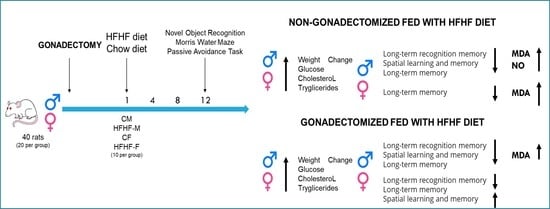
High Fructose and High Fat Diet Impair Different Types of Memory through Oxidative Stress in a Sex- and Hormone-Dependent Manner
Abstract
1. Introduction
2. Results
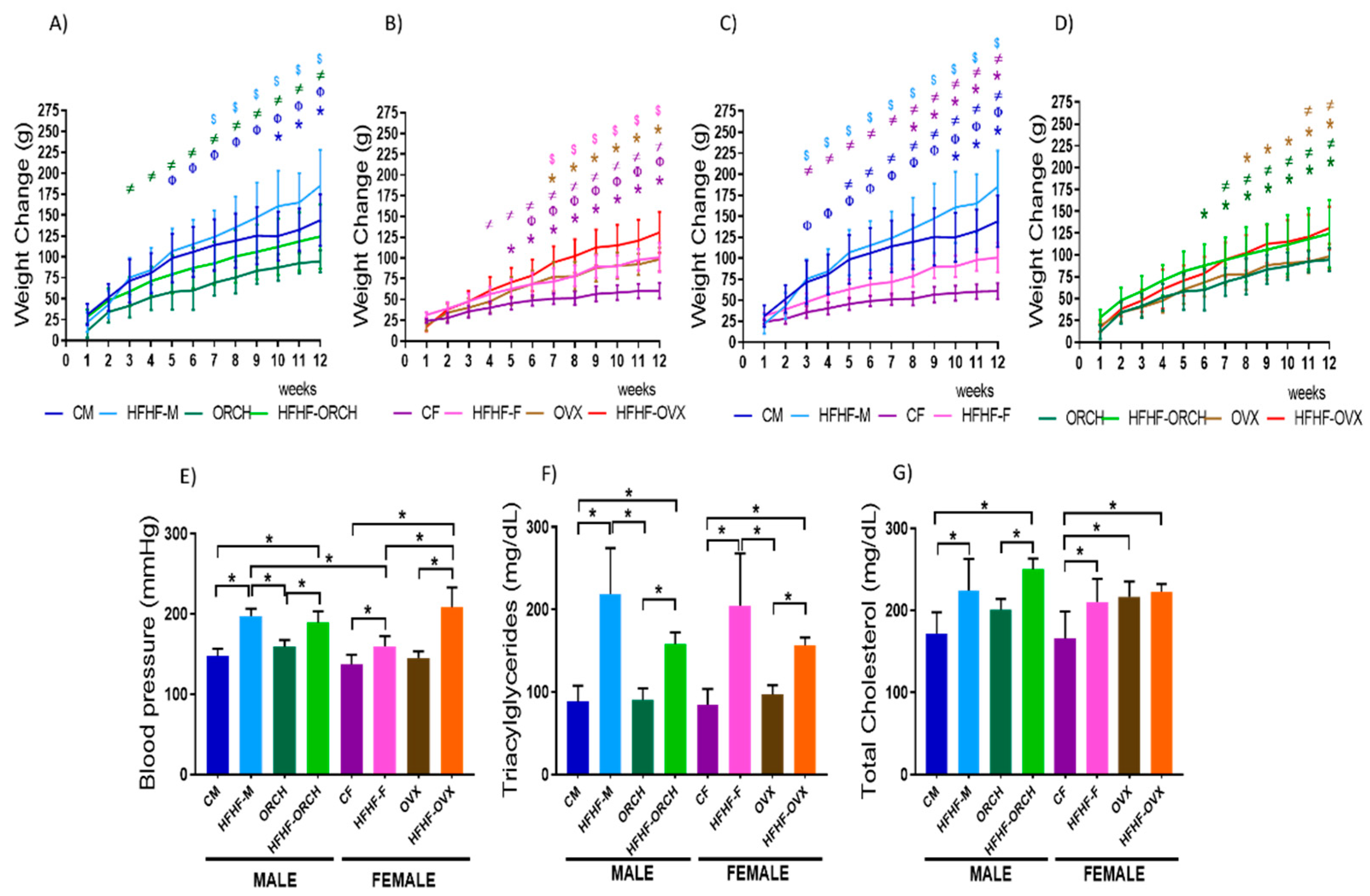
2.1. Determination of Diet and Sex-Dimorphism Effects on Somatometric and Metabolic Parameters
2.2. Determination of the Effects of the Hypercaloric Diet on Learning and Memory
