. Opin. Lipidol. 2007, 18, 271–276. [Google Scholar] [CrossRef]
- Ladanyi, A.; Mukherjee, A.; Kenny, H.A.; Johnson, A.; Mitra, A.K.; Sundaresan, S.; Nieman, K.M.; Pascual, G.; Benitah, S.A.; Montag, A.; et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 2018, 37, 2285–2301. [Google Scholar] [CrossRef] [PubMed]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res. 2011, 71, 2455. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhi, Z.; Wang, C.; Xing, H.; Song, G.; Yu, X.; Zhu, Y.; Wang, X.; Zhang, X.; Di, Y. Exogenous lipids promote the growth of breast cancer cells via CD36. Oncol. Rep. 2017, 38, 2105–2115. [Google Scholar] [CrossRef]
- Yang, P.; Su, C.; Luo, X.; Zeng, H.; Zhao, L.; Wei, L.; Zhang, X.; Varghese, Z.; Moorhead, J.F.; Chen, Y.; et al. Dietary oleic acid-induced CD36 promotes cervical cancer cell growth and metastasis via up-regulation Src/ERK pathway. Cancer Lett. 2018, 438, 76–85. [Google Scholar] [CrossRef]
- Kristiansen, G.; Rose, M.; Geisler, C.; Fritzsche, F.R.; Gerhardt, J.; Lüke, C.; Ladhoff, A.M.; Knüchel, R.; Dietel, M.; Moch, H.; et al. Endogenous myoglobin in human breast cancer is a hallmark of luminal cancer phenotype. Br. J. Cancer 2010, 102, 1736–1745. [Google Scholar] [CrossRef]
- Jiménez, B.; Volpert, O.V.; Crawford, S.E.; Febbraio, M.; Silverstein, R.L.; Bouck, N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat. Med. 2000, 6, 41–48. [Google Scholar] [CrossRef]
- Uray, I.P.; Liang, Y.; Hyder, S.M. Estradiol down-regulates CD36 expression in human breast cancer cells. Cancer Lett. 2004, 207, 101–107. [Google Scholar] [CrossRef]
- Liang, Y.; Han, H.; Liu, L.; Duan, Y.; Yang, X.; Ma, C.; Zhu, Y.; Han, J.; Li, X.; Chen, Y. CD36 plays a critical role in proliferation, migration and tamoxifen-inhibited growth of ER-positive breast cancer cells. Oncogenesis 2018, 7, 98. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Ye, T. MiR-22 down-regulates the proto-oncogene ATP citrate lyase to inhibit the growth and metastasis of breast cancer. Am. J. Transl. Res. 2018, 10, 659–669. [Google Scholar]
- Wang, Y.; Wang, Y.; Shen, L.; Pang, Y.; Qiao, Z.; Liu, P. Prognostic and therapeutic implications of increased ATP citrate lyase expression in human epithelial ovarian cancer. Oncol. Rep. 2012, 27, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Qiao, Z.; Li, J.; Liu, J.; Song, S.; Zhao, X.; Miao, P.; Tang, T.; Wang, L.; Liu, W.; et al. miR-22 inhibits tumor growth and metastasis by targeting ATP citrate lyase: Evidence in osteosarcoma, prostate cancer, cervical cancer and lung cancer. Oncotarget 2016, 7, 44252–44265. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.L.; Poon, I.K.; Modesitt, S.C.; Tomsig, J.L.; Chow, J.D.; Healy, M.E.; Baker, W.D.; Atkins, K.A.; Lancaster, J.M.; Marchion, D.C.; et al. Metabolic vulnerabilities in endometrial cancer. Cancer Res. 2014, 74, 5832–5845. [Google Scholar] [CrossRef] [PubMed]
- Chajes, V.; Cambot, M.; Moreau, K.; Lenoir, G.M.; Joulin, V. Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res. 2006, 66, 5287–5294. [Google Scholar] [CrossRef]
- Li, S.; Qiu, L.H.; Wu, B.C.; Shen, H.R.; Zhu, J.; Zhou, L.; Gu, L.Y.; Di, W. TOFA suppresses ovarian cancer cell growth in vitro and in vivo. Mol. Med. Rep. 2013, 8, 373–378. [Google Scholar] [CrossRef]
- Milgraum, L.Z.; Witters, L.A.; Pasternack, G.R.; Kuhajda, F.P. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1997, 3, 2115–2120. [Google Scholar]
- Gonzalez-Guerrico, A.M.; Espinoza, I.; Schroeder, B.; Park, C.H.; Kvp, C.M.; Khurana, A.; Corominas-Faja, B.; Cuyàs, E.; Alarcón, T.; Kleer, C.; et al. Suppression of endogenous lipogenesis induces reversion of the malignant phenotype and normalized differentiation in breast cancer. Oncotarget 2016, 7, 71151–71168. [Google Scholar] [CrossRef]
- Bauerschlag, D.O.; Maass, N.; Leonhardt, P.; Verburg, F.A.; Pecks, U.; Zeppernick, F.; Morgenroth, A.; Mottaghy, F.M.; Tolba, R.; Meinhold-Heerlein, I.; et al. Fatty acid synthase overexpression: Target for therapy and reversal of chemoresistance in ovarian cancer. J. Transl. Med. 2015, 13, 146. [Google Scholar] [CrossRef]
- Pizer, E.S.; Wood, F.D.; Heine, H.S.; Romantsev, F.E.; Pasternack, G.R.; Kuhajda, F.P. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996, 56, 1189–1193. [Google Scholar]
- Gansler, T.S.; Hardman, W., 3rd; Hunt, D.A.; Schaffel, S.; Hennigar, R.A. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum. Pathol. 1997, 28, 686–692. [Google Scholar] [CrossRef]
- Papaevangelou, E.; Almeida, G.S.; Box, C.; de Souza, N.M.; Chung, Y.L. The effect of FASN inhibition on the growth and metabolism of a cisplatin-resistant ovarian carcinoma model. Int. J. Cancer 2018, 143, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Roszik, J.; Ring, K.L.; Wani, K.M.; Lazar, A.J.; Yemelyanova, A.V.; Soliman, P.T.; Frumovitz, M.; Jazaeri, A.A. Gene Expression Analysis Identifies Novel Targets for Cervical Cancer Therapy. Front. Immunol. 2018, 9, 2102. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wang, H.; Cai, S.; Su, X.; Shen, J.; Meng, Q.; Chen, Y.; Li, L.; Yan, J.; Zhang, C.; et al. Integrated Analysis of a Competing Endogenous RNA Network Revealing a Prognostic Signature for Cervical Cancer. Front. Oncol. 2018, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Wang, J.; Wen, X.; Marcus, M.T.; Daniels, G.; Zhang, D.Y.; Ye, F.; Wang, L.H.; Du, X.; et al. Long Chain Fatty Acyl-CoA Synthetase 4 Is a Biomarker for and Mediator of Hormone Resistance in Human Breast Cancer. PLoS ONE 2013, 8, e77060. [Google Scholar] [CrossRef]
- Wang, J.; Scholtens, D.; Holko, M.; Ivancic, D.; Lee, O.; Hu, H.; Chatterton, R.T., Jr.; Sullivan, M.E.; Hansen, N.; Bethke, K.; et al. Lipid metabolism genes in contralateral unaffected breast and estrogen receptor status of breast cancer. Cancer Prev. Res. 2013, 6, 321–330. [Google Scholar] [CrossRef]
- Yen, M.C.; Kan, J.Y.; Hsieh, C.J.; Kuo, P.L.; Hou, M.F.; Hsu, Y.L. Association of long-chain acyl-coenzyme A synthetase 5 expression in human breast cancer by estrogen receptor status and its clinical significance. Oncol. Rep. 2017, 37, 3253–3260. [Google Scholar] [CrossRef]
- Maloberti, P.M.; Duarte, A.B.; Orlando, U.D.; Pasqualini, M.E.; Solano, A.R.; Lopez-Otin, C.; Podesta, E.J. Functional interaction between acyl-CoA synthetase 4, lipooxygenases and cyclooxygenase-2 in the aggressive phenotype of breast cancer cells. PLoS ONE 2010, 5, e15540. [Google Scholar] [CrossRef]
