Insight into the Evolving Role of PCSK9
Abstract
:1. Introduction
- -
- Signal peptide, responsible for the exit of enzyme from endoplasmatic reticulum (ER);
- -
- Prosegment, maintaining the conformation of peptide chain;
- -
- Catalytic domain, responsible for binding to the substrate (according to the substrate specificity and structure of catalytic domain, three types of PCs are distinguished: kexin-like, pyrolysin-like, proteinase K-like);
- -
- P domain, stabilizing PCs;
- -
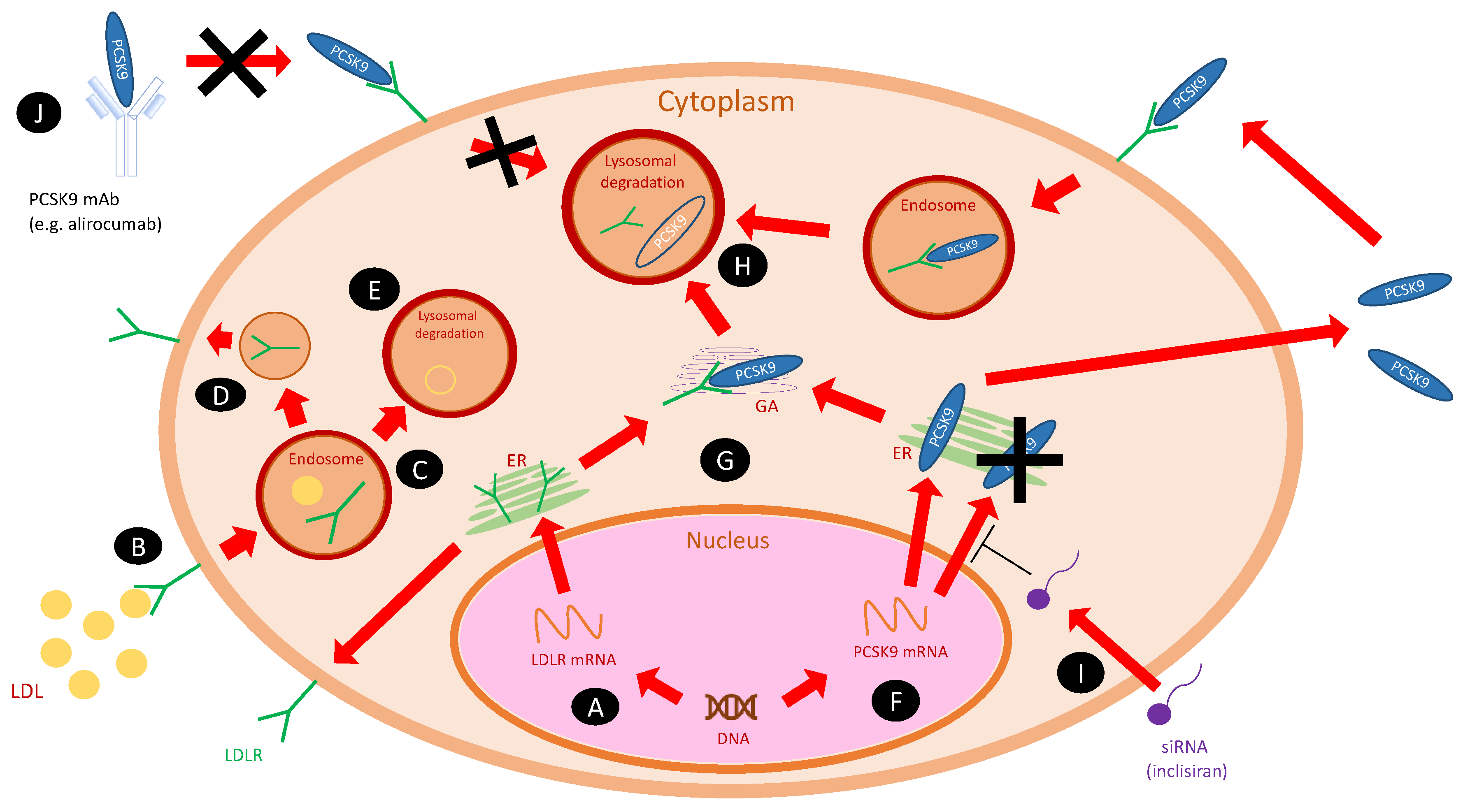
2. PCSK9 and Its Inhibitors
3. PCSK9 and Atherosclerosis
3.1. Inflammation
3.2. Monocytes, Macrophages and Foam Cells
3.3. Endothelial Cells
3.4. Smooth Muscle Cells (SMCs)
3.5. Coagulation and Platelet Aggregation
4. PCSK9 and Heart
5. PCSK9 and Gastrointestinal System
5.1. Pancreas
5.2. Small Intestine
5.3. Liver
6. PCSK9 and Kidneys
7. PCSK9 and the Endocrine System
7.1. Thyroid Function
7.2. Sex Hormones
7.3. Adrenals
7.4. Polycystic Ovary Syndrome (PCOS)
8. PCSK9 and Central Nervous System (CNS)
9. PCSK9 and Cancer
10. PCSK9 and Infections
10.1. Bacterial Infections
10.2. Viral Infections:
10.2.1. Hepatitis C Virus
10.2.2. Dengue Virus
10.2.3. SARS-CoV-2
10.3. Parasites
11. New Approaches to PCSK9 Inhibition
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seidah, N.G.; Prat, A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 2012, 11, 367–383. [Google Scholar] [CrossRef]
- Małuch, I.; Walewska, A.; Sikorska, E.; Prahl, A. Konwertazy probiałkowe—Rodzina proteaz serynowych o szerokim spektrum funkcji fizjologicznych. Post. Bioch. 2016, 62, 472–481. [Google Scholar]
- Seidah, N.G.; Prat, A. The multifaceted biology of PCSK9. Endocr. Rev. 2021, bnab035. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Benjannet, S.; Wickham, L.; Marcinkiewicz, J.; Jasmin, S.B.; Stifani, S.; Basak, A.; Prat, A.; Chretien, M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): Liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA 2003, 100, 928–933. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, P.; van de Sluis, B.; Dullaart, R.P.F.; van den Born, J. Novel aspects of PCSK9 and lipoprotein receptors in renal disease-related dyslipidemia. Cell Signal. 2019, 55, 53–64. [Google Scholar] [CrossRef]
- Poirier, S.; Mayer, G.; Benjannet, S.; Bergeron, E.; Marcinkiewicz, J.; Nassoury, N.; Mayer, H.; Nimpf, J.; Prat, A.; Seidah, N.G. The proprotein convertase PCSK9 induces the degradation of low-density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J. Biol. Chem. 2008, 283, 2363–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abifadel, M.; Varret, M.; Rabés, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK-9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef]
- Uribe, K.B.; Chemello, K.; Larrea-Sebal, A.; Benito-Vicente, A.; Galicia-Garcia, U.; Bourane, S.; Jaafar, A.K.; Lambert, G.; Martín, C. A Systematic Approach to Assess the Activity and Classification of PCSK9 Variants. Int. J. Mol. Sci. 2021, 22, 13602. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Feng, X.; Zhou, Y. PCSK9 Variants in Familial Hypercholesterolemia: A Comprehensive Synopsis. Front. Genet. 2020, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, K.D.; Wolf, A.; Schreckenberg, R. Coming Back to Physiology: Extra Hepatic Functions of Proprotein Convertase Subtilisin/Kexin Type 9. Front. Physiol. 2020, 11, 598649. [Google Scholar] [CrossRef]
- Cui, C.J.; Li, S.; Li, J.J. PCSK9 and its modulation. Clin. Chim. Acta 2015, 440, 79–86. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Zhu, Q.Q.; Zhu, L.; Chen, J.Z.; Chen, Q.H.; Li, G.N.; Xie, J.; Kang, L.N.; Xu, B. Safety and efficacy of anti-PCSK9 antibodies: A meta-analysis of 25 randomized, controlled trials. BMC Med. 2015, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Inclisiran: First Approval. Drugs 2021, 81, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S. PCSK9 inhibitors: Clinical evidence and implementation. Nat. Rev. Cardiol. 2019, 16, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Gencer, B.; Kronenberg, F.; Stroes, E.S.; Mach, F. Lipoprotein(a): The revenant. Eur. Heart J. 2017, 38, 1553–1560. [Google Scholar] [CrossRef]
- Hardy, J.; Niman, S.; Pereira, E.; Lewis, T.; Reid, J.; Choksi, R.; Goldfaden, R.F. A Critical Review of the Efficacy and Safety of Inclisiran. Am. J. Cardiovasc. Drugs 2021, 21, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, Y.; Pantea Stoian, A.; Cicero, A.; Fogacci, F.; Nikolic, D.; Sachinidis, A.; Rizvi, A.A.; Janez, A.; Rizzo, M. Inclisiran: A small interfering RNA strategy targeting PCSK9 to treat hypercholesterolemia. Expert Opin. Drug Saf. 2022, 21, 9–20. [Google Scholar] [CrossRef]
- Giglio, R.V.; Pantea Stoian, A.; Al-Rasadi, K.; Banach, M.; Patti, A.M.; Ciaccio, M.; Rizvi, A.A.; Rizzo, M. Novel Therapeutical Approaches to Managing Atherosclerotic Risk. Int. J. Mol. Sci. 2021, 22, 4633. [Google Scholar] [CrossRef] [PubMed]
- Nguy, J.; Hitchen, S.A.; Lan, N.; Dwivedi, G.; Larbalestier, R.; Yeap, B.B.; Fegan, P.G. Barriers to prescribing proprotein convertase subtilisin-kexin type 9 inhibitors after coronary revascularization. Intern. Med. J. 2022. epub ahead of print. [Google Scholar] [CrossRef]
- Caso, V.M.; Sperlongano, S.; Liccardo, B.; Romeo, E.; Padula, S.; Arenga, F.; D’Andrea, A.; Caso, P.; Golino, P.; Nigro, G. The Impact of the COVID-19 Outbreak on Patients’ Adherence to PCSK9 Inhibitors Therapy. J. Clin. Med. 2022, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- German, C.A.; Shapiro, M.D. Small interfering RNA therapeutic inclisiran: A new approach to targeting PCSK9. BioDrugs 2020, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Steffens, D.; Bramlage, P.; Scheeff, C.; Kasner, M.; Hassanein, A.; Friebel, J.; Rauch-Kröhnert, U. PCSK9 inhibitors and cardiovascular outcomes. Expert Opin. Biol. Ther. 2020, 20, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ardes, D.; Santilli, F.; Guagnano, M.T.; Bucci, M.; Cipollone, F. From endothelium to lipids, through microRNAs and PCSK9: A fascinating travel across atherosclerosis. High Blood Press. Cardiovasc. Prev. 2020, 27, 1–8. [Google Scholar] [CrossRef]
- Cheng, J.M.; Oemrawsingh, R.M.; Garcia-Garcia, H.M.; Boersma, E.; van Geuns, R.J.; Serruys, P.W.; Kardys, I.; Akkerhuis, K.M. PCSK9 in relation to coronary plaque inflammation: Results of the ATHEROREMOIVUS study. Atherosclerosis 2016, 248, 117–122. [Google Scholar] [CrossRef]