2.2.1. Determination of the Effects of the Hypercaloric Diet on the Object Recognition Memory by Novel Object Recognition Task
2.2.2. Determination of the Effects of the Hypercaloric Diet on Spatial Learning and Memory by Morris Water Maze
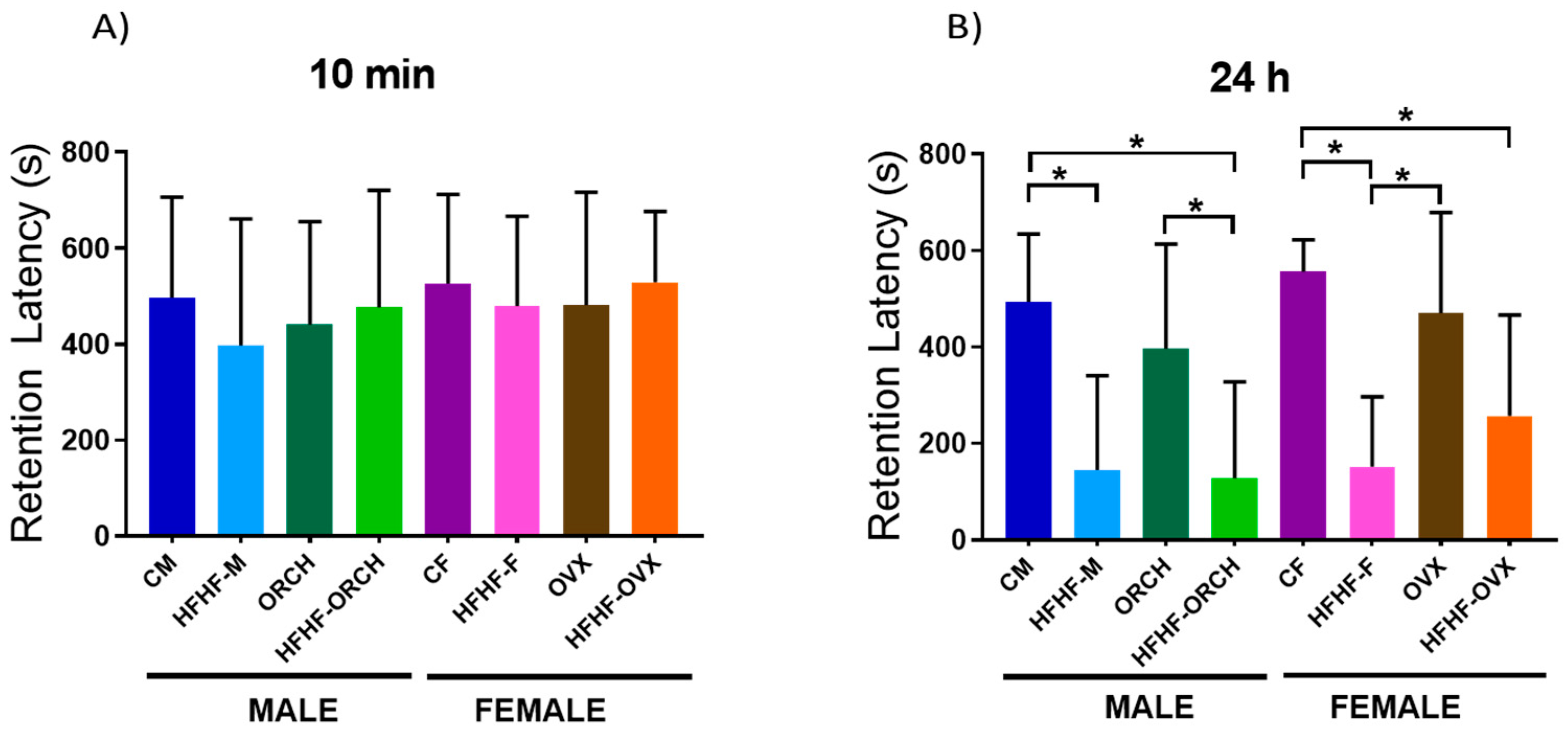
2.2.3. Determination of the Effects of the Hypercaloric Diet on the Short- and Long-Term Memory by Passive Avoidance Task
2.3. Determination of the Correlation of Oxidative Stress Parameters with the Different Types of Memory
