Untargeted Metabolomics Reveals Metabolic Stress Alleviation by Prepartum Exercise in Transition Dairy Cows
Abstract
1. Introduction
2. Results
2.1. Plasma Analyses
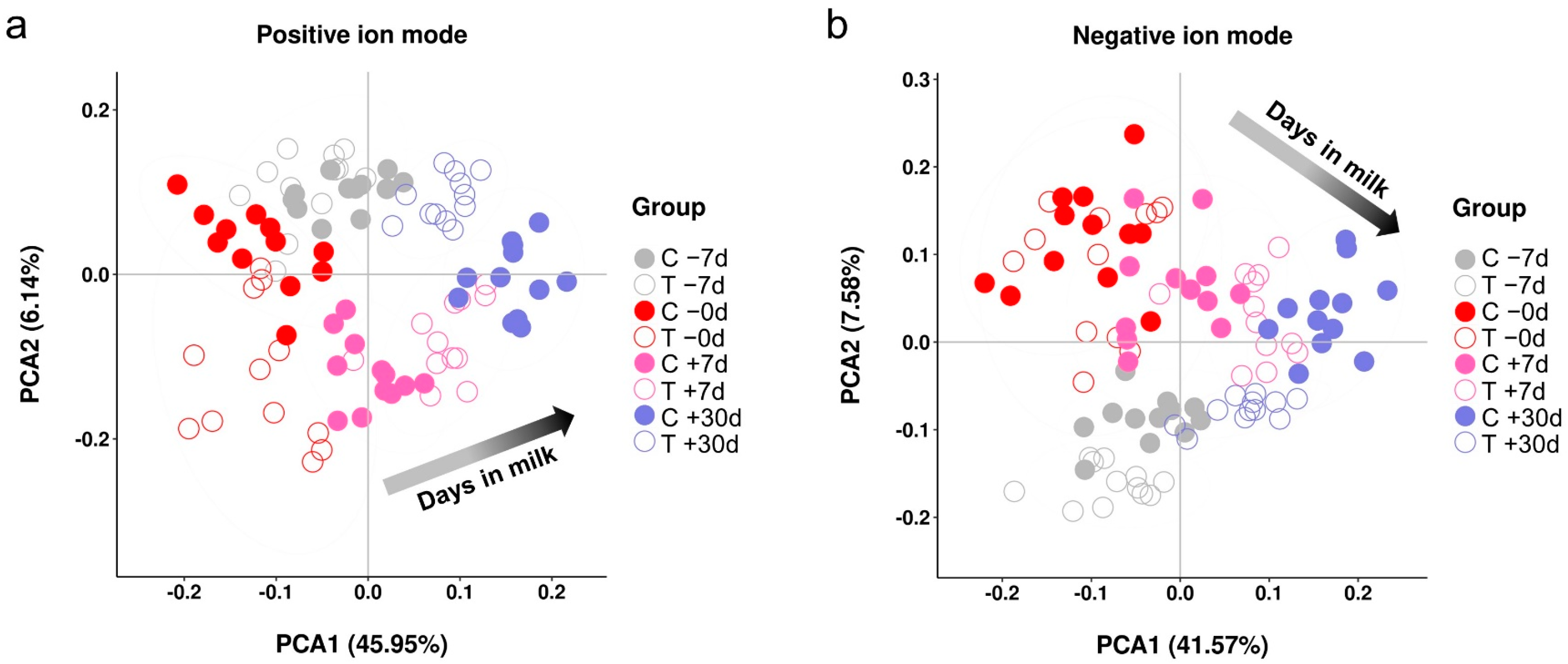
2.2. Metabolic Profiles
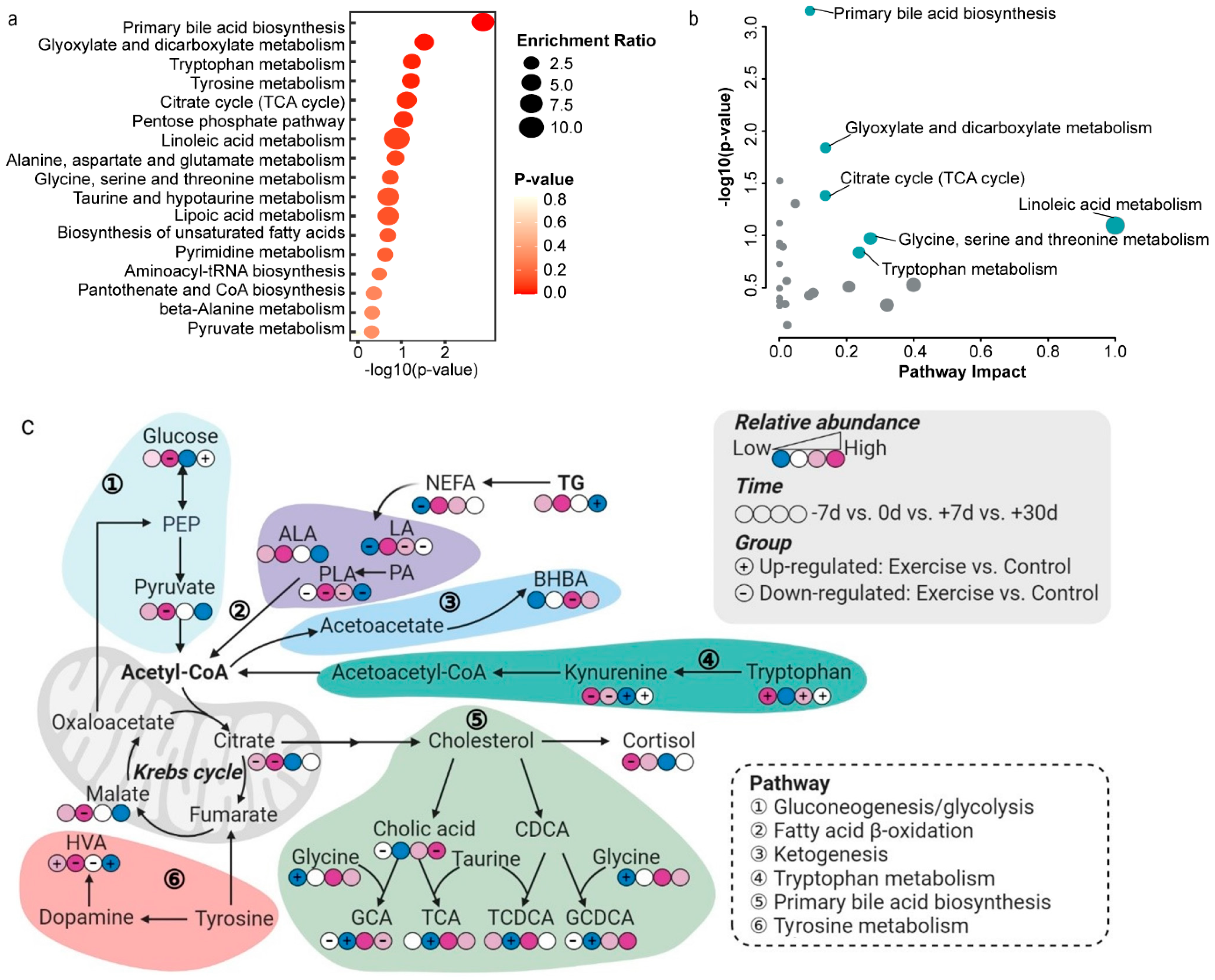
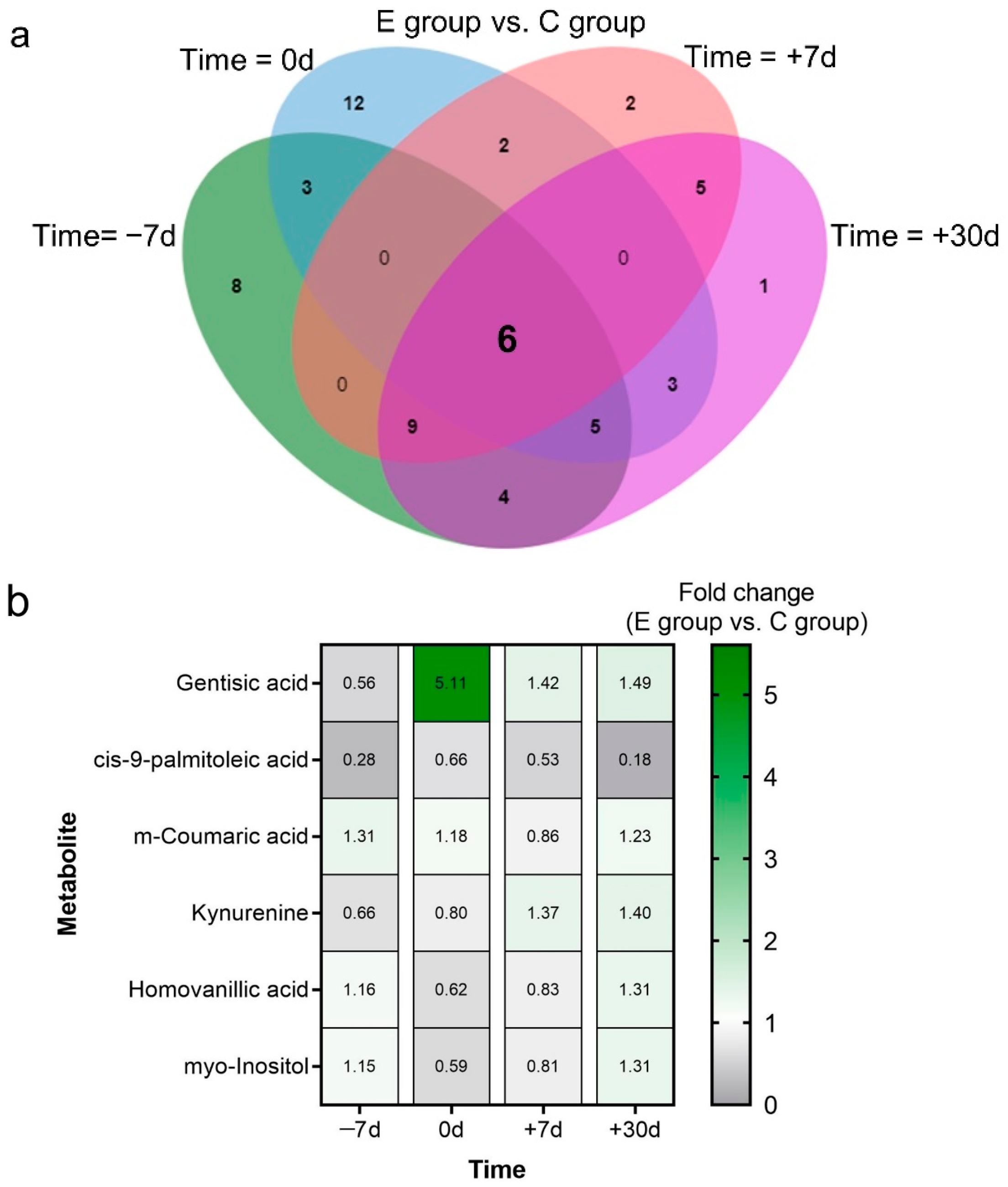
2.3. Differential Metabolites and Pathway Analysis
2.4. Key Metabolites and Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Animals, Housing, and Management
4.2. Experimental Treatments and Sample Collection
4.3. Plasma Analysis
4.4. Sample Pretreatment and Metabolomics Profiling
4.5. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drackley, J.K. Biology of dairy cows during the transition period: The Final Frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Huzzey, J.; Von Keyserlingk, M.; Weary, D. Changes in feeding, drinking, and standing behavior of dairy cows during the transition period. J. Dairy Sci. 2005, 88, 2454–2461. [Google Scholar] [CrossRef]
- Hayirli, A.; Grummer, R.R.; Nordheim, E.V.; Crump, P.M. Animal and dietary factors affecting feed intake during the prefresh transition period in Holsteins. J. Dairy Sci. 2002, 85, 3430–3443. [Google Scholar] [CrossRef]
- McArt, J.A.; Nydam, D.V.; Oetzel, G.R.; Overton, T.R.; Ospina, P.A. Elevated non-esterified fatty acids and beta-hydroxybutyrate and their association with transition dairy cow performance. Vet. J. 2013, 198, 560–570. [Google Scholar] [CrossRef]
- Herdt, T.H. Ruminant adaptation to negative energy balance: Influences on the etiology of ketosis and fatty liver. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 215–230. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Shen, L.H.; Jiang, J.; Huang, Y.X.; Bai, L.P.; Yu, S.M.; Yao, X.P.; Ren, Z.H.; Yang, Y.X.; Cao, S.Z. Plasma metabolite changes in dairy cows during parturition identified using untargeted metabolomics. J. Dairy Sci. 2019, 102, 4639–4650. [Google Scholar] [CrossRef]