- Denis, M.; Marcinkiewicz, J.; Zaid, A.; Gauthier, D.; Poirier, S.; Lazure, C.; Seidah, N.G.; Prat, A. Gene inactivation of proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice. Circulation 2012, 125, 894–901. [Google Scholar] [CrossRef] [Green Version]
- Tavori, H.; Giunzioni, I.; Predazzi, I.M.; Plubell, D.; Shivinsky, A.; Miles, J.; Devay, R.M.; Liang, H.; Rashid, S.; Linton, M.F.; et al. Human PCSK9 promotes hepatic lipogenesis and atherosclerosis development via apoE- and LDLR-mediated mechanisms. Cardiovasc. Res. 2016, 110, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Karagiannis, A.D.; Liu, M.; Toth, P.P.; Zhao, S.; Agrawal, D.K.; Libby, P.; Chatzizisis, Y.S. Pleiotropic anti-atherosclerotic effects of PCSK9 inhibitors from molecular biology to clinical translation. Curr. Atheroscler. Rep. 2018, 20, 20. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Wiviott, S.D.; Raal, F.J.; Blom, D.J.; Robinson, J.; Ballantyne, C.M.; Somaratne, R.; Legg, J.; Wasserman, S.M.; et al. Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1500–1509. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bittner, V.A.; Diaz, R.; Goodman, S.G.; Kim, Y.U.; Jukema, J.W.; Pordy, R.; Roe, M.T.; et al. Peripheral Artery Disease and Venous Thromboembolic Events after Acute Coronary Syndrome: Role of Lipoprotein(a) and Modification by Alirocumab: Prespecified Analysis of the ODYSSEY OUTCOMES Randomized Clinical Trial. Circulation 2020, 141, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Kühnast, S.; Van Der Hoorn, J.W.; Pieterman, E.J.; van den Hoek, A.M.; Sasiela, W.J.; Gusarova, V.; Peyman, A.; Schäfer, H.L.; Schwahn, U.; Jukema, J.W.; et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J. Lipid Res. 2014, 55, 2103–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, H.; Horinaka, S.; Ishimitsu, T. Effect of evolocumab therapy on coronary fibrous cap thickness assessed by optical coherence tomography in patients with acute coronary syndrome. J. Cardiol. 2020, 75, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Barale, C.; Bonomo, K.; Frascaroli, C.; Morotti, A.; Guerrasio, A.; Cavalot, F.; Russo, I. Platelet function and activation markers in primary hypercholesterolemia treated with anti-PCSK9 monoclonal antibody: A 12-month follow-up. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Koskinas, K.C.; Windecker, S.; Buhayer, A.; Gencer, B.; Pedrazzini, G.; Mueller, C.; Cook, S.; Muller, O.; Matter, C.M.; Räber, L.; et al. Design of the randomized, placebo-controlled evolocumab for early reduction of LDL-cholesterol levels in patients with acute coronary syndromes (EVOPACS) trial. Clin. Cardiol. 2018, 41, 1513–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basiak, M.; Kosowski, M.; Cyrnek, M.; Bułdak, Ł.; Maligłówka, M.; Machnik, G.; Okopień, B. Pleiotropic Effects of PCSK-9 Inhibitors. Int. J. Mol. Sci. 2021, 22, 3144. [Google Scholar] [CrossRef] [PubMed]
- Omori, H.; Ota, H.; Hara, M.; Kawase, Y.; Tanigaki, T.; Hirata, T.; Sobue, Y.; Okubo, M.; Kamiya, H.; Matsuo, H.; et al. Effect of PCSK-9 Inhibitors on Lipid-Rich Vulnerable Coronary Plaque Assessed by Near-Infrared Spectroscopy. JACC Cardiovasc. Imaging 2020, 13, 1639–1641. [Google Scholar] [CrossRef]
- Kim, H.L.; Lim, W.H.; Seo, J.B.; Kim, S.H.; Zo, J.H.; Kim, M.A. Prognostic value of arterial stiffness according to the cardiovascular risk profiles. J. Hum. Hypertens. 2021, 35, 978–984. [Google Scholar] [CrossRef]
- Mikael, L.R.; Paiva, A.; Gomes, M.M.; Sousa, A.; Jardim, P.; Vitorino, P.; Euzébio, M.B.; Sousa, W.M.; Barroso, W. Vascular Aging and Arterial Stiffness. Arq. Bras. Cardiol. 2017, 109, 253–258. [Google Scholar] [CrossRef]
- Ruscica, M.; Ferri, N.; Fogacci, F.; Rosticci, M.; Botta, M.; Marchiano, S.; Magni, P.; D’Addato, S.; Giovannini, M.; Borghi, C.; et al. Circulating Levels of Proprotein Convertase Subtilisin/Kexin Type 9 and Arterial Stiffness in a Large Population Sample: Data From the Brisighella Heart Study. J. Am. Heart Assoc. 2017, 6, e005764. [Google Scholar] [CrossRef] [Green Version]
- Scicali, R.; Russo, G.I.; Di Mauro, M.; Manuele, F.; Di Marco, G.; Di Pino, A.; Ferrara, V.; Rabuazzo, A.M.; Piro, S.; Morgia, G.; et al. Analysis of Arterial Stiffness and Sexual Function after Adding on PCSK9 Inhibitor Treatment in Male Patients with Familial Hypercholesterolemia: A Single Lipid Center Real-World Experience. J. Clin. Med. 2020, 9, 3597. [Google Scholar] [CrossRef] [PubMed]
- Mandraffino, G.; Scicali, R.; Rodríguez-Carrio, J.; Savarino, F.; Mamone, F.; Scuruchi, M.; Cinquegrani, M.; Imbalzano, E.; Di Pino, A.; Piro, S.; et al. Arterial stiffness improvement after adding on PCSK9 inhibitors or ezetimibe to high-intensity statins in patients with familial hypercholesterolemia: A Two-Lipid Center Real-World Experience. J. Clin. Lipidol. 2020, 14, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Scicali, R.; Di Pino, A.; Ferrara, V.; Rabuazzo, A.M.; Purrello, F.; Piro, S. Effect of PCSK9 inhibitors on pulse wave velocity and monocyte-to-HDL-cholesterol ratio in familial hypercholesterolemia subjects: Results from a single-lipid-unit real-life setting. Acta Diabetol. 2021, 58, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Geovanini, G.R.; Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Ruscica, M.; Camera, M.; Rossetti, L.; Macchi, C.; Colciago, A.; Zanotti, I.; Lupo, M.G.; Adorni, M.P.; Cicero, A.F.; et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci. Rep. 2018, 8, 2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.H.; Peng, J.; Ren, Z.; Yang, J.; Li, T.T.; Li, T.H.; Wang, Z.; Wei, D.H.; Liu, L.S.; Zheng, X.L.; et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis 2017, 262, 113–122. [Google Scholar] [CrossRef]
- Hovland, A.; Retterstø, K.; Mollnes, T.E.; Halvorsen, B.; Aukrust, P.; Lappegård, K.T. Anti-inflammatory effects of non-statin low-density lipoprotein cholesterol-lowering drugs: An unused potential? Scand. Cardiovasc. J. 2020, 54, 274–279. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Vitiello, M.; Casale, R.; Servillo, L.; Giovane, A.; Balestrieri, M.L. Sirtuins in vascular diseases: Emerging roles and therapeutic potential. Biochim. Biophys. Acta 2015, 1852, 1311–1322. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.X.; Li, S.; Liu, H.H.; Li, J.J. Impact of PCSK-9 monoclonal antibodies on circulating hs-CRP levels: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2018, 8, e022348. [Google Scholar] [CrossRef] [Green Version]
