1. Introduction
Cancer cells have much more intense growth than normal cells. Consequently, a tumor could be diagnosed by detecting related changes in the metabolic pathways with the use of analytical techniques. Currently, analytical procedures lean towards omics approaches: metabolomics, lipidomics and proteomics. Proteomics is one of the analytical tools widely implemented in tumor diagnoses. In this approach, different sample matrices, such as blood, urine, seminal plasma and exosomes, are found to be suitable, and its application has been deeply discussed.
However, due to the biodiversity of cancer cells and the use of various analytical techniques, the results obtained within proteomic study are frequently not convergent [
1]. For this reason, the application of bioinformatics and statistical analysis is necessary in order to interpret the obtained data. The use of bioinformatics in the case of radiomic, genomic and radiogenomic research for the detection of prostate cancer was reported by Ferro et al. [
2].
The results presented by Alkhateeb et al. [
3] indicate a correlation between differences in gene expression and cancer stage. The authors indicated that Prostaglandin F receptor (PTGFR), Neuronal regeneration related protein (NREP), Small Cajal body-specific RNA 22 (SCARNA22), Dedicator of cytokinesis 9 (DOCK9), Feline leukemia virus subgroup C cellular receptor family, member 2 (FLVCR2), IKAROS family zinc finger 3 (Aiolos) (IKZF3), Ubiquitin-specific peptidase 13 (USP13) and Cytoplasmic linker associated protein 1 (CLASP1) were found to be potential biomarkers, which may support the diagnosis of cancer. Their determination enables differentiation in accordance with the advancement stage of cancer as well as to evaluate the specificity of the potential biomarkers in order to predict prostate cancer progression [
3].
Recently, great interest has been focused on lipidomics. Prostate cancer (PCa) can be considered as a lipid-enriched type of tumor. Fatty particles play a crucial role in cell differentiation and tumor progression. Moreover, increased lipogenesis is observed within carcinogenesis. For this reason, lipids are found to be promising indicators for the detection of PCa in the early stages of the disease [
4].
Zhou et al. [
5] performed lipidomic analysis on 141 plasma samples. On the basis of the results, followed by the bioinformatic and statistical analysis, 12 lipid species were identified as individual plasma lipid biomarkers of Prostate cancer (PCa) [
5]. Buszewska-Forajta et al. used matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) technique for the detection of specific indicators of PCa through the use of a lipidomics approach [
6]. Owing to the obtained findings, it was observed that prostate cancer appears to be linked both to elevated de novo biosynthesis of steroid hormone-related fatty acids and the lipid metabolism—particularly in mechanisms involving lipid oxidation.
Moreover, phospholipids and fatty acids presented themselves as discriminative features for PCa, indicating potential biomarkers of this type of neoplasm. On the other hand, PCa is known as a hormone-related type of cancer that is regulated by steroid hormones, mainly androgens. Moreover, it is assumed that the development of PCa can be related to prolactin. Prolactin receptors are found in the prostate tissue; increased levels of this hormone in pre-cancerous lesions were reported.
Cerrato et al. (2021) showed significance in detecting zwitterionic and positively charged species in the early diagnosis of prostate cancer [
7]. Franz et al. indicated the key contribution of zinc and its transporters in the development and prognosis of PCa. Other substances reported as strongly associated with the development of PCa include citric acid (CA) and its metabolites.
Monitoring changes in the CA concentration in various biological materials could provide information regarding the molecular mechanisms related to the tumor development. The fast detection of PCa is crucial due to the high mortality associated with this disease among men. The treatment of PCa is found to be challenging, especially in the advanced stages of the disease. In addition, its diagnosis is also problematic due to the asymptomatic development of the disease. For this reason, further knowledge regarding the role of CA in PCa becomes relevant [