- Chen, W.-C.; Wang, C.-Y.; Hung, Y.-H.; Weng, T.-Y.; Yen, M.-C.; Lai, M.-D. Systematic Analysis of Gene Expression Alterations and Clinical Outcomes for Long-Chain Acyl-Coenzyme A Synthetase Family in Cancer. PLoS ONE 2016, 11, e0155660. [Google Scholar] [CrossRef]
- Gassler, N.; Yang, S.H.; Keith, M.; Helmke, B.M.; Schirmacher, P.; Obermuller, N. Expression of acyl-CoA synthetase 5 in human endometrium and in endometrioid adenocarcinomas. Histopathology 2005, 47, 501–507. [Google Scholar] [CrossRef]
- Gatza, M.L.; Silva, G.O.; Parker, J.S.; Fan, C.; Perou, C.M. An integrated genomics approach identifies drivers of proliferation in luminal-subtype human breast cancer. Nat. Genet. 2014, 46, 1051. [Google Scholar] [CrossRef]
- Linher-Melville, K.; Zantinge, S.; Sanli, T.; Gerstein, H.; Tsakiridis, T.; Singh, G. Establishing a relationship between prolactin and altered fatty acid β-Oxidation via carnitine palmitoyl transferase 1 in breast cancer cells. BMC Cancer 2011, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Vithayathil, S.; Kumar, S.; Sung, P.-L.; Dobrolecki, L.E.; Putluri, V.; Bhat, V.B.; Bhowmik, S.K.; Gupta, V.; Arora, K.; et al. Fatty Acid Oxidation-Driven Src Links Mitochondrial Energy Reprogramming and Oncogenic Properties in Triple-Negative Breast Cancer. Cell Rep. 2016, 14, 2154–2165. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Mohamed, E.M.; Xu, G.G.; Waters, M.; Jing, K.; Ma, Y.; Zhang, Y.; Spiegel, S.; Idowu, M.O.; Fang, X. Carnitine palmitoyltransferase 1A functions to repress FoxO transcription factors to allow cell cycle progression in ovarian cancer. Oncotarget 2016, 7, 3832–3846. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.Y.; Lemaitre, R.N.; Imamura, F.; King, I.B.; Song, X.; Spiegelman, D.; Siscovick, D.S.; Mozaffarian, D. Fatty acids in the de novo lipogenesis pathway and risk of coronary heart disease: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2011, 94, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Ohlrogge, J.B.; Jaworski, J.G. Regulation of Fatty Acid Synthesis. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1997, 48, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Guillou, H.; Martin, P.G.; Pineau, T. Transcriptional regulation of hepatic fatty acid metabolism. Sub-Cell. Biochem. 2008, 49, 3–47. [Google Scholar] [CrossRef]
- Mashima, T.; Seimiya, H.; Tsuruo, T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer 2009, 100, 1369–1372. [Google Scholar] [CrossRef]
- Kusakabe, T.; Maeda, M.; Hoshi, N.; Sugino, T.; Watanabe, K.; Fukuda, T.; Suzuki, T. Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2000, 48, 613–622. [Google Scholar] [CrossRef]
- Calvisi, D.F.; Wang, C.; Ho, C.; Ladu, S.; Lee, S.A.; Mattu, S.; Destefanis, G.; Delogu, S.; Zimmermann, A.; Ericsson, J.; et al. Increased Lipogenesis, Induced by AKT-mTORC1-RPS6 Signaling, Promotes Development of Human Hepatocellular Carcinoma. Gastroenterology 2011, 140, 1071–1083.e5. [Google Scholar] [CrossRef]
- Makino, K.; Nakamura, H.; Hide, T.; Yano, S.; Kuroda, J.; Iyama, K.; Kuratsu, J. Fatty acid synthase is a predictive marker for aggressiveness in meningiomas. J. Neuro-Oncol. 2012, 109, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-F.; Fang, F.-M.; Chen, Y.-Y.; Liu, T.-T.; Chan, T.-C.; Yu, S.-C.; Chen, L.-T.; Huang, H.-Y. Overexpressed Fatty Acid Synthase in Gastrointestinal Stromal Tumors: Targeting a Progression-Associated Metabolic Driver Enhances the Antitumor Effect of Imatinib. Clin. Cancer Res. 2017, 23, 4908. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Reviews. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Chypre, M.; Zaidi, N.; Smans, K. ATP-citrate lyase: A mini-review. Biochem. Biophys. Res. Commun. 2012, 422, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yin, L.; Wei, J.; Yang, Z.; Jiang, G. ATP citrate lyase is increased in human breast cancer, depletion of which promotes apoptosis. Tumour Biol. J. Int. Soc. Oncodevelop. Biol. Med. 2017, 39, 1010428317698338. [Google Scholar] [CrossRef]
- Szutowicz, A.; Kwiatkowski, J.; Angielski, S. Lipogenetic and glycolytic enzyme activities in carcinoma and nonmalignant diseases of the human breast. Br. J. Cancer 1979, 39, 681–687. [Google Scholar] [CrossRef]
- Chen, Y.; Qian, J.; He, Q.; Zhao, H.; Toral-Barza, L.; Shi, C.; Zhang, X.; Wu, J.; Yu, K. mTOR complex-2 stimulates acetyl-CoA and de novo lipogenesis through ATP citrate lyase in HER2/PIK3CA-hyperactive breast cancer. Oncotarget 2016, 7, 25224–25240. [Google Scholar] [CrossRef]
- Lucenay, K.S.; Doostan, I.; Karakas, C.; Bui, T.; Ding, Z.; Mills, G.B.; Hunt, K.K.; Keyomarsi, K. Cyclin E Associates with the Lipogenic Enzyme ATP-Citrate Lyase to Enable Malignant Growth of Breast Cancer Cells. Cancer Res. 2016, 76, 2406–2418. [Google Scholar] [CrossRef]
- Tong, L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. CMLS 2005, 62, 1784–1803. [Google Scholar] [CrossRef]
- Wang, C.; Ma, J.; Zhang, N.; Yang, Q.; Jin, Y.; Wang, Y. The acetyl-CoA carboxylase enzyme: A target for cancer therapy? Expert Rev. Anticancer Ther. 2015, 15, 667–676. [Google Scholar] [CrossRef]
- Sinilnikova, O.M.; Ginolhac, S.M.; Magnard, C.; Leone, M.; Anczukow, O.; Hughes, D.; Moreau, K.; Thompson, D.; Coutanson, C.; Hall, J.; et al. Acetyl-CoA carboxylase alpha gene and breast cancer susceptibility. Carcinogenesis 2004, 25, 2417–2424. [Google Scholar] [CrossRef] [PubMed]
- Pizer, E.S.; Thupari, J.; Han, W.F.; Pinn, M.L.; Chrest, F.J.; Frehywot, G.L.; Townsend, C.A.; Kuhajda, F.P. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000, 60, 213–218. [Google Scholar] [PubMed]
- Thupari, J.N.; Pinn, M.L.; Kuhajda, F.P. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem. Biophys. Res. Commun. 2001, 285, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Camarda, R.; Zhou, A.Y.; Kohnz, R.A.; Balakrishnan, S.; Mahieu, C.; Anderton, B.; Eyob, H.; Kajimura, S.; Tward, A.; Krings, G.; et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med. 2016, 22, 427. [Google Scholar] [CrossRef] [PubMed]
- Perrone, F.; Baldassarre, G.; Indraccolo, S.; Signoriello, S.; Chiappetta, G.; Esposito, F.; Ferrandina, G.; Franco, R.; Mezzanzanica, D.; Sonego, M.; et al. Biomarker analysis of the MITO2 phase III trial of first-line treatment in ovarian cancer: Predictive value of DNA-PK and phosphorylated ACC. Oncotarget 2016, 7, 72654–72661. [Google Scholar] [CrossRef][Green Version]