- Contreras, G.A.; Strieder-Barboza, C.; Raphael, W. Adipose tissue lipolysis and remodeling during the transition period of dairy cows. J. Anim. Sci. Biotechnol. 2017, 8, 41. [Google Scholar] [CrossRef]
- Shen, L.; Zhu, Y.; Xiao, J.; Qian, B.; You, L.; Zhang, Y.; Yu, S.; Zong, X.; Cao, S. Serum adipokines play different roles in type I and II ketosis. Asian-Australas. J. Anim. Sci. 2020, 33, 1930–1939. [Google Scholar] [CrossRef]
- Keil, N.M.; Wiederkehr, T.U.; Friedli, K.; Wechsler, B. Effects of frequency and duration of outdoor exercise on the prevalence of hock lesions in tied Swiss dairy cows. Prev. Vet. Med. 2006, 74, 142–153. [Google Scholar] [CrossRef]
- EFSA. Scientific report on the effects of farming systems on dairy cow welfare and disease. EFSA J. 2009, 7, 1143–1181. [Google Scholar] [CrossRef]
- Davidson, J.A.; Beede, D.K. Exercise training of late-pregnant and nonpregnant dairy cows affects physical fitness and acid-base homeostasis. J. Dairy Sci. 2009, 92, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; van Amstel, S.R.; Krawczel, P.D. Effect of prepartum exercise, pasture turnout, or total confinement on hoof health. J. Dairy Sci. 2017, 100, 8338–8346. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; Krawczel, P.D. Effect of prepartum exercise on lying behavior, labor length, and cortisol concentrations. J. Dairy Sci. 2019, 102, 11250–11259. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, J.M.; Menichetti, B.T.; Barragan, A.A.; Relling, A.E.; Weiss, W.P.; Bas, S.; Schuenemann, G.M. Associations of pre- and postpartum lying time with metabolic, inflammation, and health status of lactating dairy cows. J. Dairy Sci. 2019, 102, 3348–3361. [Google Scholar] [CrossRef]
- Goselink, R.M.A.; Schonewille, J.T.; Duinkerken, G.; Hendriks, W.H. Physical exercise prepartum to support metabolic adaptation in the transition period of dairy cattle: A proof of concept. J. Anim. Physiol. Anim. Nutr. 2020, 104, 790–801. [Google Scholar] [CrossRef]
