Branched-Chain Amino Acid Deprivation Decreases Lipid Oxidation and Lipogenesis in C2C12 Myotubes
Abstract
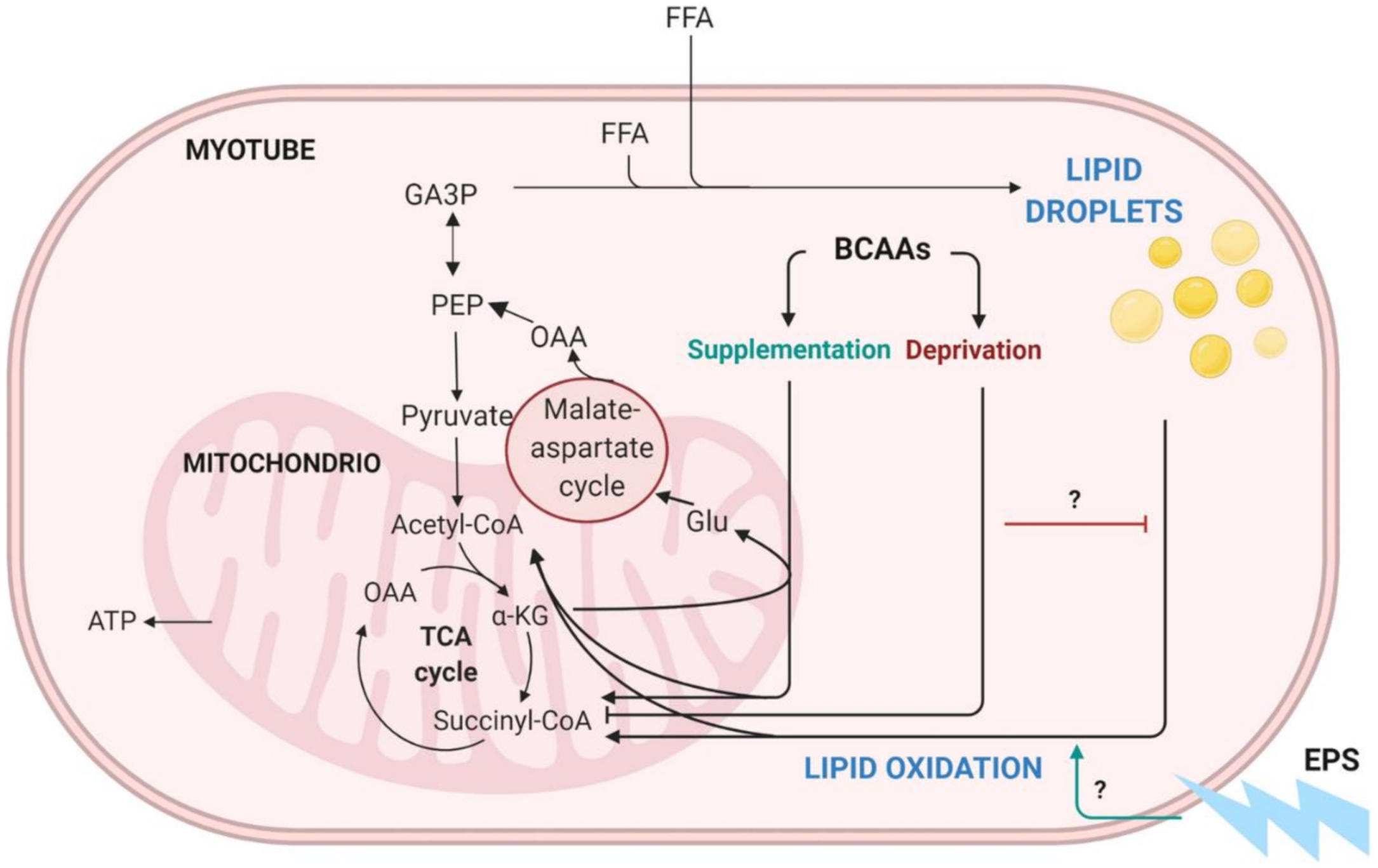
:1. Introduction
2. Results
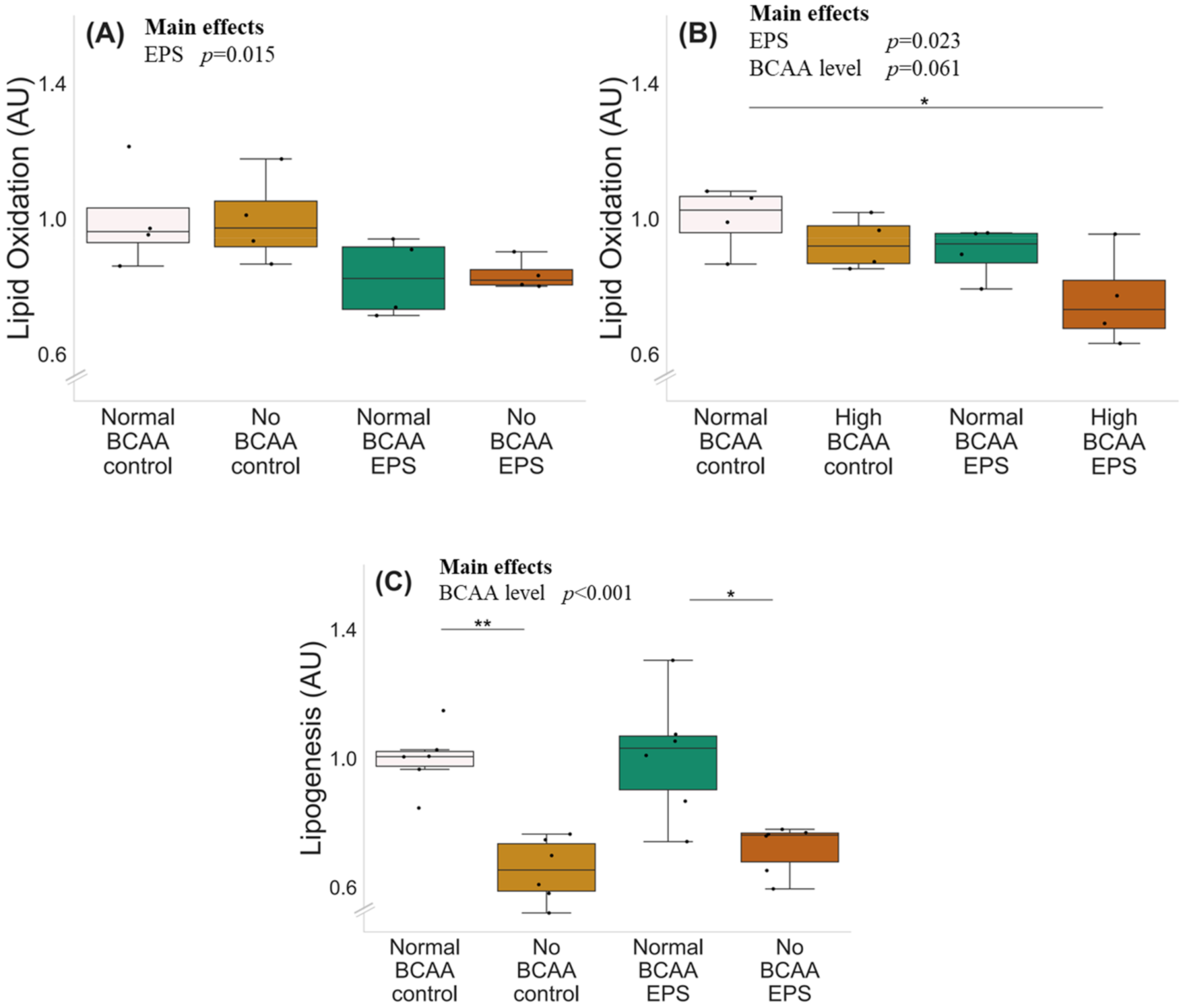
2.1. BCAA Deprivation Decreased Lipid Oxidation and Lipogenesis in C2C12 Myotubes
2.2. High BCAA Supplementation Combined with EPS Decreased Lipid Oxidation, whereas BCAA Deprivation but Not EPS Decreased Lipogenesis in C2C12 Myotubes
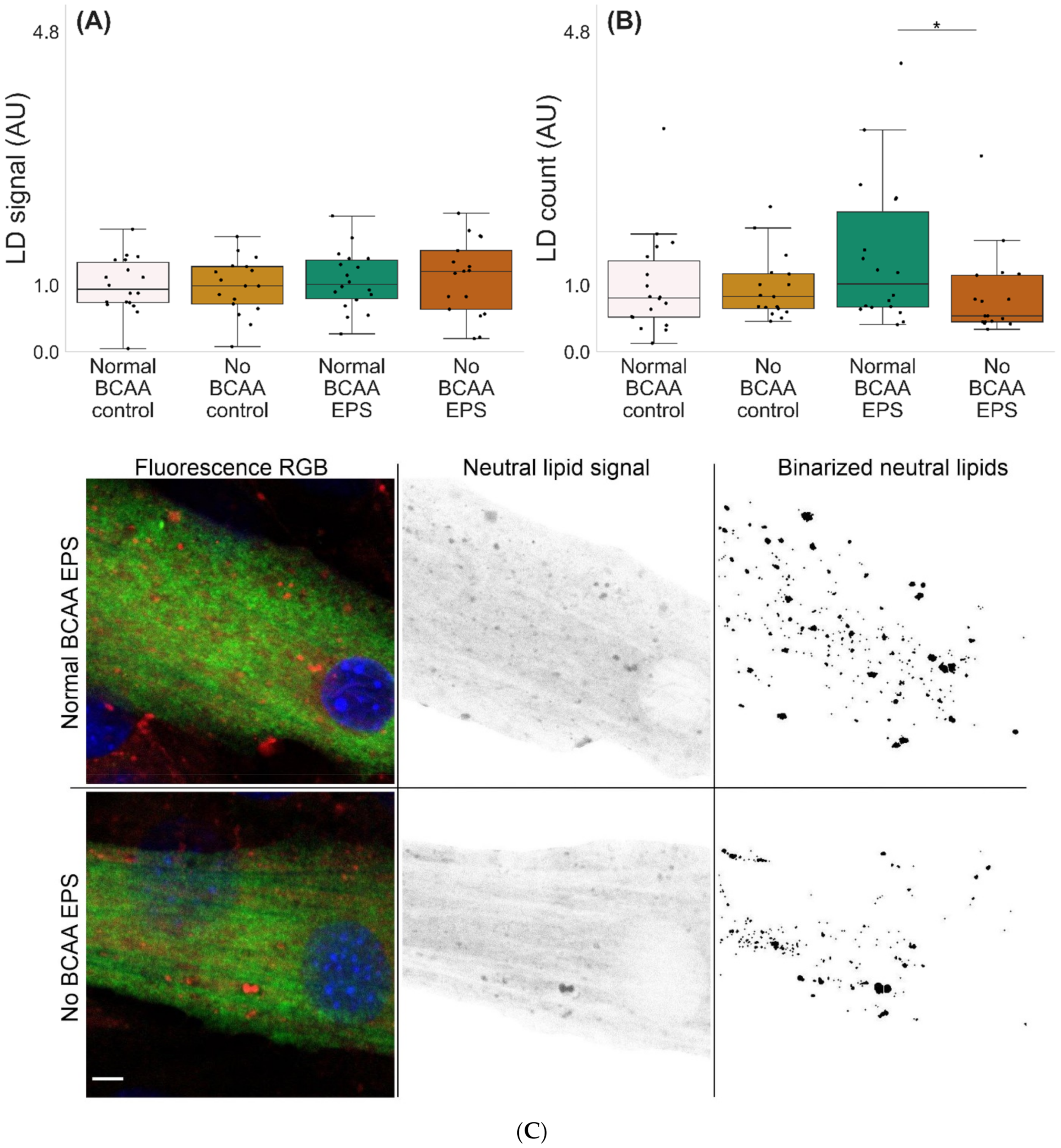
2.3. BCAA Deprivation Diminished the Number of Lipid Droplets in the EPS-Treated C2C12 Myotubes
2.4. Metabolites in C2C12 Myotubes and in Cell Culture Media
2.5. Total Protein Content, and Citrate Synthase Activity and Cell Viability Measurements
3. Discussion
3.1. The Deprivation of BCAAs Reduced Lipid Oxidation and Lipogenesis in C2C12 Myotubes
3.2. EPS Treatment Decreased Lipid Oxidation but Not Lipogenesis in C2C12 Myotubes
3.3. BCAA Deprivation Diminished the Number of Lipid Droplets in the EPS-Treated C2C12 Myotubes
4. Materials and Methods
4.1. Treatments
4.1.1. Lipid Oxidation
4.1.2. Skeletal-Muscle-Specific Exercise-like Electrical Pulse Stimulation (EPS) and Lipid Oxidation
4.1.3. Lipogenesis
4.1.4. Electrical Pulse Stimulation (EPS) and Lipogenesis
4.2. Histology and Image Analysis
4.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.4. Total Protein Content and Enzyme Activity Measurements
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savage, D.B.; Petersen, K.F.; Shulman, G.I. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 2007, 87, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv. Nutr. 2011, 2, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kainulainen, H.; Hulmi, J.J.; Kujala, U.M. Potential role of branched-chain amino acid catabolism in regulating fat oxidation. Exerc. Sport Sci. Rev. 2013, 41, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Kujala, U.M.; Makinen, V.P.; Heinonen, I.; Soininen, P.; Kangas, A.J.; Leskinen, T.H.; Rahkila, P.; Wurtz, P.; Kovanen, V.; Cheng, S.; et al. Long-term leisure-time physical activity and serum metabolome. Circulation 2013, 127, 340–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leskinen, T.; Rinnankoski-Tuikka, R.; Rintala, M.; Seppanen-Laakso, T.; Pollanen, E.; Alen, M.; Sipila, S.; Kaprio, J.; Kovanen, V.; Rahkila, P.; et al. Differences in muscle and adipose tissue gene expression and cardio-metabolic risk factors in the members of physical activity discordant twin pairs. PLoS ONE 2010, 5, e12609. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Rennie, M.J. Influence of exercise on protein and amino acid metabolism. In Handbook of Physiology; Rowell, L.B., Shepherd, J.T., Eds.; Section 12: Exercise: Regulation and Integration of Multiple Systems; American Physiological Society: Bethesda, MD, USA, 1996; Volume 20, Chapter 22; pp. 995–1035. [Google Scholar]
- Holecek, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Berggren, J.R.; Boyle, K.E.; Chapman, W.H.; Houmard, J.A. Skeletal muscle lipid oxidation and obesity: Influence of weight loss and exercise. Am. J. Physiol. Metab. 2008, 294, E726–E732. [Google Scholar] [CrossRef]
- Argiles, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Manas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [Green Version]
- She, P.; Zhou, Y.; Zhang, Z.; Griffin, K.; Gowda, K.; Lynch, C.J. Disruption of BCAA metabolism in mice impairs exercise metabolism and endurance. J. Appl. Physiol. 2010, 108, 941–949. [Google Scholar] [CrossRef] [Green Version]
- Kivelä, R.; Silvennoinen, M.; Lehti, M.; Rinnankoski-Tuikka, R.; Purhonen, T.; Ketola, T.; Pullinen, K.; Vuento, M.; Mutanen, N.; Sartor, M.A.; et al. Gene expression centroids that link with low intrinsic aerobic exercise capacity and complex disease risk. FASEB J. 2010, 24, 4565–4574. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Nagasaki, M.; Obayashi, M.; Sato, Y.; Tamura, T.; Shimomura, Y. Mechanism of activation of branched-chain alpha-keto acid dehydrogenase complex by exercise. Biochem. Biophys. Res. Commun. 2001, 287, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Kasperek, G.J.; Dohm, G.L.; Snider, R.D. Activation of branched-chain keto acid dehydrogenase by exercise. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1985, 248, R166–R171. [Google Scholar] [CrossRef] [PubMed]
- Balage, M.; Dardevet, D. Long-term effects of leucine supplementation on body composition. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 265–270. [Google Scholar] [CrossRef]