- Modesitt, S.C.; Hsu, J.Y.; Chowbina, S.R.; Lawrence, R.T.; Hoehn, K.L. Not All Fat Is Equal Differential Gene Expression and Potential Therapeutic Targets in Subcutaneous Adipose, Visceral Adipose, and Endometrium of Obese Women With and Without Endometrial Cancer. Int. J. Gynecol. Cancer 2012, 22, 732–741. [Google Scholar] [CrossRef]
- Chirala, S.S.; Wakil, S.J. Structure and function of animal fatty acid synthase. Lipids 2004, 39, 1045–1053. [Google Scholar] [CrossRef]
- Alo, P.L.; Visca, P.; Trombetta, G.; Mangoni, A.; Lenti, L.; Monaco, S.; Botti, C.; Serpieri, D.E.; Di Tondo, U. Fatty acid synthase (FAS) predictive strength in poorly differentiated early breast carcinomas. Tumori 1999, 85, 35–40. [Google Scholar] [CrossRef]
- Alo, P.L.; Visca, P.; Marci, A.; Mangoni, A.; Botti, C.; Di Tondo, U. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer 1996, 77, 474–482. [Google Scholar] [CrossRef]
- Corominas-Faja, B.; Vellon, L.; Cuyas, E.; Buxo, M.; Martin-Castillo, B.; Serra, D.; Garcia, J.; Lupu, R.; Menendez, J.A. Clinical and therapeutic relevance of the metabolic oncogene fatty acid synthase in HER2+ breast cancer. Histol. Histopathol. 2017, 32, 687–698. [Google Scholar] [CrossRef]
- Alo, P.L.; Visca, P.; Framarino, M.L.; Botti, C.; Monaco, S.; Sebastiani, V.; Serpieri, D.E.; Di Tondo, U. Immunohistochemical study of fatty acid synthase in ovarian neoplasms. Oncol. Rep. 2000, 7, 1383–1388. [Google Scholar] [PubMed]
- Tsuji, T.; Yoshinaga, M.; Togami, S.; Douchi, T.; Nagata, Y. Fatty acid synthase expression and clinicopathological findings in endometrial cancer. Acta Obs. Gynecol. Scand. 2004, 83, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, V.; Visca, P.; Botti, C.; Santeusanio, G.; Galati, G.M.; Piccini, V.; Capezzone de Joannon, B.; Di Tondo, U.; Alo, P.L. Fatty acid synthase is a marker of increased risk of recurrence in endometrial carcinoma. Gynecol. Oncol. 2004, 92, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, E.; Miliaras, D.; Meditskou, S.; Grimbizis, G. Immunohistochemical investigation of metabolic markers fatty acid synthase (FASN) and glucose transporter 1 (GLUT1) in normal endometrium, endometrial hyperplasia, and endometrial malignancy. Hippokratia 2017, 21, 169–174. [Google Scholar]
- Pizer, E.S.; Lax, S.F.; Kuhajda, F.P.; Pasternack, G.R.; Kurman, R.J. Fatty acid synthase expression in endometrial carcinoma. Cancer 1998, 83, 528–537. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Kim, H.M.; Koo, J.S. Expression of Lipid Metabolism-Related Proteins in Metastatic Breast Cancer. PLoS ONE 2015, 10, e0137204. [Google Scholar] [CrossRef]
- Zhang, D.; Tai, L.K.; Wong, L.L.; Chiu, L.L.; Sethi, S.K.; Koay, E.S. Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu-positive breast cancer. Mol. Cell. Proteom. 2005, 4, 1686–1696. [Google Scholar] [CrossRef]
- Schug, Z.T.; Peck, B.; Jones, D.T.; Zhang, Q.; Grosskurth, S.; Alam, I.S.; Goodwin, L.M.; Smethurst, E.; Mason, S.; Blyth, K.; et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 2015, 27, 57–71. [Google Scholar] [CrossRef]
- Dupuy, F.; Tabariès, S.; Andrzejewski, S.; Dong, Z.; Blagih, J.; Annis, M.G.; Omeroglu, A.; Gao, D.; Leung, S.; Amir, E.; et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell Metab. 2015, 22, 577–589. [Google Scholar] [CrossRef]
- Rahman, M.T.; Nakayama, K.; Ishikawa, M.; Rahman, M.; Katagiri, H.; Katagiri, A.; Ishibashi, T.; Iida, K.; Miyazaki, K. Fatty Acid Synthase Is a Potential Therapeutic Target in Estrogen Receptor-/Progesterone Receptor-Positive Endometrioid Endometrial Cancer. Oncology 2013, 84, 166–173. [Google Scholar] [CrossRef]
- Alli, P.M.; Pinn, M.L.; Jaffee, E.M.; McFadden, J.M.; Kuhajda, F.P. Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene 2005, 24, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Simpson, P.J.; McFadden, J.M.; Townsend, C.A.; Medghalchi, S.M.; Vadlamudi, A.; Pinn, M.L.; Ronnett, G.V.; Kuhajda, F.P. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res. 2003, 63, 7330–7337. [Google Scholar] [PubMed]
- Kuhajda, F.P.; Jenner, K.; Wood, F.D.; Hennigar, R.A.; Jacobs, L.B.; Dick, J.D.; Pasternack, G.R. Fatty acid synthesis: A potential selective target for antineoplastic therapy. Proc. Natl. Acad. Sci. USA 1994, 91, 6379–6383. [Google Scholar] [CrossRef] [PubMed]
- Pizer, E.S.; Jackisch, C.; Wood, F.D.; Pasternack, G.R.; Davidson, N.E.; Kuhajda, F.P. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res. 1996, 56, 2745–2747. [Google Scholar]
- Vazquez-Martin, A.; Colomer, R.; Brunet, J.; Lupu, R.; Menendez, J.A. Overexpression of fatty acid synthase gene activates HER1/HER2 tyrosine kinase receptors in human breast epithelial cells. Cell Prolif. 2008, 41, 59–85. [Google Scholar] [CrossRef]
- Xia, H.; Lee, K.W.; Chen, J.; Kong, S.N.; Sekar, K.; Deivasigamani, A.; Seshachalam, V.P.; Goh, B.K.P.; Ooi, L.L.; Hui, K.M. Simultaneous silencing of ACSL4 and induction of GADD45B in hepatocellular carcinoma cells amplifies the synergistic therapeutic effect of aspirin and sorafenib. Cell Death Discov. 2017, 3, 17058. [Google Scholar] [CrossRef]
- Belkaid, A.; Ouellette, R.J.; Surette, M.E. 17 beta-estradiol-induced ACSL4 protein expression promotes an invasive phenotype in estrogen receptor positive mammary carcinoma cells. Carcinogenesis 2017, 38, 402–410. [Google Scholar] [CrossRef]
- Padanad, M.S.; Konstantinidou, G.; Venkateswaran, N.; Melegari, M.; Rindhe, S.; Mitsche, M.; Yang, C.; Batten, K.; Huffman, K.E.; Liu, J.; et al. Fatty Acid Oxidation Mediated by Acyl-CoA Synthetase Long Chain 3 Is Required for Mutant KRAS Lung Tumorigenesis. Cell Rep. 2016, 16, 1614–1628. [Google Scholar] [CrossRef]
- Grevengoed, T.J.; Klett, E.L.; Coleman, R.A. Acyl-CoA metabolism and partitioning. Annu. Rev. Nutr. 2014, 34, 1–30. [Google Scholar] [CrossRef]
- Monaco, M.E.; Creighton, C.J.; Lee, P.; Zou, X.; Topham, M.K.; Stafforini, D.M. Expression of Long-chain Fatty Acyl-CoA Synthetase 4 in Breast and Prostate Cancers Is Associated with Sex Steroid Hormone Receptor Negativity. Transl. Oncol. 2010, 3, 91–98. [Google Scholar] [CrossRef]
- Orlando, U.D.; Castillo, A.F.; Medrano, M.A.R.; Solano, A.R.; Maloberti, P.M.; Podesta, E.J. Acyl-CoA synthetase-4 is implicated in drug resistance in breast cancer cell lines involving the regulation of energy-dependent transporter expression. Biochem. Pharmacol. 2019, 159, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.M.; Kim, R.N.; Kwon, M.J.; Oh, E.; Han, J.; Lee, S.K.; Choi, J.S.; Park, S.; Nam, S.J.; Gong, G.Y.; et al. Targeted exome sequencing of Korean triple-negative breast cancer reveals homozygous deletions associated with poor prognosis of adjuvant chemotherapy-treated patients. Oncotarget 2017, 8, 61538–61550. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.J.; Hou, J.; Xu, B.; Cortez, M.; Potma, E.O.; Tromberg, B.J.; Razorenova, O.V. CDCP1 drives triple-negative breast cancer metastasis through reduction of lipid-droplet abundance and stimulation of fatty acid oxidation. Proc. Natl. Acad. Sci. USA 2017, 114, E6556–E6565. [Google Scholar] [CrossRef]