- Harris, J.E.; Baer, L.A.; Stanford, K.I. Maternal exercise improves the metabolic health of adult offspring. Trends Endocrinol. Metab. 2018, 29, 164–177. [Google Scholar] [CrossRef]
- Eckel, E.F.; Ametaj, B.N. An omics approach to transition cow immunity. In Periparturient Diseases of Dairy Cows: A Systems Biology Approach, 1st ed.; Ametaj, B.N., Ed.; Springer: Cham, Switzerland, 2017; pp. 31–50. [Google Scholar] [CrossRef]
- Tomita, M.; Kami, K. Systems biology, metabolomics, and cancer metabolism. Science 2012, 336, 990–991. [Google Scholar] [CrossRef]
- Tebani, A.; Abily-Donval, L.; Afonso, C.; Marret, S.; Bekri, S. Clinical metabolomics: The new metabolic window for inborn errors of metabolism investigations in the Post-Genomic Era. Int. J. Mol. Sci. 2016, 17, 1167–1192. [Google Scholar] [CrossRef]
- Zarrin, M.; Grossen-Rosti, L.; Bruckmaier, R.M.; Gross, J.J. Elevation of blood beta-hydroxybutyrate concentration affects glucose metabolism in dairy cows before and after parturition. J. Dairy Sci. 2017, 100, 2323–2333. [Google Scholar] [CrossRef]
- Ling, T.; Hernandez-Jover, M.; Sordillo, L.M.; Abuelo, A. Maternal late-gestation metabolic stress is associated with changes in immune and metabolic responses of dairy calves. J. Dairy Sci. 2018, 101, 6568–6580. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Mann, S.; Nydam, D.V.; Overton, T.R.; McArt, J.A. Short communication: Concentrations of nonesterified fatty acids and beta-hydroxybutyrate in dairy cows are not well correlated during the transition period. J. Dairy Sci. 2015, 98, 6284–6290. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; McArt, J.A.; Overton, T.R.; Stokol, T.; Nydam, D.V. Using nonesterified fatty acids and β-hydroxybutyrate concentrations during the transition period for herd-level monitoring of increased risk of disease and decreased reproductive and milking performance. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 387–412. [Google Scholar] [CrossRef] [PubMed]
