Abstract
Accumulating evidence indicates that two major proteins are responsible for the structural coherence of bounding cardiomyocytes. These biomolecules are known as myocardial fibrillar collagen type I (COL1) and type III (COL3). In addition, fibronectin, laminin, fibrillin, elastin, glycoproteins, and proteoglycans take part in the formation of cardiac extracellular matrix (ECM). In physiological conditions, collagen synthesis and degradation in human cardiac ECM are well-regulated processes, but they can be impaired in certain cardiovascular diseases, such as heart failure (HF). Myocardial remodeling is part of the central mechanism of HF and involves cardiomyocyte injury and cardiac fibrosis due to increased fibrillar collagen accumulation. COL1 and COL3 are predominantly involved in this process. Specific products identified as collagen-derived peptides are released in the circulation as a result of abnormal COL1 and COL3 turnover and myocardial remodeling in HF and can be detected in patients’ sera. The role of these products in the pathogenesis of cardiac fibrosis and the possible clinical implications are the focus of numerous investigations. This paper reviews recent studies on COL1- and COL3-derived peptides in patients with HF. Their potential application as indicators of myocardial fibrosis and prognostic markers of HF is also highlighted.
1. Introduction
Heart failure (HF) has been recognized as a worldwide health burden that affects approximately 40 million people globally [1]. It has been estimated that the incidence of HF in adults is about 2%, and the rate rises to 6–10% over the age of 65 [2]. For those older than 75 years, the rate is more than 10% [3]. In addition, because of the increased life expectancy and risk factors such as hypertension, diabetes, dyslipidemia, and obesity, the morbidity rate is also expected to rise [4]. It has been reported that in people over the age of 65, heart failure is the leading cause of hospitalization.
Based on left ventricular ejection fraction (LVEF) values, the European Society of Cardiology (ESC) divides HF into three types: with preserved ejection fraction (HFpEF), characterized by LVEF ≥ 50%; mid-range (HFmrEF), with LVEF of 40–49%; and with reduced ejection fraction (HFrEF), with LVEF < 40% [5]. Considering the underlying etiologies, demographics, comorbidities, and responses to therapy, differentiation of HF according to LVEF has significant practical value.
2. Type I and Type III Collagen Characteristics
Collagen (COL) is the main fibrous protein in human ECM, accounting for more than one-third of total protein content in the organism [6]. Practically, it is present in all body systems containing connective tissue. Collagen is responsible for the strength and stability of the cytoskeleton and regulates normal cell and tissue development [7]. Different COL types form collagen fibers, so they represent a heterogeneous mix. However, in any given tissue, a certain type of collagen usually prevails [8].
Collagen type I is a fibrillar protein that makes up a large part of the structure of the interstitial membrane. It is known as the most common type of collagen, and is an important structural component of many tissues. COL1 can be found in almost all connective tissue structures. It is a structural protein found in bones; skin, tendons; ligaments; sclera; corneas; and blood vessels, as well as other tissues. It is a component aligned in fibers, thus forming a structural-mechanical scaffold (matrix) for bones; skin; tendons; corneas; blood vessel walls; and other connective tissues. The dominant isoform of COL1 is heterotrimers with two α1 (I) and one α2 (I) chain. In fetal tissues and some fibrous lesions, homotrimers with three α1 (I) chains have been discovered [9]. The homotrimeric isoform is known to be less susceptible to cleavage by collagenases, which may clarify its accumulation and functional role in tumors and fibrotic lesions [10].
Collagen type III has a unique molecular structure. A long protein chain is responsible for its tensile stiffness and the biomechanical characteristics of tissues. This contributes to the specific ECM properties when this type of collagen predominates. It is an important component of reticular fibers in the interstitial tissue of the lungs, liver, heart, and vessels [11].
Collagen type III is made up of only one collagen α chain. COL3 is a homotrimer made up of three α1 (III) chains overlapped in a right triple helix. It is produced by fibroblasts and other mesenchymal cells and plays an important role in inflammatory conditions such as lung damage, liver diseases, and renal and vascular fibrosis. Consequently, COL3 and COL1 are both important components of the myocardial ECM [12]. Today, immunological markers based on collagen type I and III turnover have been extensively investigated for detection of cardiac fibrosis.
3. Cardiac Extracellular Matrix: Structure and Function
The extracellular matrix (ECM) is made up of a fibrillar network along with a basement membrane, proteoglycans, and fibrous proteins such as fibronectins, collagens, elastins, fibrillins, and laminins [13]. Together they maintain the structural coherence of bounding cells, ensuring stability. The ECM has also been associated with the transmission of important biochemical signals, which are crucial for normal tissue development. ECM is present in all tissues, but each organ has a unique distribution of matrix components [14]. For example, cardiac ECM is primarily composed of collagen type I (85%) and III (11%). The traditional concept of myocardial ECM was that it was an inert mechanical scaffold providing structural cardiac integrity. Nowadays, it is considered as a dynamic network with important metabolic activity and many complex functions, such as regulation of molecular signaling; cell proliferation; differentiation, migration; adhesion; and protein interactions. Moreover, it also regulates myocardial remodeling in normal and pathological conditions. Therefore, cardiac ECM plays a fundamental role in maintaining cardiovascular homeostasis [15]. With the accumulation of additional knowledge about the structure and function of heart ECM, cardiac fibroblasts have been described as the primary source of myocardial COL1 and COL3 peptides. It can be concluded that they are the main cells producing collagen in the heart [14,15].