- Gualano, A.B.; Bozza, T.; De Campos, P.L.; Roschel, H.; Costa, A.D.S.; Marquezi, M.L.; Benatti, F.; Junior, A.H.L. Branched-chain amino acids supplementation enhances exercise capacity and lipid oxidation during endurance exercise after muscle glycogen depletion. J. Sports Med. Phys. Fit. 2011, 51, 82–88. [Google Scholar]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, J.; Masaki, T.; Arakawa, M.; Seike, M.; Yoshimatsu, H. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARalpha and uncoupling protein in diet-induced obese mice. J. Nutr. 2010, 140, 496–500. [Google Scholar] [CrossRef] [Green Version]
- Ahtiainen, J.P.; Lensu, S.; Ruotsalainen, I.; Schumann, M.; Ihalainen, J.K.; Fachada, V.; Mendias, C.L.; Brook, M.S.; Smith, K.; Atherton, P.J.; et al. Physiological adaptations to resistance training in rats selectively bred for low and high response to aerobic exercise training. Exp. Physiol. 2018, 103, 1513–1523. [Google Scholar] [CrossRef] [Green Version]
- Lensu, S.; Pekkala, S.P.; Mäkinen, A.; Karstunen, N.; Turpeinen, A.T.; Hulmi, J.J.; Silvennoinen, M.M.; Ma, H.; Kujala, U.M.; Karvinen, S.; et al. Beneficial effects of running and milk protein supplements on Sirtuins and risk factors of metabolic disorders in rats with low aerobic capacity. Metab. Open 2019, 4, 100019. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Laakso, M.; Mero, A.A.; Häkkinen, K.; Ahtiainen, J.P.; Peltonen, H. The effects of whey protein with or without carbohydrates on resistance training adaptations. J. Int. Soc. Sports Nutr. 2015, 12, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, K.S.; Short, K.R. Hormonal and signaling role of branched-chain amino acids. J. Nutr. 2005, 135, 1547S–1552S. [Google Scholar] [CrossRef] [PubMed]
- Watford, M. Lowered concentrations of branched-chain amino acids result in impaired growth and neurological problems: Insights from a branched-chain alpha-keto acid dehydrogenase complex kinase-deficient mouse model. Nutr. Rev. 2007, 65, 167–172. [Google Scholar] [CrossRef]