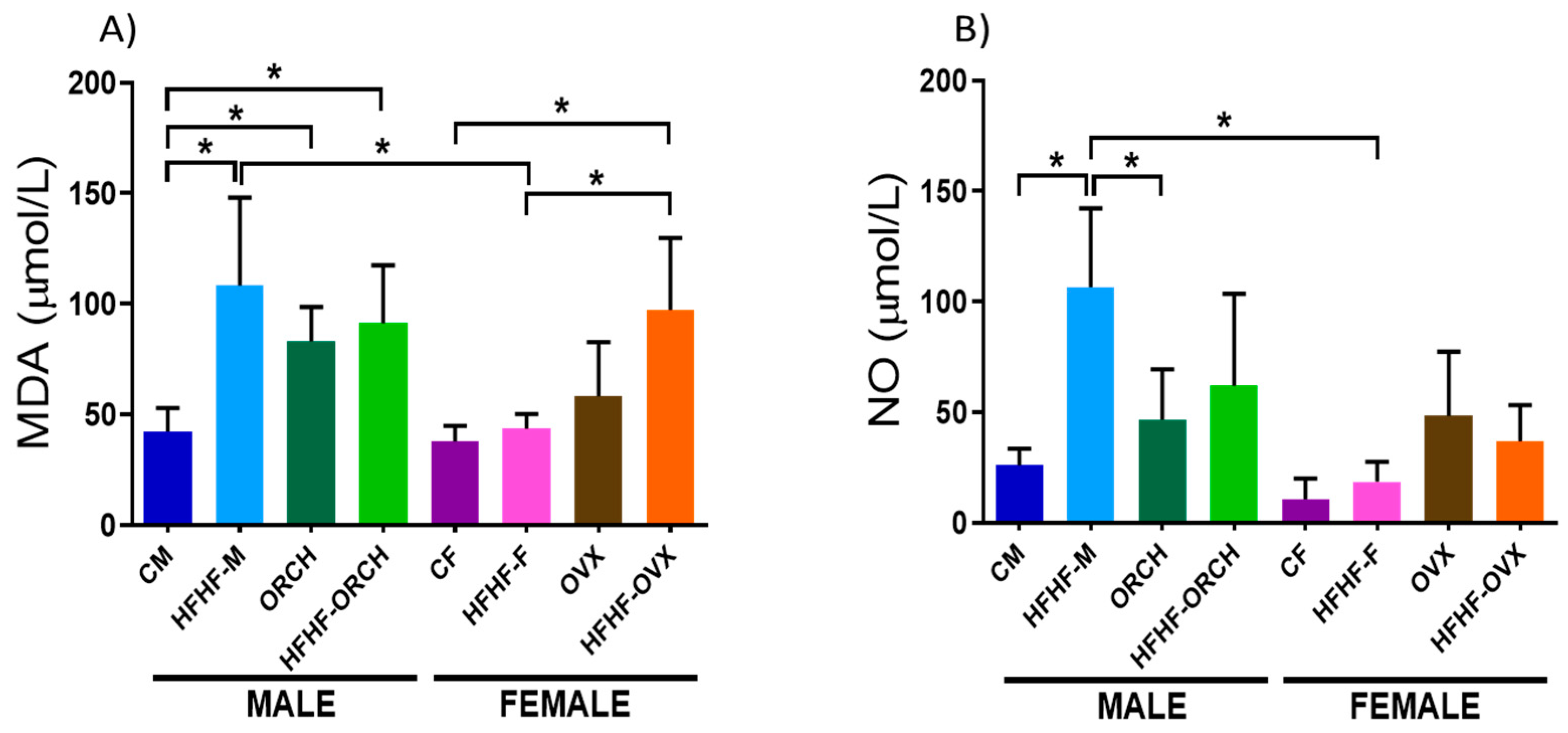
2.3.1. Determination of the Effects of the Hypercaloric Diet on the Oxidative Stress Parameters
2.3.2. Determination of the Correlation of Oxidative Stress Parameters with the Different Types of Memory
3. Discussion
Strengths and Limitations of the Study
4. Materials and Methods
4.1. Animals
4.2. Methods
- (a)
- Feeding
- (b)
- Gonadectomy
- i.