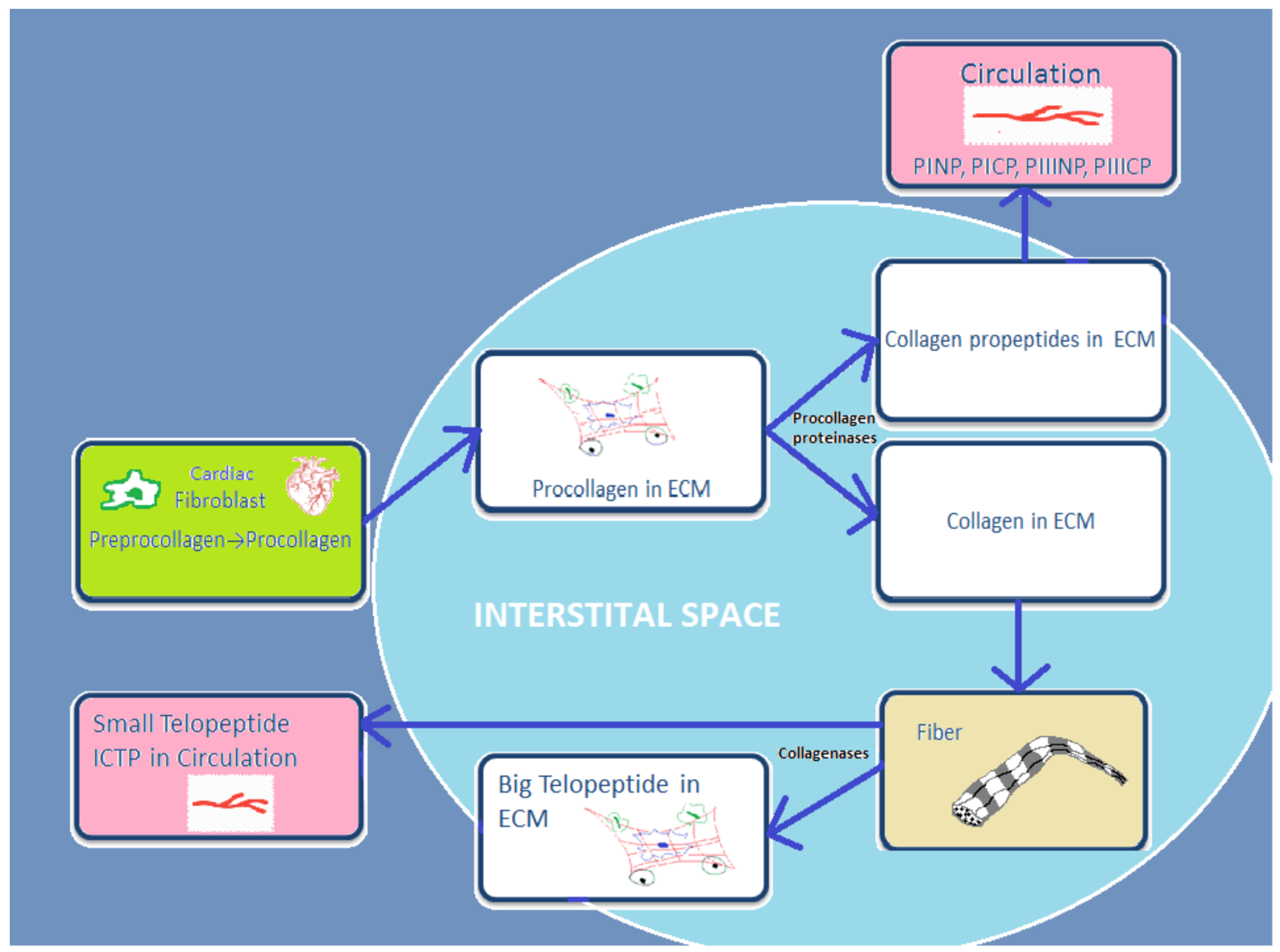
Cardiac fibroblasts are the major heart cells producing COL1 and COL3. Fibrillar collagen is initially synthetized as a procollagen, which is then split by specific proteinases into carboxy (C)- and amino (N)-terminal propeptides: N-terminal propeptides of COL1 and COL3 (PINP and PIIINP) and C-terminal propeptides (PICP and PIIICP). Thereafter, they are secreted in the circulation. Since propeptides are split, the triple helix chain “will form big collagen fibers with other collagen chains” [16]. Collagenases MMP-1, -8, and -13 degrade these collagen fibers, and telopeptides are formed during this process. Then the small telopeptides of collagen type I (ICTP, 12 kDa) are released into the circulation [17]. The big telopeptides go through spontaneous denaturation in nonhelical derivatives [18]. Subsequently, gelatinases MMP-2 and -9 completely degrade them into inactive fragments (Figure 1).
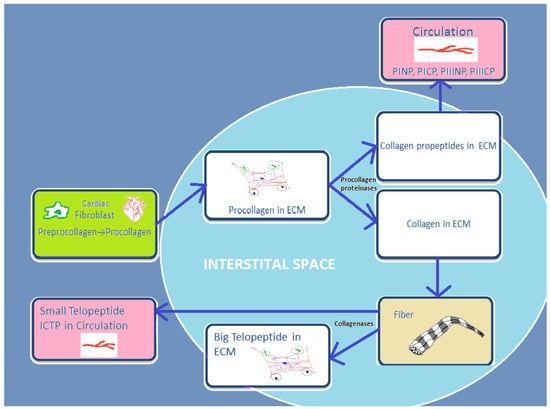
Figure 1.
Schematic presentation of basic stages from process of synthesis and degradation of collagen I and III.
4. General Concepts of Abnormal Cardiac Extracellular Matrix Changes in Heart Failure
ECM is a dynamic structure that plays a crucial role in the development and progression of many cardiovascular diseases. Accumulating data indicate that fibrosis is observed in different cardiovascular diseases (CVDs). HF is an example of abnormal collagen accumulation, which pathologically increases myocardial stiffness and impairs heart contractile properties. Several CVDs, including hypertension; coronary artery disease; valvular disease; and arrhythmias, are considered to be leading causes of HF. A link has been found between cardiac remodeling and the development of HF [19]. Cardiac remodeling is defined as “a group of molecular, cellular and interstitial changes that manifest clinically as alterations in the size, mass, geometry and function of the heart after a stressful stimulus.” [20,21]. This process can be triggered by “ischemia (myocardial infarction), inflammation (myocarditis), hemodynamic overload (workload by volume or pressure) and neurohormonal activation” [22,23,24].
Paradoxically, cardiac remodeling is thought to be both an adaptive and a maladaptive process. Initially, cellular changes in the heart structure, such as myocyte hypertrophy, necrosis, and apoptosis, occur and then extracellular matrix deposition of fibrillar collagen increases (a process often defined as “myocardial fibrosis”) [25,26,27]. This has been related to impaired collagen metabolism, manifesting as accelerated synthesis and accumulation of COL1 and COL3 in the myocardium [28,29]. Accordingly, collagen degradation is slowed down and heart function is inevitably altered in the later stages of cardiac remodeling [30].
5. Basic Underlying Mechanisms of Myocardial Fibrosis in Heart Failure: Role of Impaired Type I and III Collagen Turnover
There is a large body of evidence regarding the accelerated myocardial accumulation of fibrillar collagen in heart failure. Early research on HF showed that various MMPs are present in the myocardium of patients with chronic heart failure [31]; since then, there has been ongoing enthusiasm for the routine application of COL1 and COL3 as biological markers for assessing cardiac tissue remodeling and myocardial fibrosis. This is true for both laboratory models and clinical studies [32]. In important experiments, Douglas et al.’s [31] and Alla’s [32] findings suggested delayed degradation of collagen in patients with chronic HF, thereby contributing to the mechanism of myocardial fibrosis development.
Increased serum levels of COL1 and COL3 synthesis biomarkers (PICP, PINP, PIIINCP, PIIINP) and decreased serum levels of the COL1 degradation biomarker (ICTP) have been linked to myocardial collagen deposition and fibrosis [33,34,35]. According to these findings, the equilibrium between synthesis and degradation of cardiac collagen is disrupted in heart disease [31,32]. Importantly, heart failure is an example of CVD disease that presents with altered collagen turnover [36,37].