- Thyfault, J.P.; Bergouignan, A. Exercise and metabolic health: Beyond skeletal muscle. Diabetologia 2020, 63, 1464–1474. [Google Scholar] [CrossRef]
- De Koster, J.D.; Opsomer, G. Insulin resistance in dairy cows. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef]
- Shi, Z.; Li, X.B.; Peng, Z.C.; Fu, S.-P.; Zhao, C.X.; Du, X.L.; Fang, Z.Y.; Wang, Z.; Liu, G.W.; Li, X.W. Berberine protects against NEFA-induced impairment of mitochondrial respiratory chain function and insulin signaling in bovine hepatocytes. Int. J. Mol. Sci. 2018, 19, 1691–1705. [Google Scholar] [CrossRef]
- Archer, A.E.; Von Schulze, A.T.; Geiger, P.C. Exercise, heat shock proteins and insulin resistance. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160529–20160540. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Hugo, E.R.; Brandebourg, T.D.; Lapensee, C.R. Focus on prolactin as a metabolic hormone. Trends Endocrinol. Metab. 2006, 17, 110–116. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, X.; Qu, B.; Wang, J.; Gao, X.; Li, Q. Pten regulates development and lactation in the mammary glands of dairy cows. PLoS ONE 2014, 9, e102118. [Google Scholar] [CrossRef]
- Contreras, G.A.; Sordillo, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef]
- Esposito, G.; Raffrenato, E.; Lukamba, S.D.; Adnane, M.; Irons, P.C.; Cormican, P.; Tasara, T.; Chapwanya, A. Characterization of metabolic and inflammatory profiles of transition dairy cows fed an energy-restricted diet. J. Anim. Sci. 2020, 98, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, L.; Zhang, C.; Cheng, X.; Zhang, Y.; Guo, Y.; Long, M.; Yang, S.; He, J. Palmitic acid and β-hydroxybutyrate induce inflammatory responses in bovine endometrial cells by activating oxidative stress-mediated NF-κB signaling. Molecules 2019, 24, 2421–2435. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Huang, Q.; Guan, Y.; Lv, M.; He, X.; Fang, C.; Wang, X.; Sheng, J. Caffeine promotes conversion of palmitic acid to palmitoleic acid by inducing expression of fat-5 in caenorhabditis elegans and scd1 in mice. Front. Pharmacol. 2018, 9, 321–342. [Google Scholar] [CrossRef]