- Ovariectomy (OVX). Two lateral dorsal incisions of 1 cm were made to access the peritoneal cavity and locate each ovary. Ligatures with absorbable silk were placed in the oviducts, right below the ovaries, to prevent bleeding. Subsequently, the ovaries were gently removed using sterile scissors, verifying that no bleeding occurred. Following the ovariectomy, oviducts were introduced again in the abdominal cavity. The muscle layer was sutured with absorbable silk and the incisions made in the skin with polypropylene sutures.
- ii.
- Orchiectomy (ORCH). Incisions of 1 cm were made in the ventral side of the scrotum to expose the testicles and epididymis. Upon exposition, ligatures with absorbable silk were placed around the blood vessels, and testicle and epididymis were gently removed with sterile scissors, verifying that no bleeding occurred. The remaining content was reintroduced in the testicular sac. The incision on the scrotum was sutured with polypropylene thread.
- (c)
- Body weight, caloric intake, and blood pressure monitoring
- (d)
- Evaluation of memory and learning
- i.
- Episodic memory. Novel Object Recognition (NOR)
- ii.
- Learning and spatial memory. Morris Water Maze (MWM)
- iii.
- Short- and long-term memory. Passive avoidance test (PAT).
- (e)
- Sacrifice and blood collection
- (f)
- Triacylglycerides and cholesterol analysis
- (g)
- Determination of lipid peroxidation
- (h)
- Determination of Nitric Oxide (NO)
- (i)
- Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Wilson, O.W.A.; Garra, S.; Bopp, M.; Bopp, C.M. Incorporating the American College of Cardiology/American Heart Association Hypertension Diagnostic Criteria into Metabolic Syndrome Criteria Will Significantly Increase the Prevalence of Metabolic Syndrome among College Students. J. Hum. Hypertens. 2021, 35, 517–523. [Google Scholar] [CrossRef]
- Zhang, H.; Sairam, M.R. Sex Hormone Imbalances and Adipose Tissue Dysfunction Impacting on Metabolic Syndrome; a Paradigm for the Discovery of Novel Adipokines. Horm. Mol. Biol. Clin. Investig. 2014, 17, 89–97. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.A.; Tran, C.; Paquot, N. Fructose and Metabolic Diseases: New Findings, New Questions. Nutrition 2010, 26, 1044–1049. [Google Scholar] [CrossRef]
- Yates, K.; Sweat, V.; Yau, P.; Turchiano, M.; Convit, A. Impact of Metabolic Syndrome on Cognition and Brain: A Selceted Review of the Litterature. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2060–2067. [Google Scholar] [CrossRef]
- Yeomans, M.R. Adverse Effects of Consuming High Fat–Sugar Diets on Cognition: Implications for Understanding Obesity. Proc. Nutr. Soc. 2017, 76, 455–465. [Google Scholar] [CrossRef]
- Hawkins, M.A.W.; Keirns, N.G.; Helms, Z. Carbohydrates and Cognitive Function. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 302–307. [Google Scholar] [CrossRef]
- Pradhan, A.D. Sex Differences in the Metabolic Syndrome: Implications for Cardiovascular Health in Women. Clin. Chem. 2014, 60, 44–52. [Google Scholar] [CrossRef]
- Anderson, R.A.; Qin, B.; Canini, F.; Poulet, L.; Roussel, A.M. Cinnamon Counteracts the Negative Effects of a High Fat/High Fructose Diet on Behavior, Brain Insulin Signaling and Alzheimer-Associated Changes. PLoS ONE 2013, 8, e83243. [Google Scholar] [CrossRef]
- Stranahan, A.M. Models and Mechanisms for Hippocampal Dysfunction in Obesity and Diabetes. Neuroscience 2015, 309, 125–139. [Google Scholar] [CrossRef]