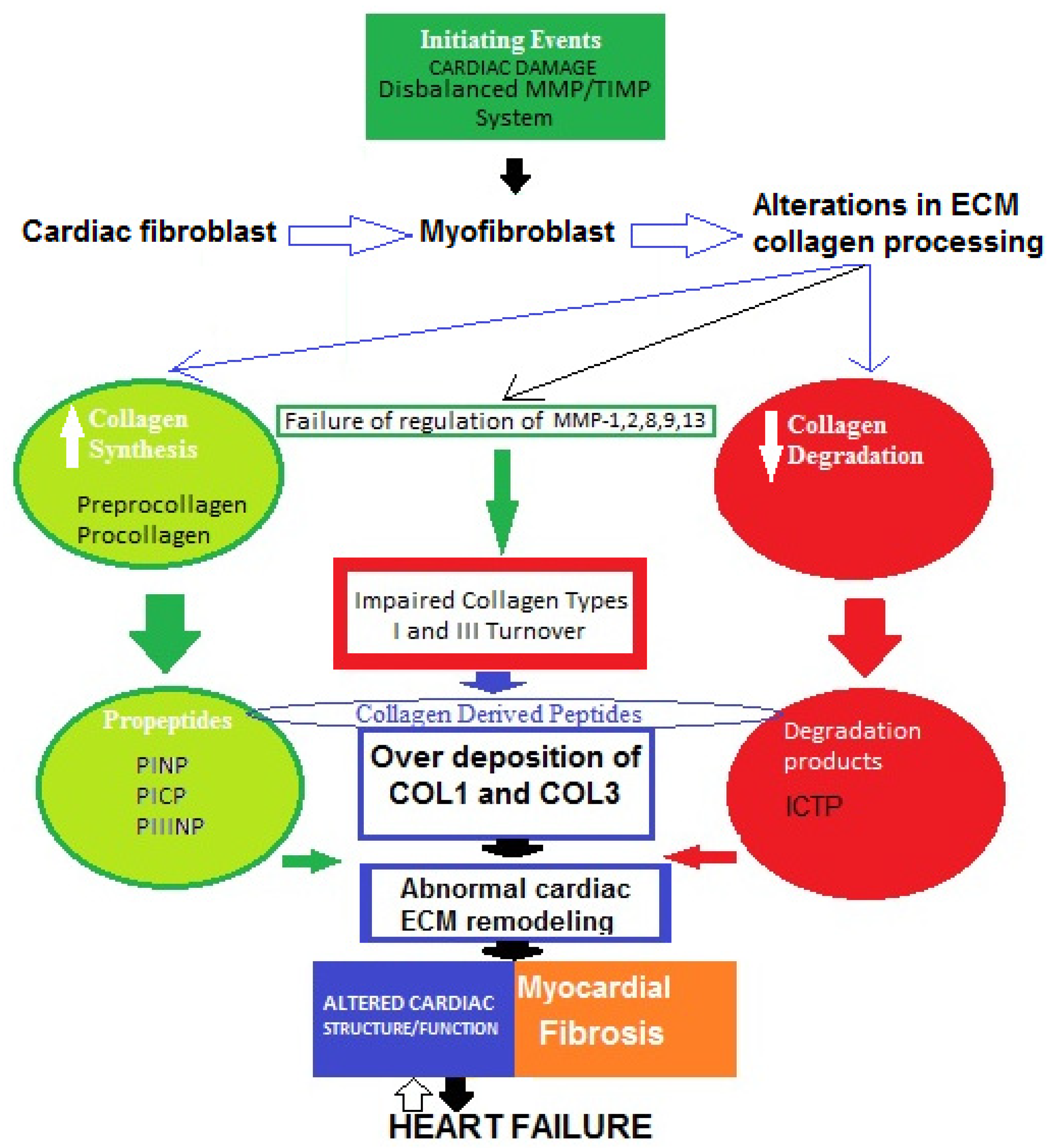
A widely held concept regarding the pathogenesis of myocardial fibrosis is that cardiac lesion is considered to be an initiating event [38]. Thus, the MMP/TIMP system fails and the degradation activity of MMP-1, -2, -8, -9, and -13 is disturbed. As a result, fibroblasts in the heart are hyperactivated and transdifferentiated into myofibroblasts, which increase the production of collagen types I and III, then degradation processes decrease and abnormal collagen deposition in myocardium occurs [39,40]. Therefore, impaired collagen turnover abnormally affects the remodeling of cardiac ECM, and COL1-/COL3-derived peptides are released into the circulation. This can trigger an incessant vicious cycle of COL1/COL3 over-deposition and consequent suppressed degradation.All of these processes might contribute tothe development of cardiac fibrosis in heart failure. However, the molecular mechanisms of the genesis and progression of myocardial fibrosis are not yet fully clear (Figure 2) [39,40,41,42,43,44,45].
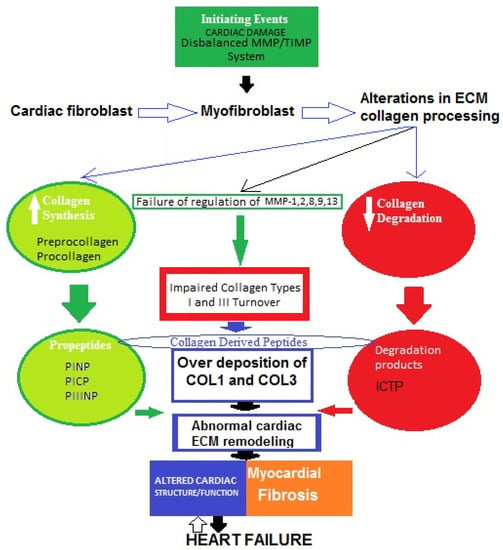
Figure 2.
Possible schematic pattern illustrating eventual mechanisms of impaired collagen I and III turnover leading to myocardial fibrosis in heart failure.
The four major collagen-derived peptides of research interest nowadays are the N-terminal propeptide of collagen type I (PINP), the N-terminal propeptide of collagen type III (PIIINP), the C-terminal propeptide of collagen type I (PICP), and the C-terminal telopeptide of collagen type I (ICTP). PINP and PICP indicate collagen type I synthesis, PIIINP reflects collagen type III synthesis, and ICTP represents collagen type I degradation. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) are other molecules produced by cardiac fibroblasts. MMPs are proteolytic enzymes that degrade ECM proteins and TIMPs are MMP inhibitors, maintaining a fine balance between synthesis and degradation processes. Thus, both MMPs and TIMPs are regulatory proteins essential for ECM homeostasis.
6. Collagen Type I and III Derived Peptides as Biomarkers in Heart Failure
6.1. Detection of Circulating PIIINP
PIIINP has been described as a parameter of COL3 synthesis. A large part of serum PIIINP is produced during the extracellular conversion of pro-COL3 to COL3 by procollagen amino-terminal proteinase. The concentration of PIIINP in serum has been associated with the myocardial area fractions of their tissue analogues [46]. In an important investigation, Klappacher et al. [47] examined the sera of patients with dilated cardiomyopathy (DCM) and found accelerated myocardial extracellular matrix turnover. Of note, PIIINP also has an effect on the determination of risk and prognosis. Additionally, it was found that, in HF patients treated with spironolactone, a decrease in collagen volume fraction was found to occur with a decrease in serum PIIINP levels [48]. Most recently, in a study involving patients with acute heart failure (AHF), Nagao et al. investigated the time-dependent prognostic utility of PIIINP and type IV collagen 7S (P4NP 7S)). P4NP 7S significantly decreased during hospitalization, whereas PIIINP did not. The authors concluded that there was no correlation between increased PIIINP levels and significant excess risk for both 90-day and 365-day outcomes [49].
Several authors have described that serum PIIINP is associated with outcomes in HF of different causes, regardless of EF. For example, Zannad et al. [50] reported decreased survival rates in patients with HFrEF with a PIIINP cut-off point of >3.85 μg/L, whereas the data of Klappacher et al. showed a PIIINP cut-off point of >7 μg/L, with the clarification that the latter’s patients all had DCM (33 idiopathic and 8 ischemic cases). It appears that patients with HF and dilated hypertrophic cardiomyopathy (HCM) have significantly higher serum PIIINP levels than healthy controls. Hypertensive patients with HFpEF also showed significantly higher serum PIIINP concentrations than both hypertensive HFrEF and HFmrEF patients [51]. With regard to HFmrEF, there is only one study with such patients, which shows decreased survival with PIIINP > 4.7 μg/L [52]. Elevated PIIINP levels are related to decreased survival rates in both HF and DCM [52,53]. Of note, serum PIIINP levels are also significantly higher in patients with acute myocardial infarction, and a PIIINP cut-off point of >5 μg/L was determined as an independent predictor of cardiac death and in-hospital development of congestive HF [54].