- Fung, E.T.; Wilson, A.M.; Zhang, F.; Harris, N.; Edwards, K.A.; Olin, J.W.; Cooke, J.P. A biomarker panel for peripheral arterial disease. Vasc. Med. 2008, 13, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Jiang, L.; Peng, J.; Ren, Z.; Wei, D.; Wu, C.; Pan, L.; Jiang, Z.; Liu, L. PCSK-9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-κB activation in THP-1-derived P-1-derived macrophages. Int. J. Mol. Med. 2012, 30, 931–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, T.; Teli, S.; Rijal, J.; Bhat, H.; Raza, M.; Khoueiry, G.; Meghani, M.; Akhtar, M.; Costantino, T. Neutrophil to lymphocyte ratio and cardiovascular diseases: A review. Expert Rev. Cardiovasc. Ther. 2013, 11, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Scicali, R.; Mandraffino, G.; Di Pino, A.; Scuruchi, M.; Ferrara, V.; Squadrito, G.; Purrello, F.; Piro, S. Impact of high neutrophil-to-lymphocyte ratio on the cardiovascular benefit of PCSK9 inhibitors in familial hypercholesterolemia subjects with atherosclerotic cardiovascular disease: Real-world data from two lipid units. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Shahanawaz, J.; Shmookler-Reis, R.J.; Varughese, K.I.; Sawamura, T.; Mehta, J.L. Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc. Res. 2015, 107, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Tibolla, G.; Pirillo, A.; Cipollone, F.; Mezzetti, A.; Pacia, S.; Corsini, A.; Catapano, A.L. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis 2012, 220, 381–386. [Google Scholar] [CrossRef]
- Yurtseven, E.; Ural, D.; Baysal, K.; Tokgözoğlu, L. An update on the role of PCSK9 in atherosclerosis. J. Atheroscler. Thromb. 2020, 27, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Adorni, M.P.; Cipollari, E.; Favari, E.; Zanotti, I.; Zimetti, F.; Corsini, A.; Ricci, C.; Bernini, F.; Ferri, N. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis 2017, 256, 1–6. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Theus, S.; Deng, X.; Fan, Y.; Zhou, S.; Mehta, J.L. PCSK9 regulates expression of scavenger receptors and ox-LDL uptake in macrophages. Cardiovasc. Res. 2018, 114, 1145–1153. [Google Scholar] [CrossRef]
- Bai, X.Q.; Peng, J.; Wang, M.M.; Xiao, J.; Xiang, Q.; Ren, Z.; Wen, H.Y.; Jiang, Z.S.; Tang, Z.H.; Liu, L.S. PCSK9: A potential regulator of apoE/apoER2 against inflammation in atherosclerosis? Clin. Chim. Acta 2018, 483, 192–196. [Google Scholar] [CrossRef]
- Bernelot Moens, S.J.; Neele, A.E.; Kroon, J.; van der Valk, F.M.; van den Bossche, J.; Hoeksema, M.A.; Hoogeveen, R.M.; Schnitzler, J.G.; Baccara-Dinet, M.T.; Manvelian, G.; et al. PCSK-9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur. Heart J. 2017, 38, 1584–1593. [Google Scholar] [CrossRef]
- Grune, J.; Meyborg, H.; Bezhaeva, T.; Kappert, K.; Hillmeister, P.; Kintscher, U.; Pieske, B.; Stawowy, P. PCSK-9 regulates the chemokine receptor CCR2 on monocytes. Biochem. Biophys. Res. Commun. 2017, 485, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K. Cholesterol, CCR2, and monocyte phenotypes in atherosclerosis. Eur. Heart J. 2017, 38, 1594–1596. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Tang, Z.H.; Jiang, L.; Li, X.F.; Jiang, Z.S.; Liu, L.S. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol. Cell. Biochem. 2012, 359, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Leiva, E.; Wehinger, S.R.; Guzmán, L.; Orrego, R. Role of oxidized LDL in atherosclerosis. In Hypercholesterolemia; Kumar, S.A., Ed.; IntechOpen Limited: London, UK, 2015; pp. 55–77. [Google Scholar]
- Campbell, J.H.; Popadynec, L.; Nestel, P.J.; Campbell, G.R. Lipid accumulation in arterial smooth muscle cells. Influence of phenotype. Atherosclerosis 1983, 47, 279–295. [Google Scholar] [CrossRef]
- Diedrich, G. How does hepatitis C virus enter cells? FEBS J. 2006, 273, 3871–3885. [Google Scholar] [CrossRef]
- Fruchart, J.C.; Sacks, F.; Hermans, M.P.; Assmann, G.; Brown, W.V.; Ceska, R.; Chapman, M.J.; Dodson, P.M.; Fioretto, P.; Ginsberg, H.N.; et al. The Residual Risk Reduction Initiative: A call to action to reduce residual vascular risk in patients with dyslipidemia. Am. J. Cardiol. 2008, 102 (Suppl. 10), 1K–34K. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Mortality differences associated with treatment responses in CANTOS and FOURIER: Insight and implications. Circulation 2018, 137, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Febbraio, M.; Li, W.; Silverstein, R.L. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ. Res. 2008, 102, 1512–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, A.; Brunssen, C.; Morawietz, H. Contribution of lectin-like oxidized low-density lipoprotein receptor-1 and LOX-1 modulating compounds to vascular diseases. Vascul. Pharmacol. 2017, S1537–S1891, 30171–30174. [Google Scholar] [CrossRef]
- Magwenzi, S.; Woodward, C.; Wraith, K.S.; Aburima, A.; Raslan, Z.; Jones, H.; McNeil, C.; Wheatcroft, S.; Yuldasheva, N.; Febbriao, M.; et al. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood 2015, 125, 2693–2703. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Xin, L.; Panigrahi, S.; Zimman, A.; Wang, H.; Yakubenko, V.P.; Byzova, T.V.; Salomon, R.G.; Podrez, E.A. Novel phosphatidylethanolamine derivatives accumulate in circulation in hyperlipidemic ApoE-/- mice and activate platelets via TLR2. Blood 2016, 127, 2618–2629. [Google Scholar] [CrossRef] [Green Version]
- Leibundgut, G.; Scipione, C.; Yin, H.; Schneider, M.; Boffa, M.B.; Green, S.; Yang, X.; Dennis, E.; Witztum, J.L.; Koschinsky, M.L.; et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a). J. Lipid Res. 2013, 54, 2815–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pęczek, P.; Leśniewski, M.; Mazurek, T.; Szarpak, L.; Filipiak, K.J.; Gąsecka, A. Antiplatelet Effects of PCSK9 Inhibitors in Primary Hypercholesterolemia. Life 2021, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Barale, C.; Bonomo, K.; Frascaroli, C.; Morotti, A.; Guerrasio, A.; Cavalot, F.; Russo, I. Effects of PCSK-9 inhibitors on platelet function in adults with hypercholesterolemia. Atherosclerosis 2017, 263, 30–31. [Google Scholar] [CrossRef]
- Cammisotto, V.; Baratta, F.; Castellani, V.; Bartimoccia, S.; Nocella, C.; D’Erasmo, L.; Cocomello, N.; Barale, C.; Scicali, R.; Di Pino, A.; et al. Proprotein Convertase Subtilisin Kexin Type 9 Inhibitors Reduce Platelet Activation Modulating ox-LDL Pathways. Int. J. Mol. Sci. 2021, 22, 7193. [Google Scholar] [CrossRef] [PubMed]