4,
6,
8].
CA is the main constituent of semen, which is the specific and unique secretion from the prostate gland. Schersten first confirmed the presence of CA in 1929 [
9]. Considering all mammals, CA and its structural isomer, isocitrate, have the highest concentration in the prostate when compared with other organs [
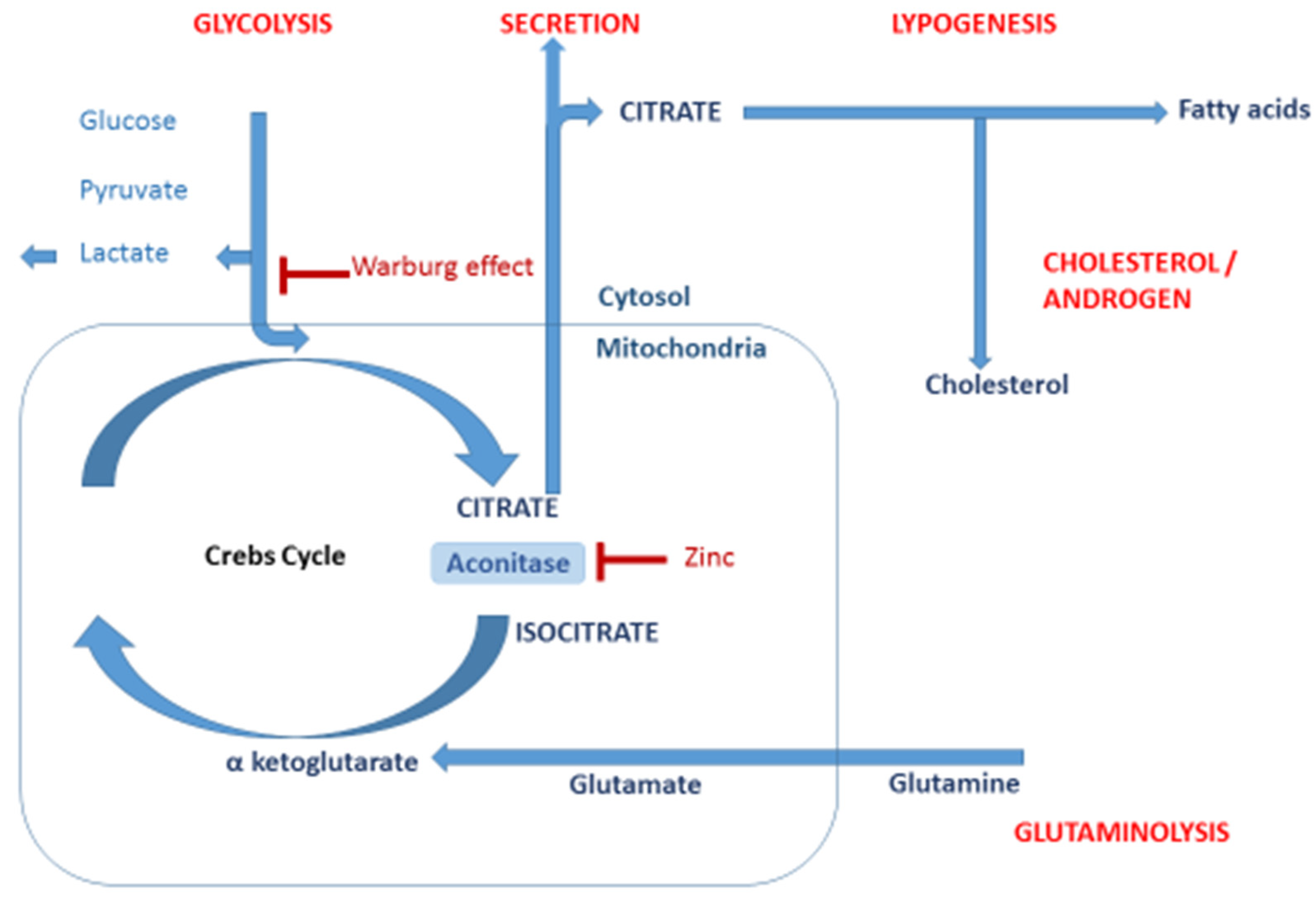
10]. From a biological point of view, citrate is crucial in the production of metabolic energy and in the synthesis of cholesterol, fatty acids and isoprenoids. These compounds have an essential role in cancer development and progress. Therefore, the determination of CA and other intermediates of the tricarboxylic acid cycle (Krebs cycle) in human tissues is a relevant approach to diagnose a prostate tumor. The scheme of the regulated pathway of the metabolism of CA in a cell is presented in
Figure 1.
Spectrophotometric techniques are found to be a promising alternative tool for the quantitative determination of CA. The application of dedicated commercial kits enables the assessment of CA in several biological matrices. Citrate links carbohydrates and the fatty acid metabolism [
11,
12]. Simultaneous quantitative analyses of intermediates have been conducted using chromatography coupled to mass spectrometry [
13,
14], nuclear magnetic resonance (NMR) [
15,
16] and spectrophotometric techniques [
17,
18].
Spectrophotometric techniques can be considered to constitute suitable alternative methods for the quantitative determination of CA. The application of dedicated commercial kits enables the assessment of CA in several biological matrices. The analysis is simple, relatively fast, cheap and reproducible. Considering this, such tests designed for the quantitative determination of CA in various biological matrices appear to be suitable for providing additional information to “omics” studies.
The present study aimed to evaluate the role of CA in the pathomechanism of PCa. For this purpose, this compound was determined in three matrices (urine, serum and tissue) with the use of a colorimetric test. Moreover, the results were correlated with other biochemical parameters in order to obtain new insights into PCa molecular mechanisms. To the best of our knowledge, this is the first time for such a type of analysis to be combined with biochemical and histopathological data. The results may explain the changes that occur during the development of the disease, with particular differences between the different stages of the disease.
3. Discussion
The characteristic features of the ideal disease indicators include specificity for the PCa, accuracy to diagnose PCa at any stage of the disease development, along with distinguishing PCa from indolent cases. Moreover, ideal biomarkers should be easy to determine in minimally invasive matrices, such as blood and urine [
19]. Recent advances in metabolomics, genomics and proteomics have provided knowledge regarding new potential biomarkers of PCa. One of the recent approaches is the application of combining the multiple gene analysis, such as SelectMDx or Mi Prostatescore for PCa prediction [
19,
20].
Another tool that can be implemented to increase the specificity of PCa diagnoses is the analysis of exosomes (Exos), which may play an important role in cancer pathophysiology [
19]. The determination of molecular factors, such as peptides, low molecular weight compounds or lipids with the use of analytical techniques may also be applied. The United States FDA approved three promising biomarkers of PCa, including the urinary Progensa Prostate Cancer Antigen 3 (PCA3) assay as well as a serum prostate health index (PHI) assay and the four-kallikrein (4Kscore).
Moreover, small molecular weight compounds, known as metabolites, should be also highlighted. One of the metabolites that can be regarded as a specific indicator of PCa is CA. Studies in recent years have shown the participation of the CA cycle in the development, proliferation and differentiation of malignant cells of prostate tissue [
21,
22,
23]. It can be assumed that CA may be a potential specific indicator of PCa.