Recently, Nikolov et al. found significantly higher circulating levels of serum PIIINP in patients with HFmrEF and coronary artery disease (CAD) with left ventricular hypertrophy (LVH) than in patients with HFmrEF and CAD without LVH [55]. Another study, by Lee et al. investigated the link between PIIINP, left ventricular end-diastolic pressure (LVEDP), and cardiovascular events in patients with acute coronary syndrome (ACS). The authors reported that PIIINP is very useful in evaluating left ventricular end-diastolic pressure. Moreover, they noted that PIIINP is related to cardiac mortality and revascularization. Hence, they provided not only an additional means of evaluating patients with ACS, but also important treatment directions [56]. Eastell et al. have described that serum PIIINP is associated with severity of HF of different causes, regardless of EF [57].
Barasch et al. found that both HFrEF and HFpEF were associated with significantly elevated amino-terminal peptide of pro-COL3 [58].
Zile et al. reported increased PIIINP levels in HF patients [59]. Previously, Schwartzkopff et al. described PIIINP as an independent predictor of HF mortality [60]. In their study, Michalski et al. examined HFrEF vs. HFpEF patients and reported that PIIINP showed a strong negative correlation with left ventricular strain [61]. Zile’s results were further confirmed by the large Multi-Ethnic Study of Atherosclerosis (MESA) in 2018. MESA evaluated the predictive value of serum PIIINP and ICTP in 3187 patients with HF who were divided into two subgroups, with and without preserved ejection fraction [62]. MESA concluded that elevated levels of circulating ICTP and PIIINP were related to the incidence of HFpEF, but not HFrEF. Table 1 presents the findings from studies determining PIIINP levels in patients with heart failure.

Table 1.
Serum PIIINP concentrations in patients with heart failure.
6.2. Detection of Circulating PINP
Procollagen type I propeptides are products derived from the COL1 molecule. This precursor is composed of a brief signal sequence and two peptides, amino-terminal propeptide (PINP) and carboxy-terminal propeptide (PICP). Propeptide extensions are later removed by specific proteinases and can be found in the circulation. Their serum levels indicate the COL1 synthesis rate [65]. PINP was cited as an indicator of COL1 synthesis. Interestingly, PINP concentration is not significantly different in patients with HCM [66]. According to Martos et al. this is also valid for hypertensive patients with or without diastolic HF, compared with healthy controls.
In order to estimate the predictive value of PINP, PIIINP, and ICTP in HF, Dupuy et al. examined these parameters together with two mediators of cardiac fibrosis, galectin-3 and soluble suppression of tumorigenicity-2 protein (sST2). The authors concluded that a multi-marker strategy demonstrated the greatest prognostic improvement when “attained with the combined addition of ICTP/PIIINP ratio and sST2 highlighting the potential role of fibrosis pathways in risk stratification” [67].
6.3. Detection of Circulating PICP
PICP acts as an indicator of COL1 synthesis. Serum C-terminal propeptide of pro-COL1 is produced during the extracellular conversion of pro-COL1 into COL1. This reaction is catalyzed by the enzyme procollagen C-terminal proteinase [46]. Querejeta et al. provided evidence that a net release from the heart into the circulation occurs in HF [68]. In 2021, He et al. studied subjects with suspected HF from the HOMAGE-HULL sub-cohort. They used ELISA to quantify the collagen synthesis biomarker PICP and collagen degradation biomarkers ICTP and matrix metalloproteinase (MMP-1). They detected a significantly positive association between heart failure death and ICTP and MMP-1, but not PICP [69].
This supports the suggestion that systemic PICP has a cardiac origin. Additionally, serum PICP levels were related to collagen volume fraction [70,71,72].
PICP circulation has been the subject of many studies. For example, Lopez et al. [73] concluded that PICP levels decreased in patients with hypertensive heart failure who receivedtorasemide treatment. Löfsjögård et al. reported that serum PICP is associated with HFrEF severity [74], whereas Krum et al. [75] noted that PICP is related to mortality in HFpEF. In 2017, Löfsjögård et al. [76] reported that PICP is linked with mortality in HFrEF. Of interest, Flevari et al. found that the serum PICP-to-PIIINP ratio is related to malignant ventricular arrhythmogenesis in HF [77]. When analyzing these data, it can be observed that PICP concentrations are significantly increased in more studies of patients with HFpEF than HFrEF. Except in the studies of Alla et al. [32] and Plaksej et al. [63], patients with HF have been shown to have higher serum PICP levels than controls. Both serum and coronary PICP are positively correlated with the myocardial collagen content [78]. The investigation by Quaretta et al. is the only research involving subjects with HFmrEF, and it reports that the abnormal increase in cardiac COL1 synthesis and deposition may play an important role in the augmentation of myocardial fibrosis in HF from hypertensive origin [79]. Similarly, analysis of PICP in patients with HCM and in patients with cardiomyopathies shows elevated serum PICP in patients with mild to moderate dilated cardiomyopathy [64,80,81].
Ruiz-Ruiz et al. examined the relationship between serum levels of propeptide of procollagen type I and outcomes in 111 patients with decompensated heart failure, considering death from any cause or due to heart failure and readmission as primary endpoints. Serum levels of propeptide of procollagen type I were significantly higher among patients who experienced either endpoint during follow-up. The investigators suggested that “a single serum measurement of propeptide of procollagen type I may possibly have prognostic value in patients with decompensated heart failure”. Accordingly, patients with higher levels of propeptide of procollagen type I at decompensation are at a higher risk of death or readmission during follow-up [82].
In another study, Raafs et al. tested whether the combination of blood PICP levels and late gadolinium enhancement (LGE) in cardiac magnetic resonance (CMR) could provide additional prognostic information in idiopathic dilated cardiomyopathy. Using those methods plus invasive endomyocardial biopsy (EMB) and collagen volume fraction, (CVF), the authors quantified fibrosis in 209 DCM patients. The major conclusion was that a “combination of myocardial fibrosis at CMR and circulating PICP levels provides additive prognostic value accompanied by a pro-fibrotic and pro-inflammatory transcriptomic profile in DCM patients with LGE and elevated PICP” [83]. Table 2 represents the findings from the studies determining PICP levels in patients with heart failure.

Table 2.
Serum PICP concentrations in subjects with heart failure.
6.4. Detection of Circulating ICTP
ICTP is an indicator of COL1 degradation. Studies have shown that ICTP concentrations are increased in both HFrEF and HFpEF patient groups. Patients with DCM and HCM also express elevated serum ICTP levels. Previously, Klappacher et al. indicated that serum ICTP is positively correlated with myocardial collagen content. Later, Kitahara et al. concluded that ICTP is a predictor of mortality at levels > 7.6 μg/L [84].
Plaksej et al. evaluated serum ICTP levels in hypertensive patients with HF and reported increased concentrations in those with New York Heart Association (NYHA) class IV HF [63]. On the other hand, Barasch et al. did not find an association between ICTP and HFrEF or HFpEF. Manhenke et al. [85] demonstrated that ICTP is an independent predictor of total and cardiovascular mortality in patients with an acute myocardial infarction.