- Kotani, K.; Banach, M. Lipoprotein(a) and inhibitors of proprotein convertase subtilisin/kexin type 9. J. Thorac. Dis. 2017, 9, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Folsom, A.R.; Lutsey, P.L.; Astor, B.C.; Cushman, M. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thromb. Haemost. 2009, 102, 615–619. [Google Scholar]
- Prandoni, P.; Bilora, F.; Marchiori, A.; Bernardi, E.; Petrobelli, F.; Lensing, A.W.; Prins, M.H.; Girolami, A. An association between atherosclerosis and venous thrombosis. N. Engl. J. Med. 2003, 348, 1435–1441. [Google Scholar] [CrossRef] [Green Version]
- Glynn, R.J.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N. Engl. J. Med. 2009, 360, 1851–1861. [Google Scholar] [CrossRef] [Green Version]
- Sofi, F.; Marcucci, R.; Abbate, R.; Gensini, G.F.; Prisco, D. Lipoprotein (a) and venous thromboembolism in adults: A meta-analysis. Am. J. Med. 2007, 120, 728–733. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.G.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Lira Pineda, A.; Wasserman, S.M.; Češka, R.; et al. Lipoprotein(a), PCSK-9Inhibition, and Cardiovascular Risk. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Siegler, J.E.; Samai, A.; Albright, K.C.; Boehme, A.K.; Martin-Schild, S. Factoring in Factor VIII with Acute Ischemic Stroke. Clin. Appl. Thromb. Hemost. 2015, 21, 597–602. [Google Scholar] [CrossRef] [Green Version]
- Schlüter, K.D.; Wolf, A.; Weber, M.; Schreckenberg, R.; Schulz, R. Oxidized low-density lipoprotein (oxLDL) affects load-free cell shortening of cardiomyocytes in a proprotein convertase subtilisin/kexin 9 (PCSK9)-dependent way. Basic Res. Cardiol. 2017, 112, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.; Wang, X.; Liu, S.; Shahanawaz, J.; Theus, S.; Fan, Y.; Deng, X.; Zhou, S.; Mehta, J.L. PCSK9 expression in the ischaemic heart and its relationship to infarct size, cardiac function, and development of autophagy. Cardiovasc. Res. 2018, 14, 1738–1751. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Zeng, Y.D.; Hu, Z.X.; Liang, H. PCSK9 promotes the secretion of pro-inflammatory cytokines by macrophages to aggravate H/R-induced cardiomyocyte injury via activating NF-κB signalling. Gen. Physiol. Biophys. 2020, 39, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yan, B.; Tai, S.; Zhou, S.; Zheng, X.L. PCSK9: Associated with cardiac diseases and their risk factors? Arch. Biochem. Biophys. 2021, 704, 108717. [Google Scholar] [CrossRef] [PubMed]
- Potere, N.; Del Buono, M.G.; Mauro, A.G.; Abbate, A.; Toldo, S. Low Density Lipoprotein Receptor-Related Protein-1 in Cardiac Inflammation and Infarct Healing. Front. Cardiovasc. Med. 2019, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Palee, S.; McSweeney, C.M.; Maneechote, C.; Moisescu, D.M.; Jaiwongkam, T.; Kerdphoo, S.; Chattipakorn, S.C.; Chattipakorn, N. PCSK9 inhibitor improves cardiac function and reduces infarct size in rats with ischaemia/reperfusion injury: Benefits beyond lipid-lowering effects. J. Cell. Mol. Med. 2019, 23, 7310–7319. [Google Scholar] [CrossRef] [Green Version]
- Bayes-Genis, A.; Núñez, J.; Zannad, F.; Ferreira, J.P.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; Lang, C.C.; Ng, L.L. The PCSK9-LDL Receptor Axis and Outcomes in Heart Failure: BIOSTAT-CHF Subanalysis. J. Am. Coll. Cardiol. 2017, 70, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Dehn, S.; Thorp, E.B. Myeloid receptor CD36 is required for early phagocytosis of myocardial infarcts and induction of Nr4a1-dependent mechanisms of cardiac repair. FASEB J. 2018, 32, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Chandrakala, A.N.; Sukul, D.; Selvarajan, K.; Sai-Sudhakar, C.; Sun, B.; Parthasarathy, S. Induction of brain natriuretic peptide and monocyte chemotactic protein-1 gene expression by oxidized low-density lipoprotein: Relevance to ischemic heart failure. Am. J. Physiol. Cell Physiol. 2012, 302, C165–C177. [Google Scholar] [CrossRef] [PubMed]
- Padmasekar, M.; Nandigama, R.; Wartenberg, M.; Schlüter, K.D.; Sauer, H. The acute phase protein alpha2-macroglobulin induces rat ventricular cardiomyocyte hypertrophy via ERK1,2 and PI3-kinase/Akt pathways. Cardiovasc. Res. 2007, 75, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Cammisotto, V.; Pastori, D.; Nocella, C.; Bartimoccia, S.; Castellani, V.; Marchese, C.; Scavalli, A.S.; Ettorre, E.; Viceconte, N.; Violi, F.; et al. PCSK9 Regulates Nox2-Mediated Platelet Activation via CD36 Receptor in Patients with Atrial Fibrillation. Antioxidants 2020, 9, 296. [Google Scholar] [CrossRef] [Green Version]
- Bovenschen, N.; Mertens, K.; Hu, L.; Havekes, L.M.; van Vlijmen, B.J. LDL receptor cooperates with LDL receptor-related protein in regulating plasma levels of coagulation factor VIII in vivo. Blood 2005, 106, 906–912. [Google Scholar] [CrossRef]
- Riddell, D.R.; Vinogradov, D.V.; Stannard, A.K.; Chadwick, N.; Owen, J.S. Identification and characterization of LRP8 (apoER2) in human blood platelets. J. Lipid Res. 1999, 40, 1925–1930. [Google Scholar] [CrossRef]
- Ljungberg, J.; Janiec, M.; Bergdahl, I.A.; Holmgren, A.; Hultdin, J.; Johansson, B.; Näslund, U.; Siegbahn, A.; Fall, T.; Söderberg, S. Proteomic Biomarkers for Incident Aortic Stenosis Requiring Valvular Replacement. Circulation 2018, 138, 590–599. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Beyer, T.P.; Bensch, W.R.; Qian, Y.W.; Lin, A.; Kowala, M.; Alborn, W.E.; Konrad, R.J.; Cao, G. Secreted proprotein convertase subtilisin/kexin type 9 reduces both hepatic and extrahepatic low-density lipoprotein receptors in vivo. Biochem. Biophys. Res. Commun. 2008, 370, 634–640. [Google Scholar] [CrossRef]
- Cnop, M.; Hannaert, J.C.; Grupping, A.Y.; Pipeleers, D.G. Low density lipoprotein can cause death of islet beta-cells by its cellular uptake and oxidative modification. Endocrinology 2002, 143, 3449–3453. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.U.; Rahman, H.; Okunrintemi, V.; Riaz, H.; Khan, M.S.; Sattur, S.; Kaluski, E.; Lincoff, A.M.; Martin, S.S.; Blaha, M.J. Association of Lowering Low-Density Lipoprotein Cholesterol with Contemporary Lipid-Lowering Therapies and Risk of Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e011581. [Google Scholar] [CrossRef] [Green Version]