- Schreurs, M.; Kuipers, F.; Van Der Leij, F.R. Regulatory enzymes of mitochondrial β-oxidation as targets for treatment of the metabolic syndrome. Obes. Rev. 2010, 11, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Casals, N.; Zammit, V.; Herrero, L.; Fadó, R.; Rodríguez-Rodríguez, R.; Serra, D. Carnitine palmitoyltransferase 1C: From cognition to cancer. Prog. Lipid Res. 2016, 61, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, K.; Yao, Y.; Reilly, P.T.; Kannan, K.; Kiarash, R.; Mason, J.; Huang, P.; Sawyer, S.K.; Fuerth, B.; Faubert, B.; et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 2011, 25, 1041–1051. [Google Scholar] [CrossRef]
- Harjes, U.; Kalucka, J.; Carmeliet, P. Targeting fatty acid metabolism in cancer and endothelial cells. Crit. Rev. Oncol. Hematol. 2016, 97, 15–21. [Google Scholar] [CrossRef]
- Carracedo, A.; Cantley, L.C.; Pandolfi, P.P. Cancer metabolism: Fatty acid oxidation in the limelight. Nat. Rev. Cancer 2013, 13, 227–232. [Google Scholar] [CrossRef]
- Pike, L.S.; Smift, A.L.; Croteau, N.J.; Ferrick, D.A.; Wu, M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 726–734. [Google Scholar] [CrossRef]
- Houten, S.M.; Wanders, R.J.A. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef]
- Rada, M.; Cha, J.; Sage, J.; Zhou, B.; Yang, W.; Orsulic, S.; Cheon, D.-J. Abstract A16: COL11A1 confers cisplatin resistance through fatty acid oxidation in ovarian cancer cells. Clin. Cancer Res. 2018, 24, A16. [Google Scholar] [CrossRef]
- Orlando, U.D.; Castillo, A.F.; Dattilo, M.A.; Solano, A.R.; Maloberti, P.M.; Podesta, E.J. Acyl-CoA synthetase-4, a new regulator of mTOR and a potential therapeutic target for enhanced estrogen receptor function in receptor-positive and -negative breast cancer. Oncotarget 2015, 6, 42632–42650. [Google Scholar] [CrossRef] [PubMed]
- Santolla, M.F.; Lappano, R.; De Marco, P.; Pupo, M.; Vivacqua, A.; Sisci, D.; Abonante, S.; Iacopetta, D.; Cappello, A.R.; Dolce, V.; et al. G Protein-coupled Estrogen Receptor Mediates the Up-regulation of Fatty Acid Synthase Induced by 17 beta-Estradiol in Cancer Cells and Cancer-associated Fibroblasts. J. Biol. Chem. 2012, 287, 43234–43245. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Lupu, R. Fatty acid synthase regulates estrogen receptor-α signaling in breast cancer cells. Oncogenesis 2017, 6, e299. [Google Scholar] [CrossRef]
- Pizer, E.S.; Lax, S.F.; Kuhajda, F.P.; Pasternack, G.R.; Kurman, R.J. Fatty acid synthase expression in endometrial carcinoma: Correlation with cell proliferation and hormone receptors. Cancer 1998, 83, 528–537. [Google Scholar]
- Lee, H.-R.; Kim, T.-H.; Choi, K.-C. Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Lab. Anim. Res. 2012, 28, 71–76. [Google Scholar] [CrossRef]
- Faulds, M.H.; Zhao, C.; Dahlman-Wright, K.; Gustafsson, J.-Å. The diversity of sex steroid action: Regulation of metabolism by estrogen signaling. J. Endocrinol. 2012, 212, 3–12. [Google Scholar] [CrossRef]
- Saha Roy, S.; Vadlamudi, R.K. Role of Estrogen Receptor Signaling in Breast Cancer Metastasis. Int. J. Breast Cancer 2012, 2012, 654698. [Google Scholar] [CrossRef]
- Bicker, A.; Nauth, T.; Gerst, D.; Aboouf, M.A.; Fandrey, J.; Kristiansen, G.; Gorr, T.A.; Hankeln, T. The role of myoglobin in epithelial cancers: Insights from transcriptomics. Int. J. Mol. Med. 2020, 45, 385–400. [Google Scholar] [CrossRef]
- Bicker, A.; Brahmer, A.M.; Meller, S.; Kristiansen, G.; Gorr, T.A.; Hankeln, T. The Distinct Gene Regulatory Network of Myoglobin in Prostate and Breast Cancer. PLoS ONE 2015, 10, e0142662. [Google Scholar] [CrossRef][Green Version]
- Sriram, R.; Kreutzer, U.; Shih, L.; Jue, T. Interaction of fatty acid with myoglobin. FEBS Lett. 2008, 582, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yang, X.; Zhang, K.; Liu, Z.; Shao, Z.; Song, C.; Wang, X.; Li, Z. Estrogen receptor α/prolactin receptor bilateral crosstalk promotes bromocriptine resistance in prolactinomas. Int. J. Med. Sci. 2020, 17, 3174–3189. [Google Scholar] [CrossRef] [PubMed]
- Barcus, C.E.; Holt, E.C.; Keely, P.J.; Eliceiri, K.W.; Schuler, L.A. Dense Collagen-I Matrices Enhance Pro-Tumorigenic Estrogen-Prolactin Crosstalk in MCF-7 and T47D Breast Cancer Cells. PLoS ONE 2015, 10, e0116891. [Google Scholar] [CrossRef] [PubMed]
- Sethi, B.K.; Chanukya, G.V.; Nagesh, V.S. Prolactin and cancer: Has the orphan finally found a home? Indian J. Endocrinol. Metab. 2012, 16, S195–S198. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Tsai-Morris, C.H.; Dufau, M.L. A novel estradiol/estrogen receptor alpha-dependent transcriptional mechanism controls expression of the human prolactin receptor. J. Biol. Chem. 2006, 281, 18825–18836. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.; Sairam, M.R. Novel genes of visceral adiposity: Identification of mouse and human mesenteric estrogen-dependent adipose (MEDA)-4 gene and its adipogenic function. Endocrinology 2012, 153, 2665–2676. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Wu, Q.; Tu, Y.; Wang, C.; Yu, X.; Li, B.; Wang, Z.; Sun, S.; Sun, S. MEDAG enhances breast cancer progression and reduces epirubicin sensitivity through the AKT/AMPK/mTOR pathway. Cell Death Dis. 2021, 12, 97. [Google Scholar] [CrossRef]
- Orlando, U.D.; Garona, J.; Ripoll, G.V.; Maloberti, P.M.; Solano, A.R.; Avagnina, A.; Gomez, D.E.; Alonso, D.F.; Podesta, E.J. The Functional Interaction between Acyl-CoA Synthetase 4, 5-Lipooxygenase and Cyclooxygenase-2 Controls Tumor Growth: A Novel Therapeutic Target. PLoS ONE 2012, 7, 14. [Google Scholar] [CrossRef]
- Yang, X.; Belosay, A.; Du, M.; Fan, T.M.; Turner, R.T.; Iwaniec, U.T.; Helferich, W.G. Estradiol increases ER-negative breast cancer metastasis in an experimental model. Clin. Exp. Metastasis 2013, 30, 711–721. [Google Scholar] [CrossRef]
- James, R.E.; Lukanova, A.; Dossus, L.; Becker, S.; Rinaldi, S.; Tjønneland, A.; Olsen, A.; Overvad, K.; Mesrine, S.; Engel, P.; et al. Postmenopausal Serum Sex Steroids and Risk of Hormone Receptor–Positive and -Negative Breast Cancer: A Nested Case–Control Study. Cancer Prev. Res. 2011, 4, 1626–1635. [Google Scholar] [CrossRef]