Another intriguing approach in the search for HF indicators is to assess the ratio of ECM components. For example, the ICTP-to-MMP-1 ratio was recently studied by López et al. for evaluation as a novel biomarker. It has been theorized that, since collagen cross-linking modifies the resistance of collagen fiber to MMP degradation, the higher the cross-linking of COL1 fibers, the lower the cleavage of ICTP by MMP-1. Consequently, the serum ICTP-to-MMP-1 ratio is inversely correlated with myocardial collagen cross-linking [86]. López et al. also found that the ICTP-to-MMP-1 ratio is independently associated with the risk of HF hospitalization. Furthermore, Ravassa et al. provided evidence for an integrative strategy combining the ICTP-to-MMP-1 ratio with PICP measurement. With this test, a decreased ICTP-to-MMP-1 ratio and elevated PICP concentrations indicated HF patients with the highest risk [87].
Similarly, Zile et al. detected increased ICTP levels, but in HFpEF patients. Schwartzkopff et al. found increased ICTP levels in HFmrEF patients. Ristelli et al. and Cornelissen et al. also analyzed connective tissue metabolites in human serum [88,89]. Batlle et al. reported elevated ICTP concentrations and increased risk of a clinical event [90]. In 2018, MESA demonstrated high levels of circulating ICTP. Table 3 presents findings from the studies determining ICTP levels in patients with heart failure.

Table 3.
Serum levels of ICTP in patients with heart failure.
Curiously, in recent years, only two studies have assessed myocardial fibrosis in patients with severe aortic stenosis and HF symptoms. First, Echegaray et al. analyzed the myocardial collagen volume fraction from left ventricular free wall transmural biopsies and suggested that in symptomatic HFpEF patients with severe aortic stenosis, diastolic dysfunction is related to intensified nonmysial deposition of COL1. This leads to increased ECM stiffness [91]. Later, Park et al. reported that extracellular volume is more closely related to predictions of clinical outcomes in severe aortic stenosis than native T1 mapping sequences generated from cardiac magnetic resonance or global longitudinal strain measured by speckle-tracking echocardiography, although the result was not statistically significant [92]. As for atrial fibrillation (AF), Ravassa et al. examined circulating COL1 biomarkers in samples from both HF patients and patients referred for AF ablation. The researchers found that serum indicators of intensified myocardial COL1 cross-linking and deposition are associated with higher AF prevalence, incidence, and recurrence after ablation [93].
Beyond the diagnosis of myocardial fibrosis, Ravassa et al., Hinderer et al., Bing et al., and Lopez et al. [39,40,41,42] highlighted the importance of circulating biomarkers of fibrillar COL1- and COL3-derived peptides as valuable non-invasive prognostic tools for determining clinical outcomes of patients with HF.
7. Limitations of and Future Prospects for the Application of COL1 and COL3 Derived Peptides as Biomarkers of Myocardial Fibrosis and Prognostic Indicators of Heart Failure
In light of the above-mentioned reports, a question of great interest arises: is it possible that circulating COL1 and COL3 peptides mark the development of myocardial fibrosis and even predict a prognosis of heart failure? Our current approach to collagen-derived peptides as parameters of COL1 and COL3 metabolism assumes that they have the potential to be used in routine clinical settings in the near future. Despite this argument, the studies discussed here contain some controversial findings and limitations. Several remarks uncover gaps in the possible application of PINP, PICP, PIIINP, and ICTP as biomarkers of myocardial fibrosis and prognostic indicators of heart failure.
Alterations in COL1 and COL3 metabolism are likely to occur early in the course of heart failure, even before clinical manifestation of HF. A large proportion of the cited research involved patients with established HF; therefore, it cannot be determined whether the change in collagen-derived peptide levels developed before or after the onset of HF. To better understand the link between these markers and concurrent HF, it would be appropriate to closely monitor a group of subjects with clinically unmanifested HF and test them for abnormal changes in COL1 and COL3 turnover biomarkers. Moreover, different studies involved diverse patient groups, such as patients with HFpEF [51,63,66,68,78,85,87]; HFmrEF [52,55,62,86]; or HFrEF [32,50,67,69,78,87]; or did not specify any subgroups [54,61,71,73,78,80,81,85,88,89]. Some studies investigated patients with DCM [47,53,77,86] or HCM [74,75]. The diversity of cohorts makes it very difficult to perform a head-to-head comparison of the results obtained from these experiments.
Whether COL1 and COL3 turnover indicators alone might provide enough prognostic information, or a combination model with other known cardiovascular markers added such as natriuretic peptides and galectins would be useful, is another exciting question. Furthermore, image tests such as contrast CMRs are also used for the detection of myocardial fibrosis. In this regard, it may be more reasonable to use an integrated model that combines blood markers and image tests [77], rather than just one of them, for better prognosis of heart failure. This theory requires additional investigation.
Considering the studies covered in this review, some major differences can be observed between the methods applied for detection of serum markers of COL1 and COL3 metabolism. For non-invasive assessment and measurement of concentrations, researchers used radioimmunoassay [32,34,47,53,62,63,68,71,72,77,78], ELISA [49,52,55,59,60,61,65,66,67,69,75], and both of these [51]. Therefore, some differences can be observed between the cut-off points for detection of serum levels of COL1 and COL3 peptides. This suggests that not only the absolute parameter levels, but also their ratios [60,63,69,70,71,72,73,74,75,76,77,78,79,80,81], may be relevant in the assessment of myocardial remodeling and the risk of HF hospitalization.
Another important feature that should also be pointed out is the etiology of heart failure, because it can affect collagen turnover in a variety of ways. Hypertension, for instance, is associated with cardiac myocyte hypertrophy and increased stiffness of the ventricular wall, implying an increase in collagen fibers. As a result, patients with underlying hypertension express higher levels of COL1 and COL3 synthesis markers such as PIIINP and PICP. The levels of serum collagen-derived peptides can also be affected by different patient characteristics, such as the type of HF treatment, comorbidities, age, and BMI. In addition, the heart failure functional class should also be taken into account. It is important to distinguish whether collagen peptides are measured in patients with NYHA class I-II or III-IV. Likewise, it is important to highlight whether studies include subjects with acute or chronic heart failure, because these conditions can affect the concentrations of circulating collagen peptides.
It is well known that cardiac remodeling is a continual process. Unfortunately, most studiesused a cross-sectional design and indicated COL1 and COL3 peptide concentrations at only a certain moment. Instead, these biomarkers should be determined in serial measurements at various time points. There is a need for larger, longitudinal investigations.
COL1- and COL3-derived peptides are not entirely cardiac-specific, and changes in their levels may represent a wide range of cardiovascular pathologies, including comorbidities. Fibrosis can affect many organs, and it is possible that impaired collagen type I and III turnover (manifested by elevated serum concentrations of these markers) may have either cardiac or extracardiac origin. Notably, collagen-derived peptides can also be produced by other organs, such as bone, liver, kidney, lung, etc. [83]. Currently, EMB with evaluation of collagen volume fraction represents the most reliable test for the diagnosis of myocardial fibrosis. However, EMB is an invasive method based on histological analysis, and non-invasive tools would be more appropriate for routine practice. CMR imaging is precise, but has a high price and requires trained personnel. That is why research is aimed at investigating new blood markers such as COL1- and COL3-derived peptides. Their serum levels have been reported to correlate with the collagen volume fraction. Although these peptides are not entirely cardiac typical, important relationships have been found between their blood concentration and histologically assessed myocardial collagen deposition, HF severity, and prognosis. In order to more completely determine the differences in leakage from organs other than the heart, more studies exploring cardiac collagen type I and III turnover are needed. They could possibly detect novel molecules as entirely myocardial specific COL1 and COL3 precursors, enzymes, or degradation products. It also remains to be clarified whether medicament treatment could affect the elimination of collagen peptides by the liver and kidneys, as well as the kinetics and dynamics of these processes in the cardiac extracellular matrix.