- Tsai, Y.W.; Lu, C.H.; Chang, R.C.; Hsu, Y.P.; Ho, L.T.; Shih, K.C. Palmitoleic acid ameliorates palmitic acid-induced proinflammation in J774A.1 macrophages via TLR4-dependent and TNF-alpha-independent signallings. Prostaglandins Leukot Essent Fat. Acids 2021, 169, 102270–102278. [Google Scholar] [CrossRef] [PubMed]
- Jesudason, E.P.; Baben, B.; Ashok, B.S.; Masilamoni, J.G.; Kirubagaran, R.; Jebaraj, W.C.; Jayakumar, R. Anti-inflammatory effect of melatonin on A beta vaccination in mice. Mol. Cell Biochem. 2007, 298, 69–81. [Google Scholar] [CrossRef]
- Akter, S.; Uddin, K.R.; Sasaki, H.; Shibata, S. Gamma oryzanol alleviates high-fat diet-induced anxiety-like behaviors through downregulation of dopamine and inflammation in the amygdala of mice. Front. Pharmacol. 2020, 11, 330–343. [Google Scholar] [CrossRef]
- Mann, S.; Yepes, F.A.; Overton, T.R.; Wakshlag, J.J.; Lock, A.L.; Ryan, C.M.; Nydam, D.V. Dry period plane of energy: Effects on feed intake, energy balance, milk production, and composition in transition dairy cows. J. Dairy Sci. 2015, 98, 3366–3382. [Google Scholar] [CrossRef]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Goodarzi, P.; Habibi, M.; Roberts, K.; Sutton, J.; Shili, C.N.; Lin, D.; Pezeshki, A. Dietary tryptophan supplementation alters fat and glucose metabolism in a low-birthweight piglet model. Nutrients 2021, 13, 2561–2583. [Google Scholar] [CrossRef]
- Badawy, A.A. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci. Rep. 2015, 35, e00261–e00277. [Google Scholar] [CrossRef]
- Joseph, K.; Hage, D.S. The effects of glycation on the binding of human serum albumin to warfarin and L-tryptophan. J. Pharm. Biomed. Anal. 2010, 53, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe 2017, 22, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294–3304. [Google Scholar] [CrossRef]