- Zhu, L.; Pollard, J.W. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 15847–15851. [Google Scholar] [CrossRef] [PubMed]
- Koobotse, M.; Holly, J.; Perks, C. Elucidating the novel BRCA1 function as a non-genomic metabolic restraInt. in ER-positive breast cancer cell lines. Oncotarget 2018, 9, 33562–33576. [Google Scholar] [CrossRef] [PubMed]
- Kasznicki, J.; Sliwinska, A.; Drzewoski, J. Metformin in cancer prevention and therapy. Ann. Transl. Med. 2014, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Monaco, M.E. Fatty acid metabolism in breast cancer subtypes. Oncotarget 2017, 8, 29487–29500. [Google Scholar] [CrossRef]
- Yamashita, Y.; Nishiumi, S.; Kono, S.; Takao, S.; Azuma, T.; Yoshida, M. Differences in elongation of very long chain fatty acids and fatty acid metabolism between triple-negative and hormone receptor-positive breast cancer. BMC Cancer 2017, 17, 589. [Google Scholar] [CrossRef]
- Koussounadis, A.; Langdon, S.P.; Um, I.H.; Harrison, D.J.; Smith, V.A. Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci. Rep. 2015, 5, 10775. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Podufaly, A.; Dillon, P.F. Estradiol Binds to Insulin and Insulin Receptor Decreasing Insulin Binding in vitro. Front. Endocrinol. 2014, 5, 118. [Google Scholar] [CrossRef]
- Huang, L.-H.; Chung, H.-Y.; Su, H.-M. Docosahexaenoic acid reduces sterol regulatory element binding protein-1 and fatty acid synthase expression and inhibits cell proliferation by inhibiting pAkt signaling in a human breast cancer MCF-7 cell line. BMC Cancer 2017, 17, 890. [Google Scholar] [CrossRef]
- Cleland, W.H.; Mendelson, C.R.; Simpson, E.R. Effects of aging and obesity on aromatase activity of human adipose cells. J. Clin. Endocrinol. Metab. 1985, 60, 174–177. [Google Scholar] [CrossRef]
- Cauley, J.A.; Gutai, J.P.; Kuller, L.H.; LeDonne, D.; Powell, J.G. The epidemiology of serum sex hormones in postmenopausal women. Am. J. Epidemiol. 1989, 129, 1120–1131. [Google Scholar] [CrossRef]
- Hautanen, A. Synthesis and regulation of sex hormone-binding globulin in obesity. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24, S64–S70. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Ellingjord-Dale, M.; Christakoudi, S.; Weiderpass, E.; Panico, S.; Dossus, L.; Olsen, A.; Tjønneland, A.; Kaaks, R.; Schulze, M.B.; Masala, G.; et al. Long-term weight change and risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Int. J. Epidemiol. 2021, 50, 1914–1926. [Google Scholar] [CrossRef] [PubMed]
- Catherine, M.O.; Christina, M.N.; David, C.W.; Roberta, N.; Celeste Leigh, P.; Malcolm, C.P.; Mary Anne, R.; Kathryn, L.T.; Anna, H.W.; Harvey, A.R.; et al. Obesity and risk of ovarian cancer subtypes: Evidence from the Ovarian Cancer Association Consortium. Endocr.-Relat. Cancer 2013, 20, 251–262. [Google Scholar] [CrossRef]
- Nagle, C.M.; Dixon, S.C.; Jensen, A.; Kjaer, S.K.; Modugno, F.; de Fazio, A.; Fereday, S.; Hung, J.; Johnatty, S.E.; Australian Ovarian Cancer Study, G.; et al. Obesity and survival among women with ovarian cancer: Results from the Ovarian Cancer Association Consortium. Br. J. Cancer 2015, 113, 817–826. [Google Scholar] [CrossRef]
- Zhou, Y.; Irwin, M.L.; Risch, H.A. Pre- and post-diagnosis body mass index, weight change, and ovarian cancer mortality. Gynecol. Oncol. 2011, 120, 209–213. [Google Scholar] [CrossRef]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D.; Million Women Study, C. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 2007, 335, 1134. [Google Scholar] [CrossRef]
- Secord, A.A.; Hasselblad, V.; Von Gruenigen, V.E.; Gehrig, P.A.; Modesitt, S.C.; Bae-Jump, V.; Havrilesky, L.J. Body mass index and mortality in endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2016, 140, 184–190. [Google Scholar] [CrossRef]
- Trentham-Dietz, A.; Nichols, H.; Hampton, J.; Newcomb, P. Weight change and risk of endometrial cancer. Int. J. Epidemiol. 2005, 35, 151–158. [Google Scholar] [CrossRef]
- Lee, J.K.; So, K.A.; Piyathilake, C.J.; Kim, M.K. Mild Obesity, Physical Activity, Calorie Intake, and the Risks of Cervical Intraepithelial Neoplasia and Cervical Cancer. PLoS ONE 2013, 8, e66555. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Fetterman, B.; Cheung, L.C.; Wentzensen, N.; Gage, J.C.; Katki, H.A.; Befano, B.; Demarco, M.; Schussler, J.; Kinney, W.K.; et al. Epidemiologic Evidence That Excess Body Weight Increases Risk of Cervical Cancer by Decreased Detection of Precancer. J. Clin. Oncol. 2018, 36, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Green, L.E.; Dinh, T.A.; Smith, R.A. An estrogen model: The relationship between body mass index, menopausal status, estrogen replacement therapy, and breast cancer risk. Comput. Math. Methods Med. 2012, 2012, 792375. [Google Scholar] [CrossRef] [PubMed]
- Vaysse, C.; Lømo, J.; Garred, Ø.; Fjeldheim, F.; Lofteroed, T.; Schlichting, E.; McTiernan, A.; Frydenberg, H.; Husøy, A.; Lundgren, S.; et al. Inflammation of mammary adipose tissue occurs in overweight and obese patients exhibiting early-stage breast cancer. NPJ Breast Cancer 2017, 3, 19. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Panahi, G.; Pasalar, P.; Zare, M.; Rizzuto, R.; Meshkani, R. High glucose induces inflammatory responses in HepG2 cells via the oxidative stress-mediated activation of NF-κB, and MAPK pathways in HepG2 cells. Arch. Physiol. Biochem. 2018, 124, 468–474. [Google Scholar] [CrossRef]
- Zhao, Y.; Nichols, J.E.; Valdez, R.; Mendelson, C.R.; Simpson, E.R. Tumor necrosis factor-alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol. Endocrinol. 1996, 10, 1350–1357. [Google Scholar] [CrossRef]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef]
- Mauro, L.; Naimo, G.D.; Gelsomino, L.; Malivindi, R.; Bruno, L.; Pellegrino, M.; Tarallo, R.; Memoli, D.; Weisz, A.; Panno, M.L.; et al. Uncoupling effects of estrogen receptor α on LKB1/AMPK interaction upon adiponectin exposure in breast cancer. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 4343–4355. [Google Scholar] [CrossRef]
- Morad, V.; Abrahamsson, A.; Dabrosin, C. Estradiol Affects Extracellular Leptin:Adiponectin Ratio in Human Breast Tissue in Vivo. J. Clin. Endocrinol. Metab. 2014, 99, 3460–3467. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, H.T.; Kim, Y.J. The role of estrogen in adipose tissue metabolism: Insights into glucose homeostasis regulation. Endocr J. 2014, 61, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Chapter Two - Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambrigde, MA, USA, 2016; pp. 35–58. [Google Scholar]