8. Conclusions
Based on the current understanding, myocardial fibrosis in heart failure is associated with impaired collagen type I and III turnover, abnormal cardiac ECM remodeling, and the development of structural and functional heart changes. The modification of myocardial collagen content is a crucial factor in these pathological processes. As a result, COL1- and COL3-derived peptides are released into the circulation. These biomolecules have promising potential as markers of myocardial fibrosis and could be useful in the prognosis of heart failure.
Author Contributions
Conceptualization, A.N.; Methodology, N.P.; Writing—original draft preparation, A.N.; Writing—review and editing, A.N.; Visualization, N.P.; Supervision, A.N. and N.P. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Medical University-Pleven, Bulgaria.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GBD 2015. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study. Lancet 2015, 388, 10053. [Google Scholar]
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.; Ponikowski, P.; Poole-Wilson, P.A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. Heart J. 2018, 29, 2388–2442. [Google Scholar]
- Metra, M.; Teerlink, J.R. Heart failure. Lancet 2017, 390, 1981–1995. [Google Scholar] [CrossRef]
- Mann, D.L.; Chakinala, M. Harrison’s Principles of Internal Medicine: Heart Failure and Cor Pulmonale, 18th ed.; McGraw-Hill: New York, NY, USA, 2012; Chapter 234; pp. 1–11. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wever, O.; Demetter, P.; Mareel, M.; Bracke, M. Stromal myofibroblasts are drivers of invasive cancer growth. Int. J. Cancer 2008, 123, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriksen, K.; Karsdal, M.A. Type I Collagen. In Biochemistry of Collagens, Laminins and Elastin Structure, Function and Biomarkers, 1st ed.; Karsdal, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 1; pp. 1–11. [Google Scholar]
- Han, S.; Makareeva, E.; Kuznetsova, N.V. Molecular mechanism of type I collagen homotrimer resistance to mammalian collagenases. J. Biol. Chem. 2010, 285, 22276–22281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Der Mark, K. Localization of collagen types in tissues. Int. Rev. Connect. Tissue Res. 1981, 9, 265–324. [Google Scholar]
- Nielsen, M.J.; Karsdal, M.A. Type III Collagen. In Biochemistry of Collagens, Laminins and Elastin Structure, Function and Biomarkers, 1st ed.; Karsdal, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 3; pp. 21–30. [Google Scholar]
- Jarvelainen, H.; Sainio, A.; Koulu, M.; Wight, T.N.; Penttinen, R. Extracellular matrix molecules: Potential targets in pharmacotherapy. Pharmacol. Rev. 2009, 61, 198–223. [Google Scholar] [CrossRef] [Green Version]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: London, UK, 2007. [Google Scholar]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.R.; Sutton, M.G.; Lie, J.T. Histopathological types of cardiac fibrosis in myocardial disease. J. Pathol. 1978, 128, 79–85. [Google Scholar] [CrossRef]
- Eghbali, M.; Czaja, M.J.; Zeydel, M.; Weiner, F.; Zern, M.A.; Seifter, S.; Blumenfefd, O.O. Colllagen chain mRNAs in isolated heart cells from young and adult rats. J. Mol. Cell. Cardiol. 1988, 20, 267–276. [Google Scholar] [CrossRef]
- Lijnen, P.; Petrov, V.; Fagard, R. Induction of cardiac fibrosis by transforming growth factor-β1. Mol. Gen. Metab. 2000, 71, 418–435. [Google Scholar] [CrossRef]
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling-concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Hockman, J.S.; Bulkley, B.H. Expansion of acute myocardial infarction: An experimental study. Circulation 1982, 65, 1446–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, M.A.; Braunwald, E. Ventricular remodeling after myocardial infarction: Experimental observations and clinical implications. Circulation 1990, 81, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Gaasch, W.H. Left ventricular radius to wall thickness ratio. Am. J. Cardiol. 1979, 43, 1189–1194. [Google Scholar] [CrossRef]
- Sayer, G.; Bhat, G. The renin-angiotensin-aldosterone system and heart failure. Cardiol. Clin. 2014, 32, 21–32. [Google Scholar] [CrossRef]
- Florea, V.G.; Cohn, J.N. The autonomic nervous system and heart failure. Circ. Res. 2014, 114, 1815–1826. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.B.; Jalil, J.E.; Pick, R. Cardiac myocyte necrosis induced by angiotensin II. Circ. Res. 1991, 69, 1185–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharov, V.G.; Sabbah, H.N.; Shimoyama, H. Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. Am. J. Pathol. 1996, 148, 141–149. [Google Scholar] [PubMed]
- Teiger, E.; Dam, T.V.; Richard, L. Apoptosis in pressure overload—Induced heart hypertrophy in the rat. J. Clin. Investig. 1996, 97, 2891–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivetti, G.; Abbi, R.; Quaini, F. Apoptosis in the failing human heart. N. Engl. J. Med. 1997, 336, 1131–1141. [Google Scholar] [CrossRef]
- Villarreal, F.J.; Kim, N.N.; Ungab, G.D. Identification of functional angiotensin II receptors on rat cardiac fibroblastos. Circulation 1993, 88, 2849–2861. [Google Scholar] [CrossRef] [Green Version]
- Weber, K.T.; Pick, R.; Silver, M.A. Fibrillar collagen and remodeling of dilated canine left ventricle. Circulation 1990, 82, 1387–1401. [Google Scholar] [CrossRef] [Green Version]
- Douglas, L.; Mann, M.D.; Spinale, F.G. Activation of Matrix Metalloproteinases in the Failing Human Heart Breaking the Tie That Binds. Circulation 1998, 98, 1699–1702. [Google Scholar]
- Alla, F. Early changes in serum markers of cardiac extra-cellular matrix turnover in patients with uncomplicated hypertension and type II diabetes. Eur. J. Heart Fail. 2006, 8, 147–153. [Google Scholar] [CrossRef]
- Diez, J.; Querejeta, R.; Lopez, B. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 2002, 105, 2512–2517. [Google Scholar] [CrossRef] [Green Version]
- Laviades, C.; Varo, N.; Fernandez, J. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation 1998, 98, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.; Takawale, A.; Lee, J. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 2012, 5, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eghbali, M. Cardiac fibroblasts: Function, regulation of gene expression, and phenotypic modulation. Basic Res. Cardiol. 1992, 87 (Suppl. S2), 183–189. [Google Scholar] [PubMed]
- Moore, L.; Fan, D.; Basu, R. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail Rev. 2012, 17, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyösi, M.; Winkler, J.; Ramos, I. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Heart Fail. 2017, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Ravassa, S.; Lopez, B.; Querejeta, R.; Echegaray, K.; San-Jose, G.; Moreno, M.U.; Beaumont, F.J.; Gonzalez, A.; Diez, J. Phenotyping of myocardial fibrosis in hypertensive patients with heart failure. Influence on clinical outcome. J. Hypertens. 2017, 35, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, S.; Schenke-Layland, K. Cardiac fibrosis—A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82. [Google Scholar] [CrossRef]