- Fontana, L.; Cummings, N.E.; Apelo, S.I.A.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016, 16, 520–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, N.E.; Williams, E.M.; Kasza, I.; Konon, E.N.; Schaid, M.D.; Schmidt, B.A.; Poudel, C.; Sherman, D.S.; Yu, D.; Apelo, S.I.A.; et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 2018, 596, 623–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Richardson, N.E.; Green, C.L.; Spicer, A.B.; Murphy, M.E.; Flores, V.; Jang, C.; Kasza, I.; Nikodemova, M.; Wakai, M.H.; et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab. 2021, 33, 905–922. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Author Correction: Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 990. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Regulation of fat metabolism in skeletal muscle. Ann. N. Y. Acad. Sci. 2002, 967, 217–235. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Zhao, M.; Nie, Y.; Liu, P.; Zhu, Y.; Zhang, X. Skeletal Muscle Lipid Droplets and the Athlete’s Paradox. Cells 2019, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.Q.; Xun, P.; Bujnowski, D.; Daviglus, M.L.; Van Horn, L.; Stamler, J.; He, K.; Group, I.C.R. Higher branched-chain amino acid intake is associated with a lower prevalence of being overweight or obese in middle-aged East Asian and Western adults. J. Nutr. 2011, 141, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerin, C.; Goldfine, A.B.; Boes, T.; Liu, M.; Kasif, S.; Dreyfuss, J.M.; De Sousa-Coelho, A.L.; Daher, G.; Manoli, I.; Sysol, J.R.; et al. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol. Metab. 2016, 5, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Connor, S.C.; Hansen, M.K.; Corner, A.; Smith, R.F.; Ryan, T.E. Integration of metabolomics and transcriptomics data to aid biomarker discovery in type 2 diabetes. Mol. Biosyst. 2010, 6, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Alcalde, I.; Tenorio-Guzman, M.R.; Tovar, A.R.; Salinas-Rubio, D.; Torre-Villalvazo, I.; Torres, N.; Noriega, L.G. Metabolic Fate of Branched-Chain Amino Acids During Adipogenesis, in Adipocytes From Obese Mice and C2C12 Myotubes. J. Cell. Biochem. 2017, 118, 808–818. [Google Scholar] [CrossRef]