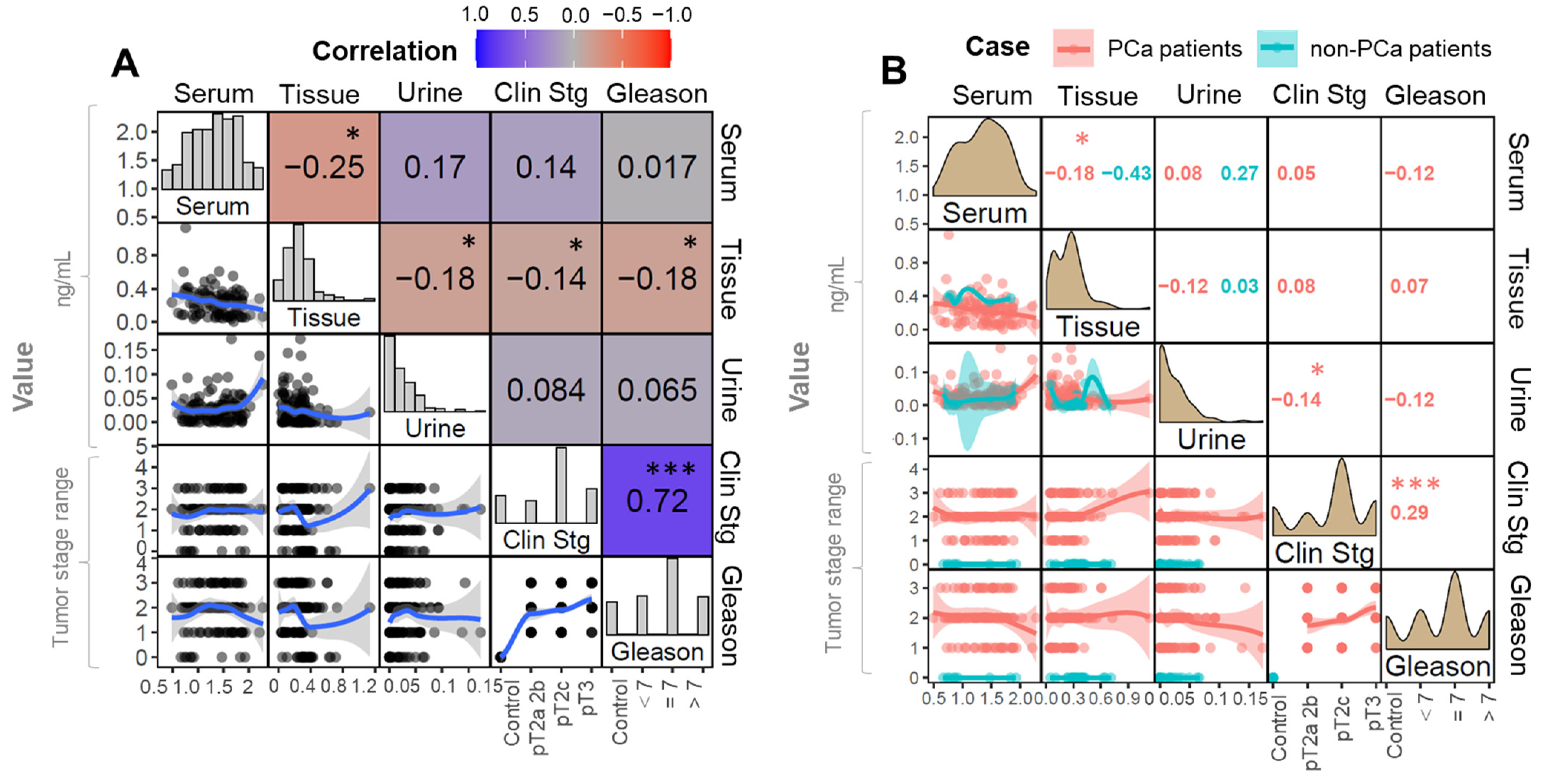
In this study, the concentration of this metabolite was determined in three biological matrices. First of all, in tissue, as the immediate focus of the disease. For this reason, it can be assumed that the changes are the most significant and reliable in the case of tissue. However, this metabolite was also investigated in the blood and urine in matrices to study the general changes in the body. The noninvasive, sample collection and the relatively unlimited access to matrices can be mentioned as an advantage. Moreover, the present research allowed verification of the usefulness of CA as an early indicator of PCa.
The results of our study demonstrated that, in the case of tissue samples, the concentration of CA significantly decreased with the progression of malignancy: we observed that the concentration of CA in tissue was negatively correlated to the pTNM staging and GS in a statistically significant manner. Our results are in accordance with the studies conducted by [
21,
23]. Carcinogenesis can be characterized by the increased proliferation of cancer cells.
On the one hand, growing cancer cells require high amounts of carbon species to produce all structural and nutritious compounds. Cell proliferation and their differentiation during cancer development causes morphological changes in the structure of the cell. As a consequence, the structure of the cell membrane is loosened, which prevents the storage of zinc ions inside the cell [
21,
22]. This leads to the activation of aconitase, which is active in the prostate cell.
Such a process is triggered by the waste of zinc ions (an essential micronutrient), which inhibits mitochondrial (m-) aconitase. The aconitase enzyme catalyzes the first step of the Krebs cycle, consisting in the oxidation of citrate to isocitrate [
20]. In this case, a major shift in energy metabolism occurs, and an increased activity of CA cycle is found in comparison to benign cells. Finally, the CA is transformed to isocitrate, which leads to a significant reduction of CA in the cell [
8,
22].
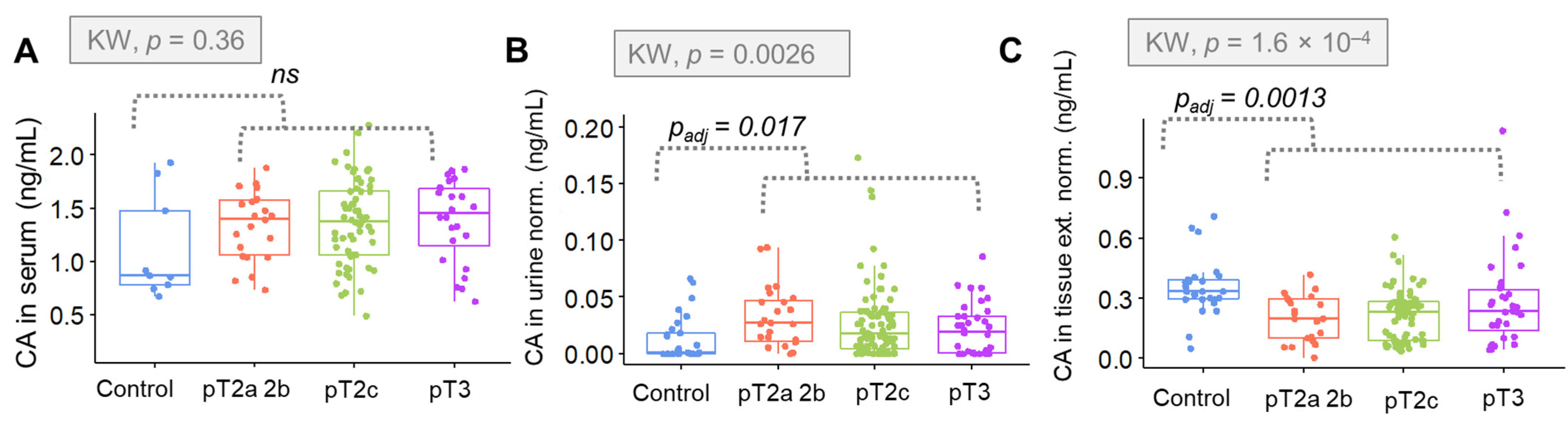
Going further, alterations in the concentration of CA in relation to PCa stage were evaluated. The results indicated that CA levels in tissue are particularly sensitive to the clinical staging and specific classification made in relation to the prostate (GS). In the case of clinical staging criteria, a positive correlation was observed. The higher the degree in the pTNM scale, the lower the concentration of CA.