- Bing, R.; Dweck, M.R. Myocardial fibrosis: Why image, how to image and clinical implications. Heart 2019, 105, 1832–1840. [Google Scholar] [CrossRef] [Green Version]
- Lopez, B.; Ravassa, S.; Moreno, M.U.; San José, G.; Beaumont, J.; González, A.; Díez, J. Diffuse myocardial fibrosis: Mechanisms, diagnosis and therapeutic approaches. Nat. Rev. Cardiol. 2021, 18, 479–498. [Google Scholar] [CrossRef]
- Espeland, T.; Lunde, I.G.; Amundsen, B.H.; Gullestad, L.; Aakhus, S. Myocardial fibrosis. Tidsskriftet 2018, 138. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac fibrosis: The fibroblast awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Song, D.; Dong, J.; Zhu, P.; Liu, J.; Liu, W.; Ma, X.; Zhao, L.; Ling, S. Current unserstandings of the pathophysiology of myocardial fibrosis and its quantitative assessment in heart failure. Front. Physiol. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Kivirikko, K.I. Collagens: Molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995, 64, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Klappacher, G.; Franzen, P.; Haab, D. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am. J. Cardiol. 1995, 75, 913–918. [Google Scholar] [CrossRef]
- Izawa, H.; Murohara, T.; Nagata, K. Mineralocorticoid eceptor antagonism ameliorates left entricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: A pilot study. Circulation 2005, 112, 2940–2945. [Google Scholar] [CrossRef] [Green Version]
- Nagao, K.; Tamura, A.; Sato, Y.; Hata, R.; Kawase, Y.; Kadota, K.; Horie, T.; Sowa, N.; Nishiga, M.; Ono, K.; et al. Utility of collagen-derived peptides as markers of organ injury in patients with acute heart failure. Open Heart 2020, 7, e001041. [Google Scholar] [CrossRef] [Green Version]
- Zannad, F.; Alla, F.; Dousset, B.; Perez, A.; Pitt, B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: Insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation 2000, 102, 2700–2706. [Google Scholar] [CrossRef] [Green Version]
- Martos, R.; Baugh, J.; Ledwidge, M.; O’Loughlin, C.; Conlon, C.; Patle, A.; Donelly, S.C.; McDonald, K. Diastolic heart failure: Evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation 2007, 115, 888–895. [Google Scholar] [CrossRef] [Green Version]
- Cicoira, M.; Rossi, A.; Bonapace, S.; Zanolla, L.; Golia, G.; Franceschini, L.; Caruso, B.; Marino, P.N.; Zardini, P. Independent and additional prognostic value of aminoterminalpropeptide of type III procollagen circulating levels in patients with chronic heart failure. J. Card. Fail. 2004, 10, 403–411. [Google Scholar] [CrossRef]
- Sato, Y.; Kataoka, K.; Matsumori, A.; Sasayama, S.; Yamada, T.; Ito, H.; Takatsu, Y. Measuring serum aminoterminal type III procollagen peptide, 7S domain of type IV collagen, and cardiac troponin T in patients with idiopathic dilated cardiomyopathy and secondary cardiomyopathy. Heart 1997, 78, 505–508. [Google Scholar] [CrossRef]
- Poulsen, S.H.; Host, N.B.; Jensen, S.E.; Egstrup, K. Relationship between serum amino-terminal propeptide of type III procollagen and changes of left ventricular function after acute myocardial infarction. Circulation 2000, 101, 1527–1532. [Google Scholar] [CrossRef]
- Nikolov, A.G.; Tzekova, M.L.; Kostov, K.M.; Popovski, N.K. Circulating serum markers of collagen type III synthesis in high atherogenic risk patients with heart failure and coronary artery disease. Atherosclerosis 2020, 315, e257. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, W.C.; Chang, S.H.; Wen, M.S.; Hung, K.C. The N-Terminal Propeptide of Type III Procollagen in Patients with Acute Coronary Syndrome: A Link between Left Ventricular End-Diastolic Pressure and Cardiovascular Events. PLoS ONE 2015, 10, e114097. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Krege, J.H.; Chen, P. Development of an algorithm for using PINP to monitor treatment of patients with teriparatide. Curr. Med. Res. Opin. 2006, 22, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Barasch, E.; Gottdiener, J.S.; Aurigemma, G.; Kitzman, D.W.; Han, J.; Kop, W.J.; Tracy, R.P. Association between elevated fibrosis markers and heart failure in the elderly: The cardiovascular health study. Circ. Heart Fail. 2009, 2, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zile, M.R.; Desantis, S.M.; Baicu, C.F.; Stroud, R.E.; Thompson, S.B.; McClure, C.D.; Mehurg, S.M.; Spinale, F.G. Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ. Heart Fail. 2011, 4, 246–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartzkopff, B.; Fassbach, M.; Pelzer, B.; Brehm, M.; Strauer, B. Elevated serum markers of collagen degradation in patients with mild to moderate dilated cardiomyopathy. Eur. J. Heart Fail. 2002, 4, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Michalski, B.; Trzciński, P.; Kupczyńska, K.; Miśkowiec, D.; Pęczek, L.; Nawrot, B.; Lipiec, P.; Kasprzak, J. The differences in the relationship between diastolic dysfunction, selected biomarkers and collagen turn-over in heart failure patients with preserved and reduced ejection fraction. Cardiol. J. 2017, 24, 35–42. [Google Scholar] [CrossRef]
- Duprez, D.A.; Gross, M.D.; Kizer, J.R.; Ix, J.H.; Hundley, W.G.; Jacobs, D.R. Predictive value of collagen biomarkers for heart failure with and without preserved ejection fraction: mESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Heart Assoc. 2018, 7, e007885. [Google Scholar] [CrossRef]
- Plaksej, R.; Kosmala, W.; Frantz, S.; Herrmann, S.; Niemann, M.; mStörk, S.; Wachter, R.; Angermann, C.E.; Ertl, G.; Bijnens, B.; et al. Relation of circulating markers of fibrosis and progression of left and right ventricular dysfunction in hypertensive patients with heart failure. J. Hypertens. 2009, 27, 2483–2491. [Google Scholar] [CrossRef]
- Fassbach, M.; Schwartzkopff, B. Elevated serum markers for collagen synthesis in patients with hypertrophic cardiomyopathy and diastolic dysfunction. Z. Kardiol. 2005, 94, 328–335. [Google Scholar] [CrossRef]
- Vasikaran, S.; Eastell, R.; Bruyere, O. IOF-IFCC Bone Marker Standards Working Group. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011, 22, 391–420. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Betocchi, S.; Cacace, A.; Losi, M.A.; Chiariello, M. Fibrosiinterstizialemiocardica e disfunzionediastolicanellacardiomiopatiaipertrofica [Myocardial interstitial fibrosis and diastolic dysfunction in hypertrophic cardiomyopathy]. Ital. Heart J. Suppl. 2003, 4, 645–650. [Google Scholar] [PubMed]
- Dupuy, A.M.; Kuster, N.; Curinier, C.; Huet, F.; Plawecki, M.; Solecki, K.; Roubille, F.; Cristol, J.P. Exploring collagen remodeling and regulation as prognosis biomarkers in stable heart failure. Clin. Chim. Acta 2019, 490, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Querejeta, R.; López, B.; González, A. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: Relation to myocardial fibrosis. Circulation 2004, 110, 1263–1268. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Melgarejo, J.D.; Clark, A.L. Serum and urinary biomarkers of collagen type-I turnover predict prognosis in patients with heart failure. Clin. Transl. Med. 2021, 11, e267. [Google Scholar] [CrossRef]