- Boulange, C.L.; Claus, S.P.; Chou, C.J.; Collino, S.; Montoliu, I.; Kochhar, S.; Holmes, E.; Rezzi, S.; Nicholson, J.K.; Dumas, M.E.; et al. Early metabolic adaptation in C57BL/6 mice resistant to high fat diet induced weight gain involves an activation of mitochondrial oxidative pathways. J. Proteome Res. 2013, 12, 1956–1968. [Google Scholar] [CrossRef]
- Aftring, R.P.; Miller, W.J.; Buse, M.G. Effects of diabetes and starvation on skeletal muscle branched-chain alpha-keto acid dehydrogenase activity. Am. J. Physiol.-Endocrinol. Metab. 1988, 254, E292–E300. [Google Scholar] [CrossRef]
- Wallace, M.; Green, C.R.; Roberts, L.S.; Lee, Y.M.; McCarville, J.L.; Sanchez-Gurmaches, J.; Meurs, N.; Gengatharan, J.M.; Hover, J.D.; Phillips, S.A.; et al. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat. Chem. Biol. 2018, 14, 1021–1031. [Google Scholar] [CrossRef]
- Green, C.R.; Wallace, M.; Divakaruni, A.S.; Phillips, S.A.; Murphy, A.N.; Ciaraldi, T.P.; Metallo, C.M. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 2016, 12, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-González, C.; Nuevo-Tapioles, C.; Herrero Martín, J.C.; Pereira, M.P.; Serrano Sanz, S.; Ramírez de Molina, A.; Cuezva, J.M.; Formentini, L. Dysfunctional oxidative phosphorylation shunts branched-chain amino acid catabolism onto lipogenesis in skeletal muscle. EMBO J. 2020, 39, e103812. [Google Scholar] [CrossRef]
- Lautaoja, J.H.; O’Connell, T.M.; Mäntyselkä, S.; Peräkylä, J.; Kainulainen, H.; Pekkala, S.; Permi, P.; Hulmi, J.J. Higher glucose availability augments the metabolic responses of the C2C12 myotubes to exercise-like electrical pulse stimulation. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E229–E245. [Google Scholar] [CrossRef]
- Abdelmoez, A.M.; Sardón Puig, L.; Smith, J.A.B.; Gabriel, B.M.; Savikj, M.; Dollet, L.; Chibalin, A.V.; Krook, A.; Zierath, J.R.; Pillon, N.J. Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism. Am. J. Physiol. Cell Physiol. 2020, 318, C615–C626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barclay, J.K.; Stainsby, W.N. Intramuscular lipid store utilization by contracting dog skeletal muscle in situ. Am. J. Physiol. 1972, 223, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Hopp, J.F.; Palmer, W.K. Electrical stimulation alters fatty acid metabolism in isolated skeletal muscle. J. Appl. Physiol. 