- Sahebkar, A.; Simental-Mendía, L.E.; Guerrero-Romero, F.; Golledge, J.; Watts, G.F. Effect of statin therapy on plasma proprotein convertase subtilisin kexin 9 (PCSK9) concentrations: A systematic review and meta-analysis of clinical trials. Diabetes Obes. Metab. 2015, 17, 1042–1055. [Google Scholar] [CrossRef]
- Ference, B.A.; Robinson, J.G.; Brook, R.D.; Catapano, A.L.; Chapman, M.J.; Neff, D.R.; Voros, S.; Giugliano, R.P.; Davey Smith, G.; Fazio, S.; et al. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N. Engl. J. Med. 2016, 375, 2144–2153. [Google Scholar] [CrossRef] [Green Version]
- Han, E.; Cho, N.H.; Moon, S.S.; Cho, H. Comparison of Serum PCSK9 Levels in Subjects with Normoglycemia, Impaired Fasting Glucose, and Impaired Glucose Tolerance. Endocrinol. Metab. 2020, 35, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Ibarretxe, D.; Girona, J.; Plana, N.; Cabré, A.; Ferré, R.; Amigó, N.; Guaita, S.; Mallol, R.; Heras, M.; Masana, L. Circulating PCSK9 in patients with type 2 diabetes and related metabolic disorders. Clin. Investig. Arterioscler. 2016, 28, 71–78. [Google Scholar] [CrossRef]
- Da Dalt, L.; Ruscica, M.; Bonacina, F.; Balzarotti, G.; Dhyani, A.; Di Cairano, E.; Baragetti, A.; Arnaboldi, L.; De Metrio, S.; Pellegatta, F.; et al. PCSK9 deficiency reduces insulin secretion and promotes glucose intolerance: The role of the low-density lipoprotein receptor. Eur. Heart J. 2019, 40, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Blom, D.J.; Koren, M.J.; Roth, E.; Monsalvo, M.L.; Djedjos, C.S.; Nelson, P.; Elliott, M.; Wasserman, S.M.; Ballantyne, C.M.; Holman, R.R. Evaluation of the efficacy, safety and glycaemic effects of evolocumab (AMG 145) in hypercholesterolaemic patients stratified by glycaemic status and metabolic syndrome. Diabetes Obes. Metab. 2017, 19, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talasaz, A.H.; Ho, A.J.; Bhatty, F.; Koenig, R.A.; Dixon, D.L.; Baker, W.L.; Van Tassell, B.W. Meta-analysis of clinical outcomes of PCSK9 modulators in patients with established ASCVD. Pharmacotherapy 2021, 41, 1009–1023. [Google Scholar] [CrossRef]
- Goldman, A.; Raschi, E.; Cukierman-Yaffe, T.; Dankner, R.; Shouval, R.; Shechter, M.; Ben-Zvi, I.; Gerstein, H.C.; Maor, E. Hyperglycaemic disorders associated with PCSK9 inhibitors: A real-world, pharmacovigilance study. Eur. J. Prev. Cardiol. 2021; epub ahead of print. [Google Scholar] [CrossRef]
- Bułdak, Ł.; Skudrzyk, E.; Machnik, G.; Bołdys, A.; Bułdak, R.J.; Okopień, B. Exenatide improves antioxidant capacity and reduces the expression of LDL receptors and PCSK9 in human insulin-secreting 1.1E7 cell line subjected to hyperglycemia and oxidative stress. Adv. Hyg. Exp. Med. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Farmer, J.A. Diabetic dyslipidemia and atherosclerosis: Evidence from clinical trials. Curr. Diab. Rep. 2008, 8, 71–77. [Google Scholar] [CrossRef]
- Athyros, V.G.; Tziomalos, K.; Karagiannis, A.; Mikhailidis, D.P. Dyslipidaemia of obesity, metabolic syndrome and type 2 diabetes mellitus: The case for residual risk reduction after statin treatment. Open Cardiovasc. Med. J. 2011, 5, 24–34. [Google Scholar] [CrossRef]
- Patti, A.M.; Giglio, R.V.; Papanas, N.; Rizzo, M.; Rizvi, A.A. Future perspectives of the pharmacological management of diabetic dyslipidemia. Expert Rev. Clin. Pharmacol. 2019, 12, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Kruit, J.K.; Groen, A.K.; van Berkel, T.J.; Kuipers, F. Emerging roles of the intestine in control of cholesterol metabolism. World J. Gastroenterol. 2006, 12, 6429–6439. [Google Scholar] [CrossRef] [PubMed]
- Suchy, D.; Łabuzek, K.; Stadnicki, A.; Okopień, B. Ezetimibe—A new approach in hypercholesterolemia management. Pharmacol. Rep. 2011, 63, 1335–1348. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Björnson, E.; Andersson, L.; Kahri, J.; Porthan, K.; Matikainen, N.; Soderlund, S.; Pietilainen, K.; Hakkarainen, A.; Lundbom, N.; et al. Impact of proprotein convertase subtilisin/kexin type 9 inhibition with evolocumab on the postprandial responses of triglyceride-rich lipoproteins in type II diabetic subjects. J. Clin. Lipidol. 2020, 14, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Tavori, H.; Brown, P.E.; Linton, M.F.; He, J.; Giunzioni, I.; Fazio, S. Proprotein convertase subtilisin kexin type 9 promotes intestinal overproduction of triglyceride-rich apolipoprotein B lipoproteins through both low-density lipoprotein receptor-dependent and -independent mechanisms. Circulation 2014, 130, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Paquette, M.; Gauthier, D.; Chamberland, A.; Prat, A.; De Lucia Rolfe, E.; Rasmussen, J.J.; Kaduka, L.; Seidah, N.G.; Bernard, S.; Christensen, D.L.; et al. Circulating PCSK9 is associated with liver biomarkers and hepatic steatosis. Clin. Biochem. 2020, 77, 20–25. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Adams, L.A.; Canbay, A.; Syn, W.K. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014, 59, 1174–1197. [Google Scholar] [CrossRef]
- Tavori, H.; Rashid, S.; Fazio, S. On the function and homeostasis of PCSK9: Reciprocal interaction with LDLR and additional lipid effects. Atherosclerosis 2015, 238, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Ruscica, M.; Ferri, N.; Macchi, C.; Meroni, M.; Lanti, C.; Ricci, C.; Maggioni, M.; Fracanzani, A.L.; Badiali, S.; Fargion, S.; et al. Liver fat accumulation is associated with circulating PCSK9. Ann. Med. 2016, 48, 384–391. [Google Scholar] [CrossRef]
- Wargny, M.; Ducluzeau, P.H.; Petit, J.M.; Le May, C.; Smati, S.; Arnaud, L.; Pichelin, M.; Bouillet, B.; Lannes, A.; Blanchet, O.; et al. Circulating PCSK9 levels are not associated with the severity of hepatic steatosis and NASH in a high-risk population. Atherosclerosis 2018, 278, 82–90. [Google Scholar] [CrossRef]
- Scicali, R.; Di Pino, A.; Urbano, F.; Ferrara, V.; Marchisello, S.; Di Mauro, S.; Scamporrino, A.; Filippello, A.; Rabuazzo, A.M.; Purrello, F.; et al. Analysis of steatosis biomarkers and inflammatory profile after adding on PCSK9 inhibitor treatment in familial hypercholesterolemia subjects with nonalcoholic fatty liver disease: A single lipid center real-world experience. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 869–879. [Google Scholar] [CrossRef]
- Shafiq, M.; Walmann, T.; Nutalapati, V.; Gibson, C.; Zafar, Y. Effects of proprotein convertase subtilisin/kexin type-9 inhibitors on fatty liver. World J. Hepatol. 2020, 12, 1258–1266. [Google Scholar] [CrossRef]