- Gancheva, S.; Galunska, B.; Zhelyazkova-Savova, M. Diets Rich in Saturated Fat and Fructose Induce Anxiety and Depression-like Behaviours in the Rat: Is There a Role for Lipid Peroxidation? Int. J. Exp. Pathol. 2017, 98, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Underwood, E.L.; Thompson, L.T. High-Fat Diet Impairs Spatial Memory and Hippocampal Intrinsic Excitability and Sex-Dependently Alters Circulating Insulin and Hippocampal Insulin Sensitivity. Biol. Sex Differ. 2016, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Underwood, E.L.; Thompson, L.T. A High-Fat Diet Causes Impairment in Hippocampal Memory and Sex-Dependent Alterations in Peripheral Metabolism. Neural Plast 2016, 7385314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Espinosa-García, C.; Fuentes-Venado, C.E.; Guerra-Araiza, C.; Segura-Uribe, J.; Chávez-Gutiérrez, E.; Farfán-García, E.D.; Estrada Cruz, N.A.; Pinto-Almazán, R. Sex Differences in the Performance of Cognitive Tasks in a Murine Model of Metabolic Syndrome. Eur. J. Neurosci. 2020, 52, 2724–2736. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.N.; Siddiqui, N.; Khan, R.A.; Kalam, A.; Jabir, N.R.; Kamal, M.A.; Firoz, C.K.; Tabrez, S. Neuroprotective Role of Steroidal Sex Hormones: An Overview. CNS Neurosci. Ther. 2016, 22, 342–350. [Google Scholar] [CrossRef]
- Lim, G.B. Role of Sex Hormones in Cardiovascular Diseases. Nat. Rev. Cardiol. 2021, 18, 385. [Google Scholar] [CrossRef]
- Bednarek-Tupikowska, G. Antioxidant Properties of Estrogens. Ginekol. Pol. 2002, 73, 61–67. [Google Scholar]
- Panchal, S.K.; Brown, L. Rodent Models for Metabolic Syndrome Research. J. Biomed. Biotechnol. 2011, 2011, 351982. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal Models of Metabolic Syndrome: A Review. Nutr Metab (Lond) 2016, 13, 65. [Google Scholar] [CrossRef]
- Wong, S.; Chin, K.-Y.; Suhaimi, F.; Ahmad, F.; Ima-Nirwana, S. The Effects of a Modified High-Carbohydrate High-Fat Diet on Metabolic Syndrome Parameters in Male Rats. Exp. Clin. Endocrinol. Diabetes 2018, 126, 205–212. [Google Scholar] [CrossRef]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome with Liver and Adipose Inflammation: Comparison to High-Fat Diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef]
- Tamaya-Mori, N.; Uemura, K.; Iguchi, A. Gender Differences in the Dietary Lard-Induced Increase in Blood Pressure in Rats. Hypertension 2002, 39, 1015–1020. [Google Scholar] [CrossRef]
- Lee, C.E.; Kang, J.S.; Kim, K. Effects of Gender, Gonadectomy and Sex Hormones on Growth and Plasma Cholesterol Level in Rats. Ann. Nutr. Metab. 2008, 53, 1–5. [Google Scholar] [CrossRef]
- Janssen, I.; Powell, L.; Crawford, S.; Lasley, B.; Sutton-Tyrrell, K. Menopause and the Metabolic Syndrome. Arch. Intern. Med. 2008, 168, 1568–1575. [Google Scholar] [CrossRef]
- do Carmo, J.M.; da Silva, A.A.; Moak, S.P.; Browning, J.R.; Dai, X.; Hall, J.E. Increased Sleep Time and Reduced Energy Expenditure Contribute to Obesity after Ovariectomy and a High Fat Diet. Life Sci. 2018, 212, 119–128. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Gender Differences in the Regulation of Blood Pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef]
- Lovejoy, J.; Champagne, C.; de Jonge, L.; Xie, H.; Smith, S. Increased Visceral Fat and Decreased Energy Expenditure during the Menopausal Transition. Int. J. Obes. Lond. 2008, 32, 949–958. [Google Scholar] [CrossRef]
- Maric-Bilkan, C.; Manigrasso, M.B. Sex Differences in Hypertension: Contribution of the Renin-Angiotensin System. Gend. Med. 2012, 9, 287–291. [Google Scholar] [CrossRef]
- Santosa, S.; Jensen, M.D. Effects of Male Hypogonadism on Regional Adipose Tissue Fatty Acid Storage and Lipogenic Proteins. PLoS ONE 2012, 7, e31473. [Google Scholar] [CrossRef]