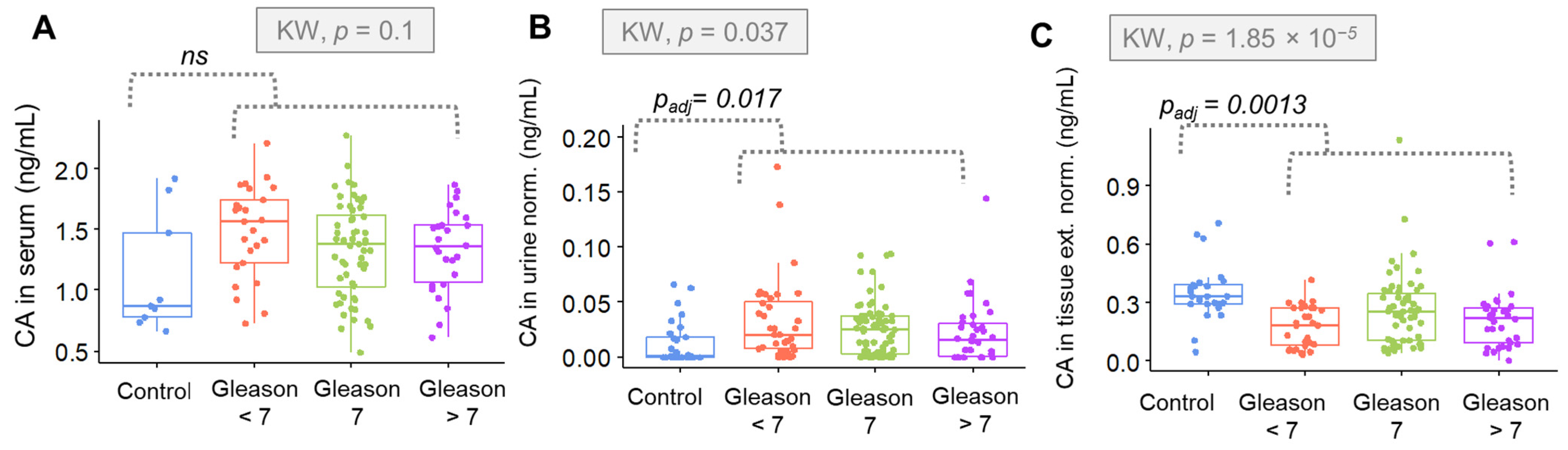
In the case of the Gleason evaluation, the concentration of CA was up to Gleason = 7, and then a plateau or even a decrease was observed. These results reflect the mechanism proposed by Costello et al. [
22]. The pTNM scale refers to the anatomic extent of the disease. It describes the degree of invasion in terms of the tumor, lymph nodes and margins. This is a general assumption that does not reflect changes at the morphological level of the cell, with a particular emphasis on differentiation. A more detailed description is possible with the Gleason scale evaluation, being more related to the molecular characterization of the tumor with a special emphasis on cell differentiation.
The results indicate that there is a strong proliferation of cells with a simultaneous inhibition of aconitase in the initial stage of the development. With the differentiation, the membrane disintegrates, which results in the loss of the ability to accumulate zinc ions and leads to the activation of aconitase. As a result, CA is a subtraction of the transformation to isocitrate. Finally, the concentration of citrate decreases with a simultaneous increase in isocitrate in the cell.
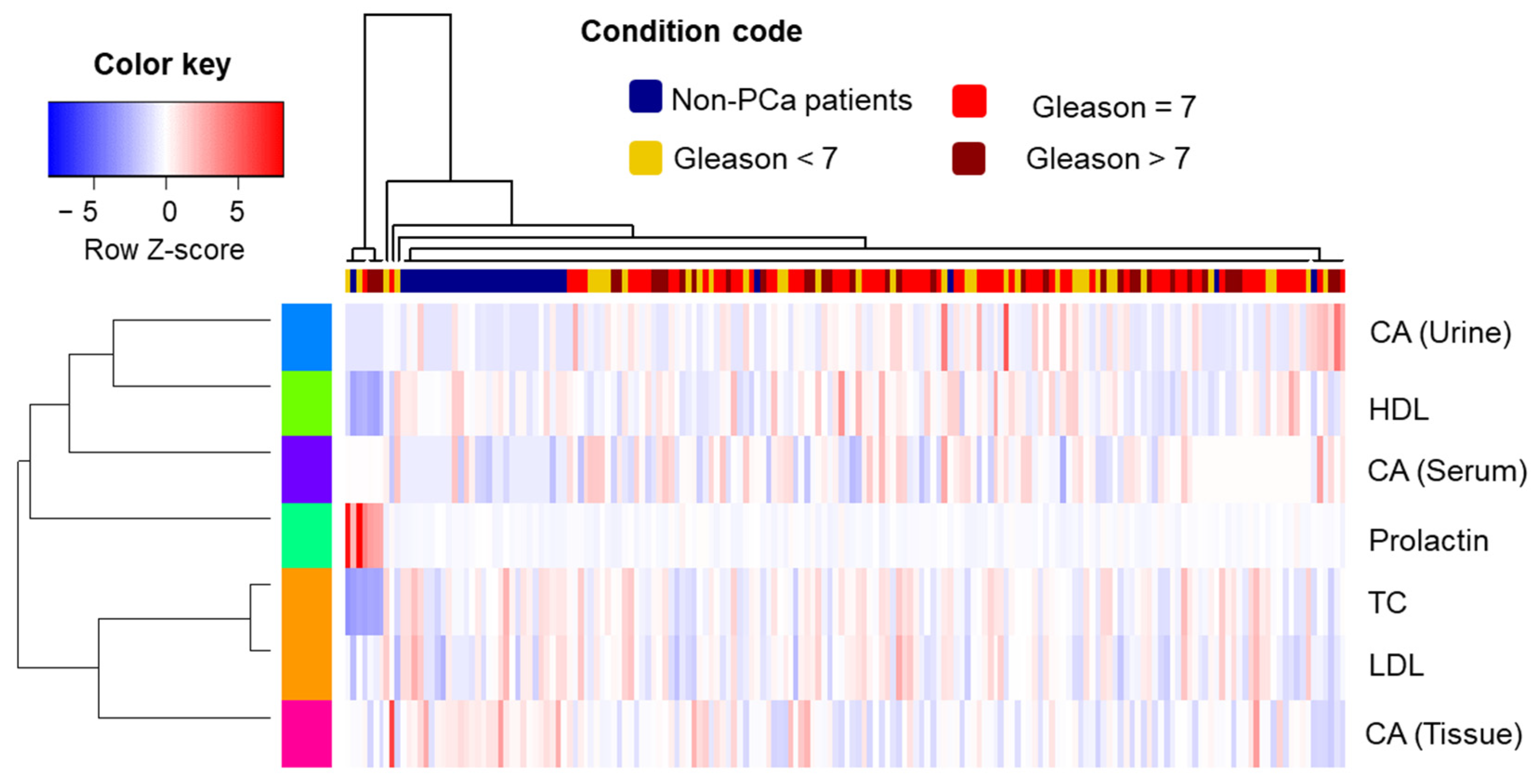
The intensity of changes is closely correlated with the degree of differentiation of neoplastic cells. The Gleason grading system can easily evaluate the differentiated morphology. Ren et al. showed that citrate can inhibit tumor growth in diverse tumor types and via multiple mechanisms by citrate supplementation [
24]. Caiazza et al. studied the long-term effects of citrate supplementation on PCa cells; they demonstrated that a chronic citrate administration might select for resistant cells [
25].