- Sekhon, A.K.; Gollapalli, A.; Kaur, D.; Kaur, D.; Janssen, B.; Stevens, M.L.; Valerio, F.; Sierra-Hoffman, M.A. A New Potential Strategy for Acute Non-Alcoholic Steatohepatitis (NASH). Am. J. Case. Rep. 2021, 22, e932961. [Google Scholar] [CrossRef]
- Lee, J.S.; Mukhopadhyay, P.; Matyas, C.; Trojnar, E.; Paloczi, J.; Yang, Y.R.; Blank, B.A.; Savage, C.; Sorokin, A.V.; Mehta, N.N.; et al. PCSK9 inhibition as a novel therapeutic target for alcoholic liver disease. Sci. Rep. 2019, 9, 17167. [Google Scholar] [CrossRef] [PubMed]
- Artunc, F. Kidney-derived PCSK9-a new driver of hyperlipidemia in nephrotic syndrome? Kidney Int. 2020, 98, 1393–1395. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhou, Y.; Pan, Y.; Li, C.; Wang, Y.; Chen, F.; Chen, X.; Yang, S.; Zhou, Z.; Liao, Y.; et al. Vaccine Against PCSK9 Improved Renal Fibrosis by Regulating Fatty Acid β-Oxidation. J. Am. Heart Assoc. 2020, 9, e014358. [Google Scholar] [CrossRef] [PubMed]
- Molina-Jijon, E.; Gambut, S.; Macé, C.; Avila-Casado, C.; Clement, L.C. Secretion of the epithelial sodium channel chaperone PCSK9 from the cortical collecting duct links sodium retention with hypercholesterolemia in nephrotic syndrome. Kidney Int. 2020, 98, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.E.; Levenson, A.E.; Sun, X.; Liao, W.H.; Rutkowski, J.M.; de Ferranti, S.D.; Schumacher, V.A.; Scherer, P.E.; Salant, D.J.; Biddinger, S.B. The role of proprotein convertase subtilisin/kexin type 9 in nephrotic syndrome-associated hypercholesterolemia. Circulation 2016, 134, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Awanami, Y.; Fukuda, M.; Nonaka, Y.; Takashima, T.; Matsumoto, K.; Yamasaki, M.; Miyazono, M.; Ikeda, Y. Successful treatment of a patient with refractory nephrotic syndrome with PCSK9 inhibitors: A case report. BMC Nephrol. 2017, 18, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharotri, V.; Collier, D.M.; Olson, D.R.; Zhou, R.; Snyder, P.M. Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexinType9(PCSK9). J. Biol. Chem. 2012, 287, 19266–19274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, J.M.; Vaillant, N.; Le May, C.; Calderon, C.; Brégeon, J.; Prieur, X.; Hadchouel, J.; Loirand, G.; Cariou, B. PCSK9-deficiency does not alter blood pressure and sodium balance in mouse models of hypertension. Atherosclerosis 2015, 239, 252–259. [Google Scholar] [CrossRef]
- Pavlakou, P.; Liberopoulos, E.; Dounousi, E.; Elisaf, M. PCSK9 in chronic kidney disease. Int. Urol. Nephrol. 2017, 49, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Zheng-Lin, B.; Ortiz, A. Lipid management in chronic kidney disease: Systematic review of PCSK9 targeting. Drugs 2018, 78, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Bułdak, Ł.; Marek, B.; Kajdaniuk, D.; Urbanek, A.; Janyga, S.; Bołdys, A.; Basiak, M.; Maligłówka, M.; Okopień, B. Endocrine diseases as causes of secondary hyperlipidemia. Endokrynol. Pol. 2019, 70, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ma, Y.; Ye, Z.; Fu, Z.; Yang, P.; Gao, B.; Guo, W.; Hu, D.; Ye, J.; Ma, S.; et al. Thyroid stimulating hormone exhibits the impact on LDLR/LDL-c via up-regulating hepatic PCSK9 expression. Metabolism 2017, 76, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Bonde, Y.; Breuer, O.; Lütjohann, D.; Sjöberg, S.; Angelin, B.; Rudling, M. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J. Lipid Res. 2014, 55, 2408–2415. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, A.M.; Koca, A.O.; Beyan, E.; Dogan, O.; Karakaya, S.; Aksoz, Z.; Ertuğrul, D.T. Association of serum proprotein convertase Subtilisin/Kexin Type 9 (PCSK9) level with thyroid function disorders. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5511–5517. [Google Scholar] [PubMed]
- Lee, G.E.; Kim, J.; Lee, J.S.; Ko, J.; Lee, E.J.; Yoon, J.S. Role of Proprotein Convertase Subtilisin/Kexin Type 9 in the Pathogenesis of Graves’ Orbitopathy in Orbital Fibroblasts. Front. Endocrinol. 2021, 11, 607144. [Google Scholar] [CrossRef]
- Lakoski, S.G.; Lagace, T.A.; Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Genetic and metabolic determinants of plasma PCSK9 levels. J. Clin. Endocrinol. Metab. 2009, 94, 2537–2543. [Google Scholar] [CrossRef]
- Ooi, T.C.; Raymond, A.; Cousins, M.; Favreau, C.; Taljaard, M.; Gavin, C.; Jolly, E.E.; Malone, S.; Eapen, L.; Chretien, M.; et al. Relationship between testosterone, estradiol and circulating PCSK9: Cross-sectional and interventional studies in humans. Clin. Chim. Acta 2015, 446, 97–104. [Google Scholar] [CrossRef]
- Peticca, P.; Raymond, A.; Gruslin, A.; Cousins, M.; Adetola, E.; Abujrad, H.; Mayne, J.; Ooi, T.C. Human Serum PCSK9 Is Elevated at Parturition in Comparison to Nonpregnant Subjects While Serum PCSK9 from Umbilical Cord Blood is Lower Compared to Maternal Blood. ISRN Endocrinol. 2013, 341632. [Google Scholar] [CrossRef]
- Persson, L.; Gälman, C.; Angelin, B.; Rudling, M. Importance of proprotein convertase subtilisin/kexin type 9 in the hormonal and dietary regulation of rat liver low-density lipoprotein receptors. Endocrinology 2009, 150, 1140–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blom, D.J.; Djedjos, C.S.; Monsalvo, M.L.; Bridges, I.; Wasserman, S.M.; Scott, R.; Roth, E. Effects of Evolocumab on Vitamin E and Steroid Hormone Levels: Results From the 52-Week, Phase 3, Double-Blind, Randomized, Placebo-Controlled DESCARTES Study. Circ. Res. 2015, 117, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Imprialos, K.P.; Stavropoulos, K.; Doumas, M.; Tziomalos, K.; Karagiannis, A.; Athyros, V.G. Sexual Dysfunction, Cardiovascular Risk and Effects of Pharmacotherapy. Curr. Vasc. Pharmacol. 2018, 16, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Z.; Shen, W.J.; Azhar, S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010, 7, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cariou, B.; Benoit, I.; Le May, C. Preserved adrenal function in fully PCSK9-deficient subject. Int. J. Cardiol. 2014, 176, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Frick, M.; Liu, M.; Saeedi Saravi, S.S.; Montrasio, G.; Preiss, H.; Pasterk, L.; Bonetti, N.; Egloff, M.; Schmid, H.R.; et al. Reduced adrenal stress response in patients on PCSK9 inhibitor therapy. Atherosclerosis 2021, 325, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ivanes, F.; Susen, S.; Mouquet, F.; Pigny, P.; Cuilleret, F.; Sautière, K.; Collet, J.P.; Beygui, F.; Hennache, B.; Ennezat, P.V.; et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur. Heart J. 2012, 33, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Izkhakov, E.; Shacham, Y.; Serebro, M.; Yaish, I.; Marcus, Y.; Shefer, G.; Tordjman, K.; Greenman, Y.; Stern, N.; Ziv-Baran, T. The Effect of the PCSK9 Inhibitor Evolocumab on Aldosterone Secretion among High Cardiovascular Risk Patients: A Pilot Study. J. Clin. Med. 2021, 10, 2504. [Google Scholar] [CrossRef]