- Yang, C.; Qiao, S.; Song, Y.; Liu, Y.; Tang, Y.; Deng, L.; Yuan, J.; Hu, F.; Yang, W. Procollagen type I carboxy-terminal propeptide (PICP) and MMP-2 are potential biomarkers of myocardial fibrosis in patients with hypertrophic cardiomyopathy. Cardiovasc Pathol. 2019, 43, 107150. [Google Scholar] [CrossRef]
- Ho, C.Y.; López, B.; Coelho-Filho, O.R.; Lakdawala, N.K.; Cirino, A.L.; Jarolim, P.; Kwong, R.; González, A.; Colan, S.D.; Seidman, J.G.; et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N. Engl. J. Med. 2010, 363, 552–563. [Google Scholar] [CrossRef] [Green Version]
- Ureche, C.; Nedelcu, A.E.; Sascău, R.A.; Stătescu, C.; Kanbay, M.; Covic, A. Role of collagen turnover biomarkers in the noninvasive assessment of myocardial fibrosis: An update. Biomark Med. 2020, 14, 1265–1275. [Google Scholar] [CrossRef]
- López, B.; Querejeta, R.; González, A. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: Potential role of lysyl oxidase. Hypertension 2012, 60, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Löfsjögård, J.; Kahan, T.; Díez, J. Biomarkers of collagen type I metabolism are related to B-type natriuretic peptide, left ventricular size, and diastolic function in heart failure. J. Cardiovasc. Med. 2014, 15, 463–469. [Google Scholar] [CrossRef]
- Krum, H.; Elsik, M.; Schneider, H.G. Relation of peripheral collagen markers to death and hospitalization in patients with heart failure and preserved ejection fraction: Results of the I-PRESERVE collagen substudy. Circ. Heart Fail. 2011, 4, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löfsjögård, J.; Kahan, T.; Díez, J. Usefulness of collagen carboxy-terminal propeptide and telopeptide to predict disturbances of long-term mortality in patients >60 years with heart failure and reduced ejection fraction. Am. J. Cardiol. 2017, 119, 2042–2048. [Google Scholar] [CrossRef]
- Flevari, P.; Theodorakis, G.; Leftheriotis, D. Serum markers of deranged myocardial collagen turnover: Their relation to malignant ventricular arrhythmias in cardioverter-defibrillator recipients with heart failure. Am. Heart J. 2012, 164, 530–537. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Querejeta, R.; González, A.; Sánchez, E.; Larman, M.; Díez, J. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J. Am. Coll. Cardiol. 2004, 43, 2028–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Querejeta, R.; Varo, N.; Lopez, B.; Larman, M.; Artinano, E.; Etayo, J.C.; Diez, J. Serum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart failure. Circulation 2000, 101, 1729–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, A.; López, B.; Ravassa, S.; San José, G.; Díez, J. The complex dynamics of myocardial interstitial fibrosis in heart failure. Focus on collagen cross-linking. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Betocchi, S.; Losi, M.A.; Tocchetti, C.G.; Aversa, M.; Miranda, M.; D’Alessndro, G.; Cacaca, A.; Chiampi, Q.; Chiarello, M. Myocardial collagen turnover in hypertrophic cardiomyopathy. Circulation 2003, 108, 1455–1460. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, F.J.; Ruiz-Laiglesia, F.J.; Samperiz-Legarre, P.; Lasierra-Diaz, P.; Flamarique-Pascual, A.; Morales-Rull, J.L.; Perez-Calvo, J.I. Propeptide of procollagen type I (PIP) and outcomes in decompensated heart failure. Eur. J. Intern. Med. 2007, 18, 129–134. [Google Scholar] [CrossRef]
- Raafs, A.G.; Verdonschot, J.A.J.; Henkens, M.T.H.M. The combination of carboxy-terminal propeptide of procollagen type I blood levels and late gadolinium enhancement at cardiac magnetic resonance provides additional prognostic information in idiopathic dilated cardiomyopathy—A multilevel assessment of myocardial fibrosis in dilated cardiomyopathy. Eur. J. Heart Fail. 2021, 23, 933–944. [Google Scholar] [CrossRef]
- Kitahara, T.; Takeishi, Y.; Arimoto, T.; Nüzeki, T.; Koyama, Y.; Sasaki, T.; Suzuki, S.; Nozaki, N.; Hirono, O.; Nitaka, J.; et al. Serum carboxy-terminal telopeptide of type I collagen (ICTP) predicts cardiac events in chronic heart failure patients with preserved left ventricular systolic function. Circ. J. 2007, 71, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Manhenke, C.; Orn, S.; Squire, I.; Radauceanu, A.; Alla, F.; Zannad, F.; Dickstein, K. The prognostic value of circulating markers of collagen turnover after acute myocardial infarction. Int. J. Cardiol. 2011, 150, 277–282. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Ravassa, S.; González, A. Myocardial collagen cross-linking is associated with heart failure hospitalization in patients with hypertensive heart failure. J. Am. Coll. Cardiol. 2016, 67, 251–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravassa, S.; González, A.; Bayés-Genís, A.; Lupón, J.; Díez, J. Myocardial interstitial fibrosis in the era of precision medicine. Biomarker-based phenotyping for a personalized treatment. Rev. Esp. Cardiol. (Engl. Ed.) 2020, 73, 248–254, (In English and Spanish). [Google Scholar] [CrossRef] [PubMed]
- Ristelli, J.; Ristelli, I. Analysing connective tissue metabolites in human serum. Biochemical, physiological and methodological aspects. J. Hepatol. 1995, 2, 77–81. [Google Scholar]
- Cornelissen, V.A.; Fagard, R.; Lijnen, P. Serum collagen-derived peptides are unaffected by physical training in older sedentary subjects. J. Sci. Med. Sports 2010, 13, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Batllea, B.; Campos, M.; Farreroc, M.; Cardonac, B. Use of serum levels of high sensitivity troponin T, galectin-3 and C-terminal propeptide of type I procollagen at long term follow-up in heart failure patients with reduced ejection fraction: Comparison with soluble AXL and BNP. Int. J. Cardiol. 2016, 225, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Echegaray, K.; Andeu, I.; Lazkano, A.; Villanueva, I.; Saenz, A.; Elizalde, M.R.; Echeverria, T.; Lopez, B.; Garro, A.; Gonzalez, A.; et al. Role of myocardial collagen in severe aortic stenosis with preserved ejection fraction and symptoms of heart failure. Rev. Esp. Cardiol. 2017, 70, 832–840. [Google Scholar] [CrossRef]
- Park, S.J.; Cho, S.W.; Kim, S.M.; Ahn, J.; Carriere, K.; Jeong, D.S.; Lee, S.C.; Park, S.W.; Choe, Y.H.; Park, P.W.; et al. Assessment of myocardial fibrosis using multimodality imaging in severe aortic stenosis comparison with histologic fibrosis. JACC Cardiovasc. Imaging 2019, 12, 109–119. [Google Scholar] [CrossRef]
- Ravassa, S.; Ballesteros, G.; Lopez, B.; Ramos, P.; Bragard, J.; Gonzalez, A.; Moreno, M.U.; Querejeta, R.; Vives, E.; Garcia-Bolao, I.; et al. Combination of circulating type I collagen-related biomarkers is associated with atrial fibrillation. J. Am. Coll. Cardiol. 2019, 73, 1398–1410. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).