Additionally, the obtained results indicate that tissue can be considered as the most reliable matrix to assess CA to derive a PCa diagnosis. The use of tissue allows for the direct assessment of the tumor environment and cancer cells. In our study, tissue was also found as the most discriminative matrix. Therefore, the use of tissue for the determination of CA for PCa diagnostics has clinical application potential.
In the case of urine and serum samples, the opposite correlation was found. In both matrices, the concentration of CA in the control samples was lower than in the samples from patients with PCa. In the above mentioned matrices, the concentration of citrate is associated with changes occurring throughout the body rather than directly at the focus of the disease.
Nevertheless, the concentration of CA in urine samples was significantly greater for the overall cancer group (p = 0.011). Moreover, a correlation with the stage of cancer was shown, especially regarding the clinical staging (p = 0.0014). Considering the GS (p = 0.02) the correlation between this parameter and the concentration or urinary CA was significantly weaker. Statistical significance found in urinary CA suggests that this is a promising approach for non-invasive and fast screening for PCa detection. These biochemical changes may be useful in the context of a rapid diagnosis of PCa.
In the case of serum samples, the concentration of CA was higher for the cancer group in general; however, these changes were not assigned as significant. Moreover, the correlation between the citrate concentration and clinical stage/GS was evaluated. We observed that the levels of CA were higher for PCa samples in comparison to control group. The concentration of CA was higher for the PCa group, but no significant changes in the concentration of citrate were observed according to the clinical stage of the tumor.
The relation was slightly different when the concentration of serum CA and the Gleason scale were considered: the concentration of citrate was higher in the case of PCa samples; nevertheless, we observed that, as the tumors progressed, the concentration of citrate decreased. This observation is complementary to the tissue data and can be easily explained by the similar complexity of these two matrices, i.e., tissue and blood. Within the study, the correlation between CA and other molecular indicators was analyzed. Due to the fact that the prostate is a hormone-related gland, the influence of hormones on PCa pathogenesis may be significant.
Normal prostate metabolism is coordinated by androgen receptor signaling and characterized by a physiological truncation of the Krebs cycle to enable the production and secretion of citrate into prostatic fluid [
26,
27,
28]. It was reported that androgens, which bind to androgen receptors, influence prostate cancer development. Although the pathomechanism remains unknown, recent studies indicate the relationship between the level of androgens and cholesterol [
27,
29].
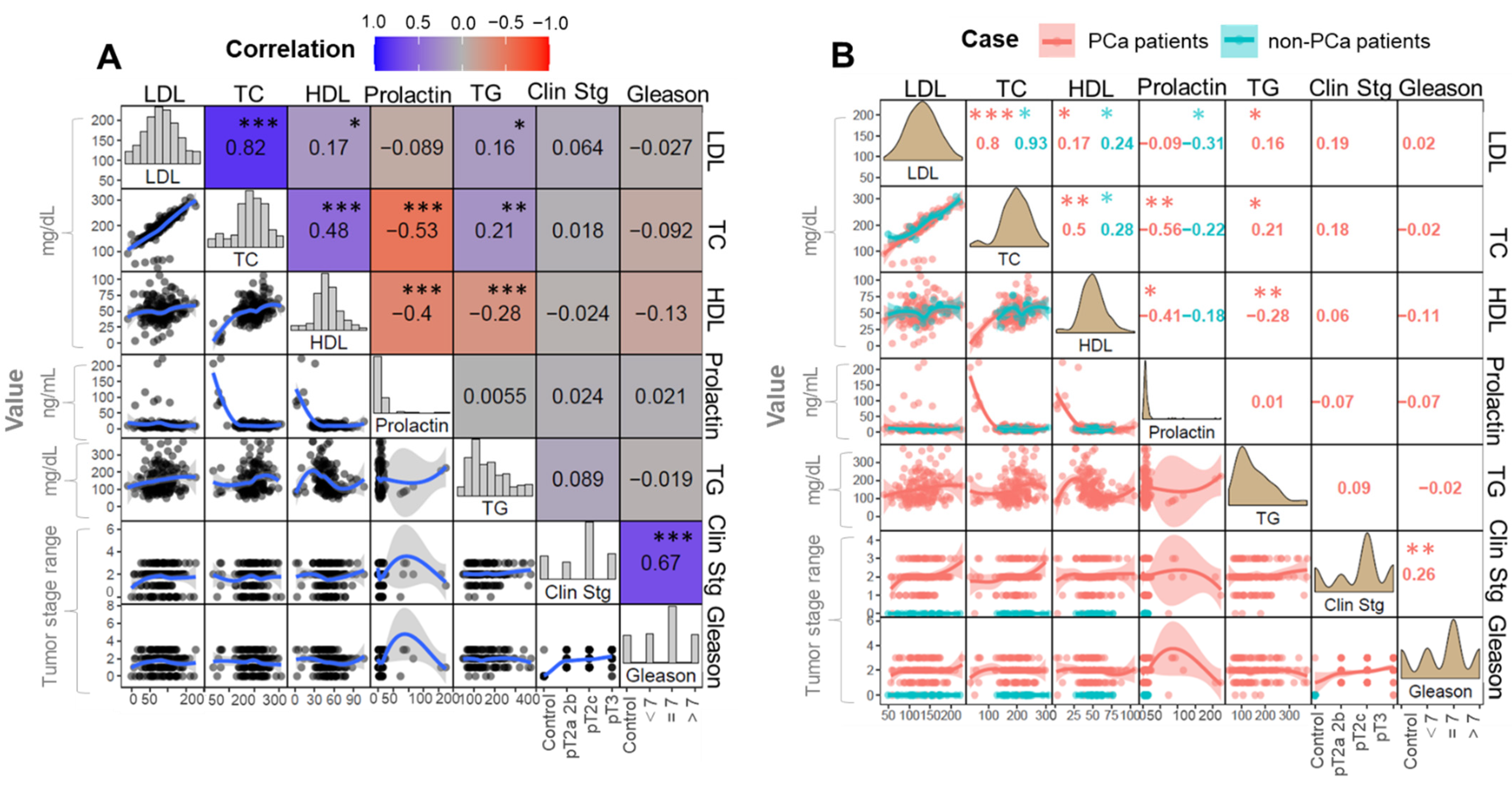
Undoubtedly androgens stimulate the lipogenic enzymes that cause the increased synthesis of fatty acids and cholesterol. For this reason, searching for the correlation between the cholesterol and other molecular factors may provide significant knowledge in terms of PCa development and progression.The role of cholesterol in this mechanism can be considered in two ways. First of all, cholesterol is the initial metabolite of hormone-related metabolic transformation pathways. On the other hand, cholesterol can be considered a nutrient that enters the body with food. Its concentration can be related with the patients’ lifestyle in terms of diet.
Therefore, recent references suggest that diet and especially consumed fats may play a role in the development and progression of prostate cancer. Cholesterol can be determined with regular tests provided by the clinical laboratories. By monitoring changes in cholesterol and its fraction levels, the hypothesis on its influence on cancerogenesis may be verified. Our results provide knowledge about the increased level of cholesterol and HDL fraction in the case of PCa patients. The opposite phenomena were observed in the case of the control group, where the LDL fraction increased. Heir et al. [
30] reported that elevated level of lipids, including free cholesterol, were associated with PCa development [
30].
In contrast, the meta-analysis reported no significant correlation between the total cholesterol fraction and the corresponding PCa staging [