- Cincović, M.; Hristovska, T.; Belić, B. Niacin, Metabolic Stress and Insulin Resistance in Dairy Cows. In B Group Vitamins—Current Uses and Perspectives; LeBlanc, J.G., de Giori, G.S., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef][Green Version]
- Petrovic, K.; Stojanovic, D.; Cincović, M.; Belić, B.; Lakic, I.; Đoković, R. Influence of niacin application on inflammatory parameters, non-esterified fatty acids and functional status of liver in cows during early lactation. Large Anim. Rev. 2021, 27, 17–21. [Google Scholar]
- Proudfoot, K.L. Maternal behavior and design of the maternity pen. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 111–124. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Huang, Y.; Ma, L.; Jiang, J.; Luo, Q.; Yang, Z.; Yong, K.; Shen, L.; Yu, S.; Yao, X.; et al. Untargeted Metabolomics Reveals Metabolic Stress Alleviation by Prepartum Exercise in Transition Dairy Cows. Metabolites 2022, 12, 309. https://doi.org/10.3390/metabo12040309
Luo Z, Huang Y, Ma L, Jiang J, Luo Q, Yang Z, Yong K, Shen L, Yu S, Yao X, et al. Untargeted Metabolomics Reveals Metabolic Stress Alleviation by Prepartum Exercise in Transition Dairy Cows. Metabolites. 2022; 12(4):309. https://doi.org/10.3390/metabo12040309
Chicago/Turabian StyleLuo, Zhengzhong, Yixin Huang, Li Ma, Jing Jiang, Qiao Luo, Zhuo Yang, Kang Yong, Liuhong Shen, Shumin Yu, Xueping Yao, and et al. 2022. "Untargeted Metabolomics Reveals Metabolic Stress Alleviation by Prepartum Exercise in Transition Dairy Cows" Metabolites 12, no. 4: 309. https://doi.org/10.3390/metabo12040309
APA StyleLuo, Z., Huang, Y., Ma, L., Jiang, J., Luo, Q., Yang, Z., Yong, K., Shen, L., Yu, S., Yao, X., Tao, J., & Cao, S. (2022). Untargeted Metabolomics Reveals Metabolic Stress Alleviation by Prepartum Exercise in Transition Dairy Cows. Metabolites, 12(4), 309. https://doi.org/10.3390/metabo12040309