- Bizoń, A.; Franik, G.; Madej, P. The role of proprotein convertase subtilisin/kexin type-9 concentration and paraoxonase 1 activities in the blood of women with polycystic ovary syndrome. Environ. Toxicol. Pharmacol. 2021, 84, 103612. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, D.; Xu, L.; Guo, W.; Nie, L.; Lei, Y.; Long, Y.; Liu, M.; Wang, Y.; Zhang, X.; et al. Role of PCSK9 in lipid metabolic disorders and ovarian dysfunction in polycystic ovary syndrome. Metabolism 2019, 94, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, L.; Cheng, D.; Liu, T.; An, L.; Li, W.P.; Zhang, C. Low-density lipoprotein receptor affects the fertility of female mice. Reprod. Fertil. Dev. 2015, 27, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Xavier, L.B.; Sóter, M.O.; Sales, M.F.; Oliveira, D.K.; Reis, H.J.; Candido, A.L.; Reis, F.M.; Silva, I.O.; Gomes, K.B.; Ferreira, C.N. Evaluation of PCSK9 levels and its genetic polymorphisms in women with polycystic ovary syndrome. Gene 2018, 644, 129–136. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Wei, X.; Li, H.; Gu, H.; Huang, T.; Zhao, G.; Liu, B.; Wang, W.; Chen, L.; Ma, W.; et al. Identification of PCSK9 as a novel serum biomarker for the prenatal diagnosis of neural tube defects using iTRAQ quantitative proteomics. Sci. Rep. 2015, 5, 17559. [Google Scholar] [CrossRef] [Green Version]
- Postmus, I.; Trompet, S.; de Craen, A.J.; Buckley, B.M.; Ford, I.; Stott, D.J.; Sattar, N.; Slagboom, P.E.; Westendorp, R.G.; Jukema, J.W. PCSK9 SNP rs11591147 is associated with low cholesterol levels but not with cognitive performance or noncardiovascular clinical events in an elderly population. J. Lipid Res. 2013, 54, 561–566. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Barger, S.D.; Ryan, C.M.; Flory, J.D.; Lehoczky, J.P.; Matthews, K.A.; Manuck, S.B. Effects of lovastatin on cognitive function and psychological well-being. Am. J. Med. 2000, 108, 538–546. [Google Scholar] [CrossRef]
- Leritz, E.C.; McGlinchey, R.E.; Salat, D.H.; Milberg, W.P. Elevated levels of serum cholesterol are associated with better performance on tasks of episodic memory. Metab. Brain Dis. 2016, 31, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Zimetti, F.; Caffarra, P.; Ronda, N.; Favari, E.; Adorni, M.P.; Zanotti, I.; Bernini, F.; Barocco, F.; Spallazzi, M.; Galimberti, D.; et al. Increased PCSK9 cerebrospinal fluid concentrations in Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 55, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.M.; Finan, C.; Schmidt, A.F.; Burgess, S.; Hingorani, A.D. Lipid lowering and Alzheimer disease risk: A Mendelian randomization study. Ann. Neurol. 2020, 87, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Gencer, B.; Mach, F.; Guo, J.; Im, K.; Ruzza, A.; Wang, H.; Kurtz, C.E.; Pedersen, T.R.; Keech, A.C.; Ott, B.R.; et al. FOURIER Investigators. Cognition after lowering LDL-cholesterol with evolocumab. J. Am. Coll. Cardiol. 2020, 75, 2283–2293. [Google Scholar] [CrossRef]
- Banach, M.; Rizzo, M.; Nikolic, D.; Howard, G.; Howard, V.J.; Mikhailidis, D.P. Intensive LDL-cholesterol lowering therapy and neurocognitive function. Pharmacol. Ther. 2017, 170, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Mannarino, M.R.; Sahebkar, A.; Bianconi, V.; Serban, M.C.; Banach, M.; Pirro, M. PCSK9 and neurocognitive function: Should it be still an issue after FOURIER and EBBINGHAUS results? J. Clin. Lipidol. 2018, 12, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, F.H.; Nijhuis, G.B.J.; Zuidema, S.U.; Luijendijk, H. Serious adverse events and deaths in PCSK9 inhibitor trials reported on ClinicalTrials.gov: A systematic review. Expert Rev. Clin. Pharmacol. 2020, 13, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.D.; Sabbagh, M.N.; Harrison, J.E.; Ginsberg, H.N.; Chapman, M.J.; Manvelian, G.; Moryusef, A.; Mandel, J.; Farnier, M. No evidence of neurocognitive adverse events associated with alirocumab treatment in 3340 patients from 14 randomized Phase 2 and 3 controlled trials: A meta-analysis of individual patient data. Eur. Heart J. 2018, 39, 374–381. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.T.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef] [Green Version]
- Giugliano, R.P.; Pedersen, T.R.; Saver, J.L.; Sever, P.S.; Keech, A.C.; Bohula, E.A.; Murphy, S.A.; Wasserman, S.M.; Honarpour, N.; Wang, H.; et al. FOURIER Investigators: Stroke prevention with the PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibitor evolocumab added to statin in high-risk patients with stable atherosclerosis. Stroke 2020, 51, 1546–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, N.S.; Patel, N.; Kalra, R.; Ahmad, A.; Venkatraman, A.; Arora, G.; Arora, P. Neurological effects of proprotein convertase subtilisin/kexin type 9 inhibitors: Direct comparisons. Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.; Noory, M.; Robertson, G. The role of cholesterol in cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef] [Green Version]
- Vitols, S.; Bjorkholm, M.; Gahrton, G.; Peterson, C. Hypocholesterolaemia in malignancy due to elevated low-density-lipoprotein-receptor activity in tumor cells: Evidence from studies in patients with leukaemia. Lancet 1985, 326, 1150–1154. [Google Scholar] [CrossRef]
- Henriksson, P.; Eriksson, S.; Stege, R.; Berglund, L.; Angelin, B. Hypocholesterolaemia and increased elimination of low-density-lipoprotein in metastatic cancer of prostate. Lancet 1989, 334, 1178–1180. [Google Scholar] [CrossRef]