- Pijacka, W.; Clifford, B.; Walas, D.; Tilburgs, C.; Joles, J.A.; McMullen, S.; Langley-Evans, S.C. Impact of Gonadectomy on Blood Pressure Regulation in Ageing Male and Female Rats. Biol. Sex Differ. 2016, 7, 64. [Google Scholar] [CrossRef]
- Sangüesa, G.; Cascales, M.; Griñán, C.; Sánchez, R.M.; Roglans, N.; Pallàs, M.; Laguna, J.C.; Alegret, M. Impairment of Novel Object Recognition Memory and Brain Insulin Signaling in Fructose- but Not Glucose-Drinking Female Rats. Mol. Neurobiol. 2018, 55, 6984–6999. [Google Scholar] [CrossRef] [PubMed]
- Arfa-Fatollahkhani, P.; Nahavandi, A.; Abtahi, H.; Anjidani, S.; Borhani, S.; Jameie, S.B.; Shabani, M.; Mehrzadi, S.; Shahbazi, A. The Effect of Luteinizing Hormone Reducing Agent on Anxiety and Novel Object Recognition Memory in Gonadectomized Rats. Basic Clin. Neurosci. 2017, 8, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.A.; Haziri, K.; Hansson, E.; Kettunen, P.; Westberg, L. Effects of Sex and Gonadectomy on Social Investigation and Social Recognition in Mice. BMC Neurosci. 2015, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, A.C.; Loughman, A.; Bernard, A.; Raipuria, M.; Abbott, K.N.; Dachtler, J.; Van, T.T.H.; Moore, R.J. An Intermittent Hypercaloric Diet Alters Gut Microbiota, Prefrontal Cortical Gene Expression and Social Behaviours in Rats. Nutr. Neurosci. 2018, 23, 613–627. [Google Scholar] [CrossRef]
- Borja-Cacho, D.; Matthews, J. Effects of Gonadectomy and Hormone Replacement on a Spontaneous Novel Object Recognition Task in Adult Male Rats. Horm. Behav. 2008, 6, 2166–2171. [Google Scholar] [CrossRef]
- Luine, V.; Attalla, S.; Mohan, G.; Costa, A.; Frankfurt, M. Dietary Phytoestrogens Enhance Spatial Memory and Spine Density in the Hippocampus and Prefrontal Cortex of Ovariectomized Rats. Brain Res. 2006, 1126, 183–187. [Google Scholar] [CrossRef]
- Wallace, M.; Luine, V.; Arellanos, A.; Frankfurt, M. Ovariectomized Rats Show Decreased Recognition Memory and Spine Density in the Hippocampus and Prefrontal Cortex. Brain Res. 2006, 1126, 176–182. [Google Scholar] [CrossRef]
- Sutcliffe, J.S.; Marshall, K.M.; Neill, J.C. Influence of Gender on Working and Spatial Memory in the Novel Object Recognition Task in the Rat. Behav. Brain Res. 2007, 177, 117–125. [Google Scholar] [CrossRef]
- Qiao, X.; McConnell, K.R.; Khalil, R.A. Sex Steroids and Vascular Responses in Hypertension and Aging. Gend. Med. 2008, 5, 46–64. [Google Scholar] [CrossRef]
- Sandstrom, N.J.; Kim, J.H.; Wasserman, M.A. Testosterone Modulates Performance on a Spatial Working Memory Task in Male Rats. Horm. Behav. 2006, 50, 18–26. [Google Scholar] [CrossRef]
- Moradpour, F.; Naghdi, N.; Fathollahi, Y.; Javan, M.; Choopani, S.; Gharaylou, Z. Pre-Pubertal Castration Improves Spatial Learning during Mid-Adolescence in Rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 105–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bimonte-Nelson, H.A.; Singleton, R.S.; Hunter, C.L.; Price, K.L.; Moore, A.B.; Granholm, A.-C.E. Ovarian Hormones and Cognition in the Aged Female Rat: I. Long-Term, but Not Short-Term, Ovariectomy Enhances Spatial Performance. Behav. Neurosci. 2003, 117, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex Differences in Lipid and Lipoprotein Metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Argüeso, A.R.; Díaz, D.J.; Díaz, P.J.; Rodríguez, G.A.; Castro, M.M.; Diz-Lois, F. Lipids, Cholesterol and Lipoproteins. Galicia Clínica Socieda De Galega De Med. Interna 2011, 72, 7–17. [Google Scholar]
- Higaki, A.; Mogi, M.; Iwanami, J.; Min, L.-J.; Bai, H.-Y.; Shan, B.-S.; Kan-No, H.; Ikeda, S.; Higaki, J.; Horiuchi, M. Recognition of Early Stage Thigmotaxis in Morris Water Maze Test with Convolutional Neural Network. PLoS ONE 2018, 13, e0197003. [Google Scholar] [CrossRef]