- Dai, Q.; Gao, Y.-T.; Shu, X.-O.; Yang, G.; Milne, G.; Cai, Q.; Wen, W.; Rothman, N.; Cai, H.; Li, H.; et al. Oxidative stress, obesity, and breast cancer risk: Results from the Shanghai Women's Health Study. J. Clin. Oncol 2009, 27, 2482–2488. [Google Scholar] [CrossRef] [PubMed]
- Sateesh, R.; Rao Bitla, A.; Budugu, S.; Mutheeswariah, Y.; Narendra, H.; Phaneedra, B.; Lakshmi, A. Oxidative stress in relation to obesity in breast cancer. Indian J. Cancer 2019, 56, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Pandey, M.K.; Tyagi, A.K.; Deb, L. Oxidative Stress and Cancer: Advances and Challenges. Oxidative Med. Cell. Longev. 2016, 2016, 5010423. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, C.; Gramignano, G.; Floris, C.; Murenu, G.; Sollai, G.; Macciò, A. Role of inflammation and oxidative stress in post-menopausal oestrogen-dependent breast cancer. J. Cell Mol. Med. 2014, 18, 2519–2529. [Google Scholar] [CrossRef] [PubMed]
- Dierks, T.; Lecca, M.R.; Schlotterhose, P.; Schmidt, B.; von Figura, K. Sequence determinants directing conversion of cysteine to formylglycine in eukaryotic sulfatases. EMBO J. 1999, 18, 2084–2091. [Google Scholar] [CrossRef]
- Maiti, S.; Nazmeen, A. Impaired redox regulation of estrogen metabolizing proteins is important determinant of human breast cancers. Cancer Cell Int. 2019, 19, 111. [Google Scholar] [CrossRef]
- Castelli, S.; De Falco, P.; Ciccarone, F.; Desideri, E.; Ciriolo, M.R. Lipid Catabolism and ROS in Cancer: A Bidirectional Liaison. Cancers 2021, 13, 5484. [Google Scholar] [CrossRef]
- Gupta, R.K.; Patel, A.K.; Kumari, R.; Chugh, S.; Shrivastav, C.; Mehra, S.; Sharma, A.N. Interactions between oxidative stress, lipid profile and antioxidants in breast cancer: A case control study. Asian Pac. J. Cancer Prev 2012, 13, 6295–6298. [Google Scholar] [CrossRef]
- Jeanne, A.; Schneider, C.; Martiny, L.; Dedieu, S. Original insights on thrombospondin-1-related antireceptor strategies in cancer. Front. Pharmacol. 2015, 6, 252. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, J.; Henkin, J.; Lawler, J.; Moorehead, R.; Petrik, J. ABT-510 induces tumor cell apoptosis and inhibits ovarian tumor growth in an orthotopic, syngeneic model of epithelial ovarian cancer. Mol. Cancer Ther. 2009, 8, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.E.; Greenaway, J.; Henkin, J.; Moorehead, R.A.; Petrik, J. The thrombospondin-1 mimetic ABT-510 increases the uptake and effectiveness of cisplatin and paclitaxel in a mouse model of epithelial ovarian cancer. Neoplasia 2010, 12, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ebbinghaus, S.; Hussain, M.; Tannir, N.; Gordon, M.; Desai, A.A.; Knight, R.A.; Humerickhouse, R.A.; Qian, J.; Gordon, G.B.; Figlin, R. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 6689–6695. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.H.; Rowinsky, E.K.; Mendelson, D.; Humerickhouse, R.A.; Knight, R.A.; Qian, J.; Carr, R.A.; Gordon, G.B.; Demetri, G.D. Randomized, phase II study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced soft tissue sarcoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 5583–5588. [Google Scholar] [CrossRef]
- Markovic, S.N.; Suman, V.J.; Rao, R.A.; Ingle, J.N.; Kaur, J.S.; Erickson, L.A.; Pitot, H.C.; Croghan, G.A.; McWilliams, R.R.; Merchan, J.; et al. A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma. Am. J. Clin. Oncol. 2007, 30, 303–309. [Google Scholar] [CrossRef]
- Rusk, A.; McKeegan, E.; Haviv, F.; Majest, S.; Henkin, J.; Khanna, C. Preclinical evaluation of antiangiogenic thrombospondin-1 peptide mimetics, ABT-526 and ABT-510, in companion dogs with naturally occurring cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 7444–7455. [Google Scholar] [CrossRef]
- Campbell, N.; Greenaway, J.; Henkin, J.; Petrik, J. ABT-898 induces tumor regression and prolongs survival in a mouse model of epithelial ovarian cancer. Mol. Cancer Ther. 2011, 10, 1876–1885. [Google Scholar] [CrossRef][Green Version]
- Isenberg, J.; Yifeng, J.; Fukuyama, J.; Switzer, C.; Wink, D.; Roberts, D. Thrombospondin-1 Inhibits Nitric Oxide Signaling via CD36 by Inhibiting Myristic Acid Uptake. J. Biol. Chem. 2007, 282, 15404–15415. [Google Scholar] [CrossRef]
- Hanai, J.I.; Doro, N.; Seth, P.; Sukhatme, V.P. ATP citrate lyase knockdown impacts cancer stem cells in vitro. Cell Death Dis. 2013, 4, e696. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Bukowska-Strakova, K.; Majka, M. Metformin and caffeic acid regulate metabolic reprogramming in human cervical carcinoma SiHa/HTB-35 cells and augment anticancer activity of Cisplatin via cell cycle regulation. Food Chem. Toxicol. 2017, 106, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zhong, Z.; Wang, S.; Suo, Z.; Yang, X.; Hu, X.; Wang, Y. Berberine Regulated Lipid Metabolism in the Presence of C75, Compound C, and TOFA in Breast Cancer Cell Line MCF-7. Evid. Based Compl. Altern. Med. 2015, 2015, 396035. [Google Scholar] [CrossRef] [PubMed]
- Schcolnik-Cabrera, A.; Chavez-Blanco, A.; Dominguez-Gomez, G.; Taja-Chayeb, L.; Morales-Barcenas, R.; Trejo-Becerril, C.; Perez-Cardenas, E.; Gonzalez-Fierro, A.; Duenas-Gonzalez, A. Orlistat as a FASN inhibitor and multitargeted agent for cancer therapy. Expert Opin. Investig. Drugs 2018, 27, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Umekita, Y.; Guo, J.; Kokontis, J.M.; Hiipakka, R.A. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 1995, 96, 239–243. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Singh, A.K.; Sharma, A.; Warren, J.; Gaddipati, J.P.; Maheshwari, R.K. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007, 245, 232–241. [Google Scholar] [CrossRef]
- Alwarawrah, Y.; Hughes, P.; Loiselle, D.; Carlson, D.A.; Darr, D.B.; Jordan, J.L.; Xiong, J.; Hunter, L.M.; Dubois, L.G.; Thompson, J.W.; et al. Fasnall, a Selective FASN Inhibitor, Shows Potent Anti-tumor Activity in the MMTV-Neu Model of HER2(+) Breast Cancer. Cell Chem. Biol. 2016, 23, 678–688. [Google Scholar] [CrossRef]
- NCT03179904. FASN Inhibitor TVB-2640, Paclitaxel, and Trastuzumab in Treating Patients with HER2 Positive Advanced Breast Cancer. Available online: https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/v?id=NCI-2017-00944&r=1 (accessed on 31 December 2021).