31]. These results are in accordance with the results presented by us. When considering the part played by cholesterol in the PCa development and progression, the role of prolactin should also be taken into account. Prolactin is an anterior pituitary hormone that influences the function of several organs; therefore, this hormone may be significant in the regulation of prostatic cholesterol, especially free cholesterol, by the stimulatory effect of androgens [
32,
33].
According to the literature, prolactin stimulates prostate growth activity, possibly due to its association with the prostate cell proliferation and the regulation of the angiogenesis process [
34]. It was found that prolactin receptors are present in prostate tissue. Moreover, the precancerous state of prostate tissue contains an increased amount of these types of receptors. For this reason, prolactin is believed to be involved in the neoplastic process. The obtained results show normal prolactin levels in both groups; however, in the case of PCa patients, the level of prolactin was higher (9.53 ng/mL) than in the controls (8.51 ng/mL).
Additionally, it was observed that the level of prolactin was not associated with the stage of cancer. Similar results were obtained by [
34]. The authors reported that circulating prolactin was not correlated with an increase in the PCa risk. The influence of prolactin on the PCa development was the aim of the study conducted by [
35]. The study was performed with murine model transgenic mice.
The results provide the knowledge that the overexpression of prolactin induces the disorganized amplification of the basal/stem cell compartment, which is found to be tumor-initiating cells in the prostate. The influence of prolactin on PCa development is still unknown. For this reason, there is a need to conduct research on a large set of samples, with special emphasis placed on the role of androgens. As the androgen receptor regulates the prostate, its role may be greater than expected in the neoplastic process.
4. Materials and Methods
4.1. Biological Material
The participants enrolled in the study were recruited by a specialist of urology from the Nicolaus Copernicus Specialist Municipal Hospital (Toruń, Poland). Patients were matched in terms of age and body mass index (BMI). A total of 184 biological samples were collected from individuals with PCa (n = 154) and control subjects (n = 30). All diagnoses, both PCa and benign prostatic hyperplasia (BPH), were histologically confirmed by prostate biopsy. Blood biochemical indicators of all participants were obtained within the physical examination.
Basic laboratory tests were performed to determine the general condition of the patient qualified for the study. Relevant information, such as age and weight as well as levels of the prostate specific antigen (PSA), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglyceride (TGs), total cholesterol (TC), creatinine (CREA) and prolactin (PRL) were obtained. Patients from the PCa group presented elevated PSA levels during screening tests and positive assessment in ultrasound and digital rectal examinations.
Each patient had their PSA level determined according to the routine laboratory protocol. The mean PSA concentration in the PCa group was 14.80 ± 12 ng/mL (max. 45.53 ng/mL and min. 0.008 ng/mL). The control group consisted of patients with diagnosed BPH. In this group, the average concentration of PSA was relatively lower (6.34 ± 3.82 ng/mL, max. 18 ng/mL and min. 0.868 ng/mL). Non-cancer patients enrolled in the control group were declared to be in good health condition.
All participants gave signed written informed consent forms and filled a standard questionnaire. The study was conducted in accordance with the Declaration of Helsinki Ethical Principles. The Independent Commission positively reviewed the research for Bioethics Research, Medical University of Gdansk (number of consent: NKBBN/432/2016). All experiments were performed by following the relevant guidelines and regulations. Three types of biological materials (tissue, blood and urine) were collected from each patient.
In the case of PCa patients, the tissue was collected during radical prostatectomy surgery, while control tissue was obtained during biopsy. In the case of the control group, the presence of cancer cells was ruled out owing to the histopathological evaluation. The tissue was preserved in accordance with recommended diagnostic histopathology protocols, based on fixation and paraffin embedding procedures (
https://www.leicabiosystems.com/knowledge-pathway/an-introduction-to-specimen-preparation/, accessed on 17 February 2022). A detailed description is presented in Buszewska-Forajta et al. [
4].