- Ganji, A.; Salehi, I.; Nazari, M.; Taheri, M.; Komaki, A. Effects of Hypericum Scabrum Extract on Learning and Memory and Oxidant/Antioxidant Status in Rats Fed a Long-Term High-Fat Diet. Metab. Brain Dis. 2017, 32, 1255–1265. [Google Scholar] [CrossRef]
- Jiang, F.; Lim, H.K.; Morris, M.J.; Prior, L.; Velkoska, E.; Wu, X.; Dusting, G.J. Systemic Upregulation of NADPH Oxidase in Diet-Induced Obesity in Rats. Redox Rep. 2011, 16, 223–229. [Google Scholar] [CrossRef]
- Mancini, A.; Festa, R.; di Donna, V.; Leone, E.; Littarru, G.P.; Silvestrini, A.; Meucci, E.; Pontecorvi, A. Hormones and Antioxidant Systems: Role of Pituitary and Pituitary-Dependent Axes. J. Endocrinol. Investig. 2010, 33, 422–433. [Google Scholar] [CrossRef]
- Udomkasemsab, A.; Ngamlerst, C.; Adisakwattana, P.; Aroonnual, A.; Tungtrongchitr, R.; Prangthip, P. Maoberry (Antidesma Bunius) Ameliorates Oxidative Stress and Inflammation in Cardiac Tissues of Rats Fed a High-Fat Diet. BMC Complementary Altern. Med. 2018, 18, 344. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Wang, K.; Niu, T.; Huang, D. Moderate- and Low-Dose of Atorvastatin Alleviate Cognition Impairment Induced by High-Fat Diet via Sirt1 Activation. Neurochem. Res. 2019, 44, 1065–1078. [Google Scholar] [CrossRef]
- Bodur, A.; İnce, İ.; Kahraman, C.; Abidin, İ.; Aydin-Abidin, S.; Alver, A. Effect of a High Sucrose and High Fat Diet in BDNF (+/-) Mice on Oxidative Stress Markers in Adipose Tissues. Arch. Biochem. Biophys. 2019, 665, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Kraegen, E.W.; Clark, P.W.; Jenkins, A.B.; Daley, E.A.; Chisholm, D.J.; Storlien, L.H. Development of Muscle Insulin Resistance after Liver Insulin Resistance in High-Fat-Fed Rats. Diabetes 1991, 40, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Ochoa, E.; Hernández-Ortega, K.; Ferrera, P.; Morimoto, S.; Arias, C. Short-Term High-Fat-and-Fructose Feeding Produces Insulin Signaling Alterations Accompanied by Neurite and Synaptic Reduction and Astroglial Activation in the Rat Hippocampus. J. Cereb. Blood Flow Metab. 2014, 34, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Wang, O.; Liu, J.; Cheng, Q.; Guo, X.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Effects of Ferulic Acid and γ-Oryzanol on High-Fat and High-Fructose Diet-Induced Metabolic Syndrome in Rats. PLoS ONE 2015, 10, e0118135. [Google Scholar] [CrossRef]
- Elizabeth, M.; Rodrigues, S.; Bekhbat, M.; Houser, M.C.; Chang, J.; Walker, D.I.; Jones, D.P.; Oller Do Nascimento, C.M.P.; Barnum, C.J.; Tansey, M.G. Chronic Psychological Stress and High-Fat High-Fructose Diet Disrupt Metabolic and Inflammatory Gene Networks in the Brain, Liver, and Gut and Promote Behavioral Deficits in Mice. Brain Behav. Immun. 2017, 59, 158–172. [Google Scholar] [CrossRef]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and Nutritional Overview of Diet-Induced Metabolic Syndrome Models in Rats: What Is the Best Choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef]
- Estrada-Cruz, N.A.; Manuel-Apolinar, L.; Segura-Uribe, J.J.; Almanza-Pérez, J.C.; Fortis-Barrera, Á.; Orozco-Suárez, S.; Bautista-Poblet, G.; Coyoy-Salgado, A.; Guerra-Araiza, C. Short-Term Administration of Tibolone Reduces Inflammation and Oxidative Stress in the Hippocampus of Ovariectomized Rats Fed High-Fat and High-Fructose. Nutr. Neurosci. 2022, 127, 396–404. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A New One-Trial Test for Neurobiological Studies of Memory in Rats. 1: Behavioral Data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Mathiasen, J.R.; Dicamillo, A. Novel Object Recognition in the Rat: A Facile Assay for Cognitive Function. Curr. Protoc. Pharmacol. 2010, 49, 5–59. [Google Scholar] [CrossRef]