- Ventura, R.; Mordec, K.; Waszczuk, J.; Wang, Z.; Lai, J.; Fridlib, M.; Buckley, D.; Kemble, G.; Heuer, T.S. Inhibition of de novo Palmitate Synthesis by Fatty Acid Synthase Induces Apoptosis in Tumor Cells by Remodeling Cell Membranes, Inhibiting Signaling Pathways, and Reprogramming Gene Expression. EBioMedicine 2015, 2, 808–824. [Google Scholar] [CrossRef]
- Zhou, W.; Han, W.F.; Landree, L.E.; Thupari, J.N.; Pinn, M.L.; Bililign, T.; Kim, E.K.; Vadlamudi, A.; Medghalchi, S.M.; El Meskini, R.; et al. Fatty Acid Synthase Inhibition Activates AMP-Activated Protein Kinase in SKOV3 Human Ovarian Cancer Cells. Cancer Res. 2007, 67, 2964–2971. [Google Scholar] [CrossRef]
- Dattilo, M.A.; Benzo, Y.; Herrera, L.M.; Prada, J.G.; Castillo, A.F.; Orlando, U.D.; Podesta, E.J.; Maloberti, P.M. Regulatory mechanisms leading to differential Acyl-CoA synthetase 4 expression in breast cancer cells. Sci. Rep. 2019, 9, 10324. [Google Scholar] [CrossRef]
- Reis, L.M.D.; Adamoski, D.; Ornitz Oliveira Souza, R.; Rodrigues Ascencao, C.F.; Sousa de Oliveira, K.R.; Correa-da-Silva, F.; Malta de Sa Patroni, F.; Meira Dias, M.; Consonni, S.R.; Mendes de Moraes-Vieira, P.M.; et al. Dual inhibition of glutaminase and carnitine palmitoyltransferase decreases growth and migration of glutaminase inhibition-resistant triple-negative breast cancer cells. J. Biol. Chem. 2019, 294, 9342–9357. [Google Scholar] [CrossRef]
- Ren, X.R.; Wang, J.; Osada, T.; Mook, R.A., Jr.; Morse, M.A.; Barak, L.S.; Lyerly, H.K.; Chen, W. Perhexiline promotes HER3 ablation through receptor internalization and inhibits tumor growth. Breast Cancer Res. 2015, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Baleka Mutombo, A.; Tozin, R.; Kanyiki, H.; Van Geertruyden, J.P.; Jacquemyn, Y. Impact of antiviral AV2 in the topical treatment of HPV-associated lesions of the cervix: Results of a phase III randomized placebo-controlled trial. Contemp. Clin. Trials Commun. 2019, 15, 100377. [Google Scholar] [CrossRef]
- Granchi, C. ATP citrate lyase (ACLY) inhibitors: An anti-cancer strategy at the crossroads of glucose and lipid metabolism. Eur. J. Med. Chem. 2018, 157, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, A.B.; Ak, H.; Atay, S.; Aydin, H.H. Targeting mitochondrial citrate transport in breast cancer cell lines. Anti-Cancer Agents Med. Chem. 2015, 15, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Hatzivassiliou, G.; Zhao, F.; Bauer, D.E.; Andreadis, C.; Shaw, A.N.; Dhanak, D.; Hingorani, S.R.; Tuveson, D.A.; Thompson, C.B. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 2005, 8, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Doghish, A.S.; Elsadek, E.M.E.; Salama, S.A.; Mariee, A.D. Hydroxycitric acid potentiates the cytotoxic effect of tamoxifen in MCF-7 breast cancer cells through inhibition of ATP citrate lyase. Steroids 2020, 160, 108656. [Google Scholar] [CrossRef] [PubMed]
- Lorizio, W.; Wu, A.H.B.; Beattie, M.S.; Rugo, H.; Tchu, S.; Kerlikowske, K.; Ziv, E. Clinical and biomarker predictors of side effects from tamoxifen. Breast Cancer Res. Treat. 2012, 132, 1107–1118. [Google Scholar] [CrossRef]
- Sun, H.; Wang, F.; Huang, Y.; Wang, J.; Zhang, L.; Shen, Y.; Lin, C.; Guo, P. Targeted inhibition of ACLY expression to reverse the resistance of sorafenib in hepatocellular carcinoma. J. Cancer 2022, 13, 951–964. [Google Scholar] [CrossRef]
- Wei, X.; Shi, J.; Lin, Q.; Ma, X.; Pang, Y.; Mao, H.; Li, R.; Lu, W.; Wang, Y.; Liu, P. Targeting ACLY Attenuates Tumor Growth and Acquired Cisplatin Resistance in Ovarian Cancer by Inhibiting the PI3K–AKT Pathway and Activating the AMPK–ROS Pathway. Front. Oncol. 2021, 11, 642229. [Google Scholar] [CrossRef]
- Stiede, K.; Miao, W.; Blanchette, H.S.; Beysen, C.; Harriman, G.; Harwood, H.J., Jr.; Kelley, H.; Kapeller, R.; Schmalbach, T.; Westlin, W.F. Acetyl-coenzyme A carboxylase inhibition reduces de novo lipogenesis in overweight male subjects: A randomized, double-blind, crossover study. Hepatology 2017, 66, 324–334. [Google Scholar] [CrossRef]
- Jeon, S.M.; Chandel, N.S.; Hay, N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012, 485, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Sikora, M.J.; Levine, K.M.; Tasdemir, N.; Riggins, R.B.; Wendell, S.G.; Van Houten, B.; Oesterreich, S. Key regulators of lipid metabolism drive endocrine resistance in invasive lobular breast cancer. Breast Cancer Res. 2018, 20, 106. [Google Scholar] [CrossRef] [PubMed]
- Boegel, S.; Löwer, M.; Bukur, T.; Sahin, U.; Castle, J.C. A catalog of HLA type, HLA expression, and neo-epitope candidates in human cancer cell lines. Oncoimmunology 2014, 3, e954893. [Google Scholar] [CrossRef] [PubMed]
- Kridel, S.J.; Lowther, W.T.; Pemble Iv, C.W. Fatty acid synthase inhibitors: New directions for oncology. Expert Opin. Investig. Drugs 2007, 16, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.S.; Dixon, A.F.; Dixon, J.B. Obesity management: Update on orlistat. Vasc. Health Risk Manag. 2007, 3, 817–821. [Google Scholar]
- Syed-Abdul, M.M.; Parks, E.J.; Gaballah, A.H.; Bingham, K.; Hammoud, G.M.; Kemble, G.; Buckley, D.; McCulloch, W.; Manrique, C.M. First-in-class fatty acid synthase inhibitor TVB-2640 reduces hepatic de novo lipogenesis in males with metabolic abnormalities. Hepatology 2019. [Google Scholar] [CrossRef]
- Menendez, J.A.; Oza, B.P.; Atlas, E.; Verma, V.A.; Mehmi, I.; Lupu, R. Inhibition of tumor-associated fatty acid synthase activity antagonizes estradiol- and tamoxifen-induced agonist transactivation of estrogen receptor (ER) in human endometrial adenocarcinoma cells. Oncogene 2004, 23, 4945–4958. [Google Scholar] [CrossRef]
- Lupu, R.; Menendez, J.A. Targeting Fatty Acid Synthase in Breast and Endometrial Cancer: An Alternative to Selective Estrogen Receptor Modulators? Endocrinology 2006, 147, 4056–4066. [Google Scholar] [CrossRef]
- Cairns, J.; Ingle, J.N.; Kalari, K.R.; Goetz, M.P.; Weinshilboum, R.M.; Gao, H.; Li, H.; Bari, M.G.; Wang, L. Anastrozole Regulates Fatty Acid Synthase in Breast Cancer. Mol. Cancer Ther. 2022, 21, 206–216. [Google Scholar] [CrossRef]
- Kim, J.H.; Lewin, T.M.; Coleman, R.A. Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J. Biol. Chem. 2001, 276, 24667–24673. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Feng, J.; Liu, Y.; Wang, T.; Zhao, M.; Ye, L.; Zhang, X. Aspirin suppresses the abnormal lipid metabolism in liver cancer cells via disrupting an NFkappaB-ACSL1 signaling. Biochem. Biophys. Res. Commun. 2017, 486, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.K.F.; Ho, J.M.W.; Chan, F.C.H.; Sung, J.J.Y. Long-term use of low-dose aspirin for cancer prevention: A 10-year population cohort study in Hong Kong. Int. J. Cancer 2019, 145, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Eskiocak, B.; Ali, A.; White, M.A. The estrogen-related receptor α inverse agonist XCT 790 is a nanomolar mitochondrial uncoupler. Biochemistry 2014, 53, 4839–4846. [Google Scholar] [CrossRef]