The diagnosis was confirmed by the histopathological evaluation, where tissue samples were characterized in reference to the Gleason scaling. Additionally, the cancer staging was evaluated in accordance with the pTNM scale. Patients were divided into subgroups according to the histopathological assessment. The first subgroup corresponds to samples where cancer was detected only in one side of the prostate (pT2*). The second subgroup, including the most common stage pT = 2, is characterized by the presence of a tumor involving both lobes (pT2**). Other cases were described as pT3, including those found as tumors extending through the prostatic capsule.
The summarized information about the collected samples, divided in reference to each parameter, is listed in
Table 3 and
Table 4.
Table 3 shows subgroups divided in accordance with the Gleason scale, while
Table 4 presents subgroups classified in relation to the description of the primary tumor.
The formalin-fixed, paraffin-embedded (FFPE) blocks containing tissue samples were stored at room temperature (20–22 °C). Prior to the analysis, FFPE blocks were cut to obtain 20 µm sections, which were implemented to the qualitative analysis of CA. Regarding the remaining matrices, 1.5 mL of morning urine and serum samples were collected in Eppendorf tubes and kept frozen at −80 °C until the analyses were conducted. The other two biological materials, namely urine and blood, were collected before the biopsy.
Blood samples were collected from each of the participants enrolled in the study. Due to the daily changes in hormone levels, each sample was drawn from fasting patients, at the same time period of the day (7–9 AM). Venous blood was collected into two polypropylene tubes containing Ethylenediaminetetraacetic acid (EDTA). Within 2 h to 4 h, blood samples collected “on clot” were centrifuged at the speed of 2500 rpm for 15 min for the collection of serum. Sera were transferred to Eppendorf tubes and stored at −80 °C up to the day of citric acid determination. The second tube of venous blood was used to determine parameters, such as the total cholesterol (TC), LDL, HDL, prolactin (PRL) and triglycerides (TG). These measurements were performed in the hospital laboratory, following routine standard protocols.
Additionally, morning urine samples were collected and vortex-mixed during 15 s. The pH of each urine sample was measured. the urine pH in the control group ranged from 5.0–5.5. In the prostate cancer group, pH values were relatively higher (5.5 to 6.2).
Next, creatinine levels were determined with the use of the routine protocol applied in the hospital laboratory. All tests were performed in accordance with the relevant guidelines and regulations. Collected urine samples were divided into probes and stored at −80 °C until analysis. On the day of the analysis, samples (urine and serum) were thawed on ice for 2 h, centrifuged and filtered.
4.5. Statistical Analysis and Validation
The obtained datasets were normalized accordingly. In the case of tissue, the data were normalized in accordance with the weight of tissue. In the case of urine samples, the data were normalized by the creatinine value determined in each analyzed sample. The normality of the data distribution was assessed using Kolmogorov–Smirnov and Shapiro–Wilk tests, employing IBM SPSS Statistics; version 24 (BM Corp., Armonk, NY, USA). The following methods were performed in R environment, using RStudio 1.1.463 console (Boston, MA, USA).
The correlation analysis was evaluated using Spearman’s rank correlation coefficient (rho) in order to assess associations existing between the levels of CA found in the different matrices, biochemical parameters and cancer staging. Plots were generated using “ggplot2” package, with the “corrmorant” extension applied to create customized correlation matrices. The Spearman method allows evaluation of the nature of the connections existing between two sets of data. It assumes a monotonic relationship between two parameters of interest.
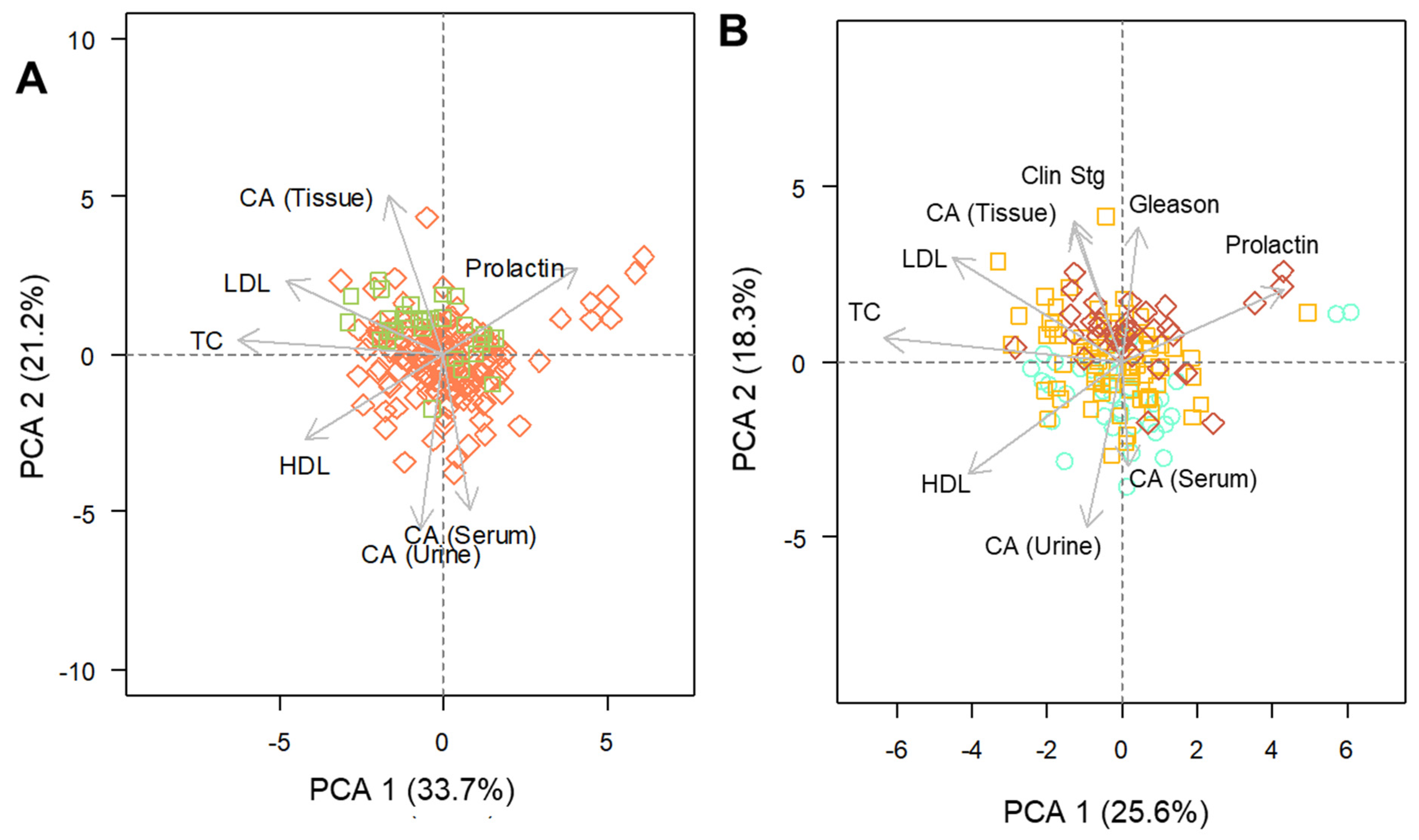
The strength and the direction of a correlation is provided by the calculated Spearman’s coefficient. This is a non-parametric approach and therefore indicated in the case of data with non-normal distribution and/or measured in different scales. The principal components analysis (PCA) was performed using the “prcomp” function. For this method, only data corresponding to at least 10% of the total abundance was included, and missing values were imputed by the variable’s means.