1990, 68, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L.; Heigenhauser, G.J.; Jones, N.L. Endogenous triacylglycerol utilization by rat skeletal muscle during tetanic stimulation. J. Appl. Physiol. 1986, 60, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Masoro, E.J.; Rowell, L.B.; McDonald, R.M.; Steiert, B. Skeletal muscle lipids. II. Nonutilization of intracellular lipid esters as an energy source for contractile activity. J. Biol. Chem. 1966, 241, 2626–2634. [Google Scholar] [CrossRef]
- Marš, T.; Miš, K.; Meznarič, M.; Prpar Mihevc, S.; Jan, V.; Haugen, F.; Rogelj, B.; Rustan, A.C.; Thoresen, G.H.; Pirkmajer, S.; et al. Innervation and electrical pulse stimulation—In vitro effects on human skeletal muscle cells. Appl. Physiol. Nutr. Metab. 2021, 46, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, N.; Skaret Bakke, S.; Tranheim Kase, E.; Rudberg, I.; Flo Halle, I.; Rustan, A.C.; Thoresen, G.H.; Aas, V. Correction: Electrical Pulse Stimulation of Cultured Human Skeletal Muscle Cells as an In Vitro Model of Exercise. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Li, L.-J.; Ma, J.; Li, S.-B.; Chen, X.-F.; Zhang, J. Electric pulse stimulation inhibited lipid accumulation on C2C12 myotubes incubated with oleic acid and palmitic acid. Arch. Physiol. Biochem. 2021, 127, 344–350. [Google Scholar] [CrossRef]
- Dyck, D.J.; Bonen, A. Muscle contraction increases palmitate esterification and oxidation and triacylglycerol oxidation. Am. J. Physiol. 1998, 275, E888–E896. [Google Scholar] [CrossRef]
- van Loon, L.J.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.; Wagenmakers, A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef]
- Picard, M.; Gentil, B.J.; McManus, M.J.; White, K.; St Louis, K.; Gartside, S.E.; Wallace, D.C.; Turnbull, D.M. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J. Appl. Physiol. 2013, 115, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Prats, C.; Donsmark, M.; Qvortrup, K.; Londos, C.; Sztalryd, C.; Holm, C.; Galbo, H.; Ploug, T. Decrease in intramuscular lipid droplets and translocation of HSL in response to muscle contraction and epinephrine. J. Lipid Res. 2006, 47, 