- Morris, R. Developments of a Water-Maze Procedure for Studying Spatial Learning in the Rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Pinto-Almazán, R.; Rivas-Arancibia, S.; Farfán-García, E.D.; Rodríguez-Martínez, E.; Guerra-Araiza, C. Neuroprotective Effects of Tibolone against Oxidative Stress Induced by Ozone Exposure. Rev. Neurol. 2014, 58, 441–449. [Google Scholar] [PubMed]
- Fuentes-Venado, C.E.; Terán-Pérez, G.; Espinosa-Hernández, V.M.; Martínez-Herrera, E.; Segura-Uribe, J.J.; Mercadillo, R.E.; Pinto-Almazán, R.; Guerra-Araiza, C. Nutritional Status Influences Oxidative Stress and Insulin Resistance in Preschool Children. Metab. Syndr. Relat. Disord. 2021, 19, 513–523. [Google Scholar] [CrossRef]
- Wade, C.R.; van Rij, A.M. Plasma Thiobarbituric Acid Reactivity: Reaction Conditions and the Role of Iron, Antioxidants and Lipid Peroxy Radicals on the Quantitation of Plasma Lipid Peroxides. Life Sci. 1988, 43, 1085–1093. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N] Nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]


| Cognitive Evaluation | Oxidative Stress Biomarkers | HFHF-M | HFHF-ORCH | HFHF-F | HFHF-OVX | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pearson r | p | Pearson r | p | Pearson r | p | Pearson r | p | ||
| NOR 24 h | MDA | −0.2869 | 0.5327 | −0.5211 | 0.2304 | 0.5118 | 0.2403 | −0.1877 | 0.6869 |
| NO | −0.1752 | 0.7071 | 0.0526 | 0.9108 | −0.7724 | 0.0418 * | −0.9054 | 0.0130 * | |
| MWM | MDA | −0.7711 | 0.0424 * | −0.7560 | 0.0493 * | −0.1971 | 0.6719 | 0.4432 | 0.3192 |
| NO | −0.3128 | 0.4946 | 0.5641 | 0.1871 | −0.1581 | 0.7349 | −0.8632 | 0.0268 * | |
| PAT 24 h | MDA | 0.0721 | 0.8873 | 0.1112 | 0.8405 | 0.3604 | 0.4262 | −0.4643 | 0.3024 |
| NO | 0.3571 | 0.4444 | 0.4077 | 0.3714 | 0.1081 | 0.8222 | 0.2029 | 0.7000 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Gutiérrez, E.; Fuentes-Venado, C.E.; Rodríguez-Páez, L.; Guerra-Araiza, C.; Larqué, C.; Martínez-Herrera, E.; Ocharan-Hernández, M.E.; Lomelí, J.; Loza-Mejía, M.A.; Salazar, J.R.; et al. High Fructose and High Fat Diet Impair Different Types of Memory through Oxidative Stress in a Sex- and Hormone-Dependent Manner. Metabolites 2022, 12, 341. https://doi.org/10.3390/metabo12040341
Chávez-Gutiérrez E, Fuentes-Venado CE, Rodríguez-Páez L, Guerra-Araiza C, Larqué C, Martínez-Herrera E, Ocharan-Hernández ME, Lomelí J, Loza-Mejía MA, Salazar JR, et al. High Fructose and High Fat Diet Impair Different Types of Memory through Oxidative Stress in a Sex- and Hormone-Dependent Manner. Metabolites. 2022; 12(4):341. https://doi.org/10.3390/metabo12040341
Chicago/Turabian StyleChávez-Gutiérrez, Edwin, Claudia Erika Fuentes-Venado, Lorena Rodríguez-Páez, Christian Guerra-Araiza, Carlos Larqué, Erick Martínez-Herrera, María Esther Ocharan-Hernández, Joel Lomelí, Marco A. Loza-Mejía, Juan Rodrigo Salazar, and et al. 2022. "High Fructose and High Fat Diet Impair Different Types of Memory through Oxidative Stress in a Sex- and Hormone-Dependent Manner" Metabolites 12, no. 4: 341. https://doi.org/10.3390/metabo12040341
APA StyleChávez-Gutiérrez, E., Fuentes-Venado, C. E., Rodríguez-Páez, L., Guerra-Araiza, C., Larqué, C., Martínez-Herrera, E., Ocharan-Hernández, M. E., Lomelí, J., Loza-Mejía, M. A., Salazar, J. R., Meneses-Ruiz, D. M., Gallardo, J. M., & Pinto-Almazán, R. (2022). High Fructose and High Fat Diet Impair Different Types of Memory through Oxidative Stress in a Sex- and Hormone-Dependent Manner. Metabolites, 12(4), 341. https://doi.org/10.3390/metabo12040341