- Yao, C.H.; Liu, G.Y.; Wang, R.; Moon, S.H.; Gross, R.W.; Patti, G.J. Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of beta-oxidation. PLoS Biol. 2018, 16, e2003782. [Google Scholar] [CrossRef] [PubMed]
- Gugiatti, E.; Tenca, C.; Ravera, S.; Fabbi, M.; Ghiotto, F.; Mazzarello, A.N.; Bagnara, D.; Reverberi, D.; Zarcone, D.; Cutrona, G.; et al. A reversible carnitine palmitoyltransferase (CPT1) inhibitor offsets the proliferation of chronic lymphocytic leukemia cells. Haematologica 2018, 103, e531–e536. [Google Scholar] [CrossRef]
- Duan, L.; Calhoun, S.; Shim, D.; Perez, R.E.; Blatter, L.A.; Maki, C.G. Fatty acid oxidation and autophagy promote endoxifen resistance and counter the effect of AKT inhibition in ER-positive breast cancer cells. J. Mol. Cell Biol 2021, 13, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M. Omega-3 fatty acids and cancers: A systematic update review of epidemiological studies. Br. J. Nutr. 2012, 107, S228–S239. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Gao, H.-F.; Hou, A.-J.; Zhou, Y.-H. Effect of omega-3 fatty acid supplementation on cancer incidence, non-vascular death, and total mortality: A meta-analysis of randomized controlled trials. BMC Public Health 2014, 14, 204. [Google Scholar] [CrossRef]
- Laviano, A.; Rianda, S.; Molfino, A.; Fanelli, F.R. Omega-3 fatty acids in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16. [Google Scholar] [CrossRef]
- Echeverría, F.; Valenzuela, R.; Espinosa, A.; Bustamante, A.; Álvarez, D.; Gonzalez-Mañan, D.; Ortiz, M.; Soto-Alarcon, S.A.; Videla, L.A. Reduction of high-fat diet-induced liver proinflammatory state by eicosapentaenoic acid plus hydroxytyrosol supplementation: Involvement of resolvins RvE1/2 and RvD1/2. J. Nutr. Biochem 2019, 63, 35–43. [Google Scholar] [CrossRef]
- Edwards, I.J.; O'Flaherty, J.T. Omega-3 Fatty Acids and PPARgamma in Cancer. PPAR Res. 2008, 2008, 358052. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.; Thorpe, G.; Winstanley, L.; Abdelhamid, A.S.; Hooper, L.; Abdelhamid, A.; Ajabnoor, S.; Alabdulghafoor, F.; Alkhudairy, L.; Biswas, P.; et al. Omega-3, omega-6 and total dietary polyunsaturated fat on cancer incidence: Systematic review and meta-analysis of randomised trials. Br. J. Cancer 2020, 122, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Ochiai, A.; Boku, N.; Ohtsu, A.; Tahara, M.; Yoshida, S.; Okabe, H.; Takechi, T.; Fukushima, M. Discrepancies between the gene expression, protein expression, and enzymatic activity of thymidylate synthase and dihydropyrimidine dehydrogenase in human gastrointestinal cancers and adjacent normal mucosa. Int. J. Oncol. 2001, 18, 705–713. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.-J.; Jang, H. A New View of Pathway-Driven Drug Resistance in Tumor Proliferation. Trends Pharm. Sci. 2017, 38, 427–437. [Google Scholar] [CrossRef]

| Enzyme | Cancer Type | Expression Level | Cancer-Associated Phenotype | Selected References |
|---|---|---|---|---|
| CD36 | Breast | ↑ | Increased proliferation Increased migration | [34] |
| Ovarian | ↑ | Enhanced metastasis | [31] | |
| Cervical | ↑ | Enhanced metastasis | [35] | |
| Endometrial | N/A | N/A | N/A | |
| ACLY | Breast | ↑ | Enhanced metastasis | [40] |
| Ovarian | ↑ | Increased proliferation | [41] | |
| Cervical | ↑ | Enhanced metastasis | [42] | |
| Endometrial | ↑ | N/A | [43] | |
| ACC | Breast | ↑ | Increased proliferation | [44] |
| Ovarian | ↑ | Enhanced tumour growth | [45] | |
| Cervical | N/A | N/A | N/A | |
| Endometrial | ↑ | N/A | [43] | |
| FASN | Breast | HER2+-↑ Luminal A, TNBC-↓ | Enhanced metastasis | [46,47] |
| Ovarian | ↑ | Increased tumour growth | [48,49,50,51] | |
| Cervical | ↑ | N/A | [52,53] | |
| Endometrial | ↑ | N/A | [43] | |
| ACS | Breast | QNBC-ACSL4 ↑, ER (-)-ACSL1↑, ACSL3 ↑, ACSL4 ↑, ACSL5 ↑ | Increased migration Increased invasion | [54,55,56,57,58] |
| Ovarian | ASCL3 ↓, ASCL 5↓ | Increased proliferation | [58] | |
| Cervical | N/A | N/A | N/A | |
| Endometrial | ACS5 ↓ | N/A | [59] | |
| CPTI | Breast | ↑ | Enhanced metastasis | [60,61,62] |
| Ovarian | ↑ | Increased proliferation | [63] | |
| Cervical | N/A | N/A | N/A | |
| Endometrial | N/A | N/A | N/A |
| Target Protein | Intervention | Cancer Type | Preclinical Model | Clinical Trial | References |
|---|---|---|---|---|---|
| CD36 | ABT-526 | Breast | Breast cancer-bearing dogs | - | [188] |
| ABT898 | Ovarian | Xenografts | - | [189] | |
| ACLY | Hydroxycitrate | Breast | In Vitro | - | [191] |
| Metformin | Cervical | In Vitro | - | [192] | |
| ACC | TOFA | Ovarian, Breast | Xenografts | - | [45,193] |
| FASN | Orlistat | Breast, Ovarian | Xenografts | - | [194] |
| Rigallocatechin Gallate | Breast | Xenografts | - | [195,196] | |
| Fasnall | Breast | Xenografts | - | [197] | |
| TVB-2640 | Breast | - | Phase II | [198] | |
| TVB-3166 | Ovarian | Xenografts | - | [199] | |
| C93 | Ovarian | Xenografts | - | [200] | |
| DHA Supplementation | Breast | In Vitro | - | [148] | |
| ACS | Triacsin C | Breast | In Vitro | - | [111,201] |
| CPTI | Etomoxir | Breast | Xenografts | - | [202] |
| Perhexiline | Breast | Xenografts | - | [203] | |
| Eugenol | Breast | In Vitro | - | [44] | |
| Cervical | - | Phase III | [204] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozihim, A.K.; Chung, I.; Said, N.A.B.M.; Jamil, A.H.A. Reprogramming of Fatty Acid Metabolism in Gynaecological Cancers: Is There a Role for Oestradiol? Metabolites 2022, 12, 350. https://doi.org/10.3390/metabo12040350
Mozihim AK, Chung I, Said NABM, Jamil AHA. Reprogramming of Fatty Acid Metabolism in Gynaecological Cancers: Is There a Role for Oestradiol? Metabolites. 2022; 12(4):350. https://doi.org/10.3390/metabo12040350
Chicago/Turabian StyleMozihim, Azilleo Kristo, Ivy Chung, Nur Akmarina B. M. Said, and Amira Hajirah Abd Jamil. 2022. "Reprogramming of Fatty Acid Metabolism in Gynaecological Cancers: Is There a Role for Oestradiol?" Metabolites 12, no. 4: 350. https://doi.org/10.3390/metabo12040350
APA StyleMozihim, A. K., Chung, I., Said, N. A. B. M., & Jamil, A. H. A. (2022). Reprogramming of Fatty Acid Metabolism in Gynaecological Cancers: Is There a Role for Oestradiol? Metabolites, 12(4), 350. https://doi.org/10.3390/metabo12040350