In addition, the data were scaled prior to the analysis. PCA is a multivariate approach that works in reducing the dimensionality of attributes from datasets containing numerous variables. This statistical method permits the visualization of data from a more informative perspective, allowing the observation of relationships existing between all parameters in a comprehensive manner, thus, facilitating the recognition of latent patterns within the data.
Hierarchical cluster analysis (HCA) combined with a heatmap was produced using the “gplots” package, applying z-score normalized data as input. Spearman correlation coefficient was used to calculate the cluster distance, and the unweighted pair group method with the arithmetic mean (“average” method) was used as the clustering criterion. The Kruskal–Wallis test was used to indicate differences among study groups (controls and different stages of cancer) according to the levels of CA found in the biospecimens.
The Mann–Whitney test was applied to investigate further differences between the control cohort and the positive sub-groups. Box plots were generated with the aid of the “ggpubr” package. The adjustment of the
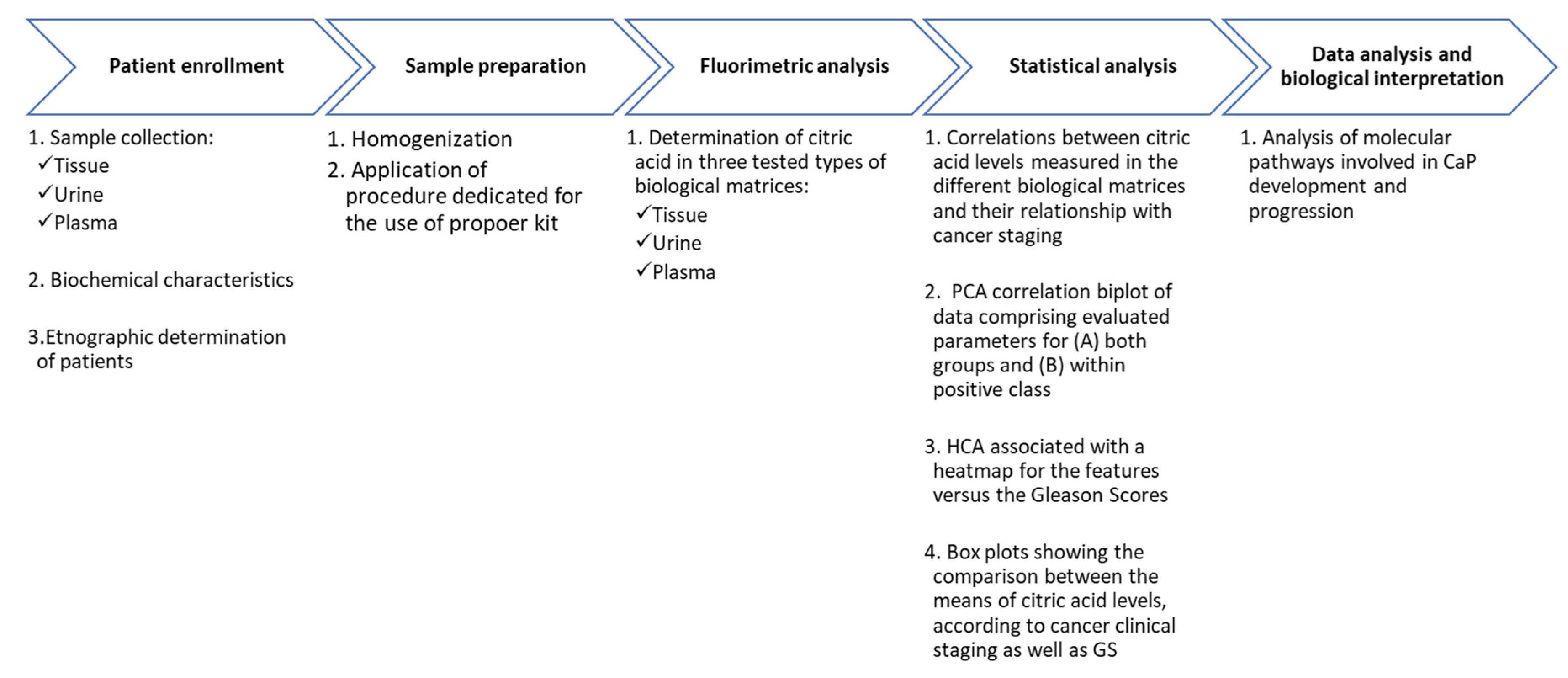
p-value was performed using the Benjamini–Hochberg (B-H) procedure. For the statistical analysis, cancer staging parameters were properly encoded as ordinal variables, as follows: control cases = 0, pT2a 2b = 1, pT2c = 2 and pT3a, b or c = 3; Gleason scores (GSs) lower than 7 = 1, scores equal to 7 = 2 and scores greater than 7 = 3. The general workflow of the applied methods is presented in
Figure 8.