2392–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraenkel, M.; Weiss, R.; Leizerman, I.; Anaby, D.; Golomb, E.; Leibowitz, G.; Kaiser, N. Scanning electron microscopic analysis of intramyocellular lipid droplets in an animal model of type 2 diabetes. Obesity 2008, 16, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Akie, T.E.; Cooper, M.P. Determination of Fatty Acid Oxidation and Lipogenesis in Mouse Primary Hepatocytes. JoVE 2015, 102, e52982. [Google Scholar] [CrossRef] [Green Version]
- Spandl, J.; White, D.J.; Peychl, J.; Thiele, C. Live cell multicolor imaging of lipid droplets with a new dye, LD540. Traffic 2009, 10, 1579–1584. [Google Scholar] [CrossRef]
- Tamura, K.; Goto-Inoue, N.; Miyata, K.; Furuichi, Y.; Fujii, N.L.; Manabe, Y. Effect of treatment with conditioned media derived from C2C12 myotube on adipogenesis and lipolysis in 3T3-L1 adipocytes. PLoS ONE 2020, 15, e0237095. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karvinen, S.; Fachada, V.; Sahinaho, U.-M.; Pekkala, S.; Lautaoja, J.H.; Mäntyselkä, S.; Permi, P.; Hulmi, J.J.; Silvennoinen, M.; Kainulainen, H. Branched-Chain Amino Acid Deprivation Decreases Lipid Oxidation and Lipogenesis in C2C12 Myotubes. Metabolites 2022, 12, 328. https://doi.org/10.3390/metabo12040328
Karvinen S, Fachada V, Sahinaho U-M, Pekkala S, Lautaoja JH, Mäntyselkä S, Permi P, Hulmi JJ, Silvennoinen M, Kainulainen H. Branched-Chain Amino Acid Deprivation Decreases Lipid Oxidation and Lipogenesis in C2C12 Myotubes. Metabolites. 2022; 12(4):328. https://doi.org/10.3390/metabo12040328
Chicago/Turabian StyleKarvinen, Sira, Vasco Fachada, Ulla-Maria Sahinaho, Satu Pekkala, Juulia H. Lautaoja, Sakari Mäntyselkä, Perttu Permi, Juha J. Hulmi, Mika Silvennoinen, and Heikki Kainulainen. 2022. "Branched-Chain Amino Acid Deprivation Decreases Lipid Oxidation and Lipogenesis in C2C12 Myotubes" Metabolites 12, no. 4: 328. https://doi.org/10.3390/metabo12040328
APA StyleKarvinen, S., Fachada, V., Sahinaho, U.-M., Pekkala, S., Lautaoja, J. H., Mäntyselkä, S., Permi, P., Hulmi, J. J., Silvennoinen, M., & Kainulainen, H. (2022). Branched-Chain Amino Acid Deprivation Decreases Lipid Oxidation and Lipogenesis in C2C12 Myotubes. Metabolites, 12(4), 328. https://doi.org/10.3390/metabo12040328