- De Gonzalo-Calvo, D.; Lopez-Vilaro, L.; Nasarre, L.; Perez-Olabarria, M.; Vazquez, T.; Escuin, D.; Badimon, L.; Barnadas, A.; Lerma, E.; Llorente-Cortes, V. Intratumor cholestyr ester accumulation is associated with human breast cancer proliferation and aggressive potential: A molecular and clinicopathological study. BMC Cancer 2015, 15, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, S.; Li, J.; Lee, S.; Lee, H.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.; Liu, X.; Ratliff, T.; et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Xu, N.; Zhang, X.; Wu, C.; Zhejiang, J. Lipids changes in liver cancer. Univ. Sci. B. 2007, 8, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Murai, T. Cholesterol lowering: Role in cancer prevention and treatment. Biol. Chem. 2015, 396, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Murai, T. The role of lipid rafts in cancer cell adhesion and migration. Int. J. Cell Biol. 2012, 763283. [Google Scholar] [CrossRef] [Green Version]
- Abramson, H. The lipogenesis pathway as a cancer target. J. Med. Chem. 2011, 54, 5615–5638. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Maruyama, Y.; Mio, K.; Nishiyama, H.; Suga, M.; Sato, C. Low cholesterol triggers membrane microdomain-dependent CD44 shedding and suppresses tumor cell migration. J. Biol. Chem. 2011, 286, 1999–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahboobnia, K.; Pirro, M.; Marini, E.; Grignani, F.; Bezsonov, E.; Jamialahmadi, T.; Sahebkar, A. PCSK9 and cancer: Rethinking the link. Biomed. Pharmacother. 2021, 140, 111758. [Google Scholar] [CrossRef] [PubMed]
- Ranheim, T.; Mattingsdal, M.; Lindvall, J.; Holla, O.; Berge, K.; Kulseth, M.; Leren, T. Genome-wide expression analysis of cells expressing gain of function mutant D374Y-PCSK9. J. Cell Physiol. 2008, 271, 459–467. [Google Scholar] [CrossRef]
- Mbikay, M.; Sirois, F.; Mayne, J.; Wang, G.; Chen, A.; Dewpura, T.; Prat, A.; Seidah, N.G.; Chretien, M.; Scott, F. PCSK9-deficient mice exhibit impaired glucose tolerance and pancreatic islet abnormalities. FEBS Lett. 2009, 584, 701–706. [Google Scholar] [CrossRef]
- Poirier, S.; Prat, A.; Marcinkiewicz, E.; Paquin, J.; Chitramuthu, B.; Baranowski, D.; Cadieux, B.; Bennett, H.; Seidah, N.G. Implication of the proprotein convertase NARC-1/PCSK-9 in the development of the nervous system. J. Neurochem. 2006, 98, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Bingham, B.; Shen, R.; Kotnis, S.; Lo, C.; Ozenberger, B.; Ghosh, N.; Kennedy, N.; Jacobsen, J.; Grenier, J.; DiStefano, P.; et al. Proapoptotic effects of NARC 1 (=PCSK9), the gene encoding a novel serine proteinase. Cytometry 2006, 69, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Essalmani, R.; Day, R.; Khatib, A.; Seidah, N.G.; Prat, A. Proprotein convertase subtilisin/kexin type 9 deficiency reduces melanoma metastasis in liver. Neoplasia 2012, 14, 1122–1131. [Google Scholar] [CrossRef] [Green Version]
- Piao, M.; Bai, J.; Zhang, P.; Zhang, Y. PCKS9 regulates apoptosis in human neuroglioma u251 cells via mitochondrial signaling pathways. Int. J. Clin. Exp. Pathol. 2015, 8, 2787–2794. [Google Scholar] [PubMed]
- Xu, X.; Cui, Y.; Cao, L.; Zhang, Y.; Yin, Y.; Hu, X. PCSK9 regulates apoptosis in human lung adenocarcinoma A549 cells via endoplasmic reticulum stress and mitochondrial signaling pathways. Exp. Ther. Med. 2017, 13, 1993–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, M.; Nicolas, S.; Marcus, V.; Deschenes, M.; Tan, X.; Bouteaud, J.; Negi, S.; Zuhier, A.; Aikin, R.; Kwan, J.; et al. Decreased PCSK9 expression in human hepatocellular carcinoma. BMC Gastroenterol. 2015, 15, 176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhu, X.; Feng, L.; Li, X.; Liu, X.; Sun, H.; Tang, Z. PCSK9 promoter tumor growth by inhibiting tumor cell apoptosis in hepatocellular carcinoma. Exp. Hematol. Oncol. 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, A.; Subbannayya, Y.; Sahasrabuddhe, N.; Balakrishnan, L.; Syed, N.; Sekhar, N.; Katte, T.; Pinto, S.; Srikanth, S.; Kumar, P.; et al. SILAC-based quantitative proteomic analysis of gastric cancer secretome. Proteom. Clin. Appl. 2013, 7, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Amersfoort, E.S.; Van Berkel, T.J.; Kuiper, J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 2003, 16, 379–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savva, A.; Roger, T. Targeting toll-like receptors: Promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front. Immunol. 2013, 4, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levels, J.H.; Marquart, J.A.; Abraham, P.R.; van den Ende, A.E.; Molhuizen, H.O.; van Deventer, S.J.; Meijers, J.C. Lipopolysaccharide is transferred from high-density to low-density lipoproteins by lipopolysaccharide-binding protein and phospholipid transfer protein. Infect. Immun. 2005, 73, 2321–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, D.J.; Grin, P.M.; Khan, M.; Prat, A.; Zhou, J.; Fox-Robichaud, A.E.; Seidah, N.G.; Liaw, P.C. Differential Expression of PCSK9 Modulates Infection, Inflammation, and Coagulation in a Murine Model of Sepsis. Shock 2016, 46, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Walley, K.R.; Thain, K.R.; Russell, J.A.; Reilly, M.P.; Meyer, N.J.; Ferguson, J.F.; Christie, J.D.; Nakada, T.A.; Fjell, C.D.; Thair, S.A.; et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci. Transl. Med. 2014, 6, 258ra143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://clinicaltrials.gov/ct2/show/NCT03634293 (accessed on 15 January 2022).
- Blom, D.J.; Hala, T.; Bolognese, M.; Lillestol, M.J.; Toth, P.D.; Burgess, L.; Ceska, R.; Roth, E.; Koren, M.J.; Ballantyne, C.M.; et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N. Engl. J. Med. 2014, 370, 1809–1819. [Google Scholar] [CrossRef] [Green Version]
- Giugliano, R.P.; Desai, N.R.; Kohli, P.; Rogers, W.J.; Somaratne, R.; Huang, F.; Liu, T.; Mohanavelu, S.; Hoffman, E.B.; McDonald, S.T.; et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): A randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet 2012, 380, 2007–2017. [Google Scholar]
- Khademi, F.; Momtazi-Borojeni, A.A.; Reiner, Ž.; Banach, M.; Al-Rasadi, K.A.; Sahebkar, A. PCSK9 and infection: A potentially useful or dangerous association? J. Cell Physiol. 2018, 233, 2920–2927. [Google Scholar] [CrossRef]
- Ploss, A.; Evans, M.J. Hepatitis C virus host cell entry. Curr. Opin. Virol. 2012, 2, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Roger, S.; Ducancelle, A.; Le Guillou-Guillemette, H.; Gaudy, C.; Lunel, F. HCV virology and diagnosis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101626. [Google Scholar] [CrossRef]
- Burlone, M.E.; Budkowska, A. Hepatitis C virus cell entry: Role of lipoproteins and cellular receptors. J. Gen. Virol. 2009, 90, 1055–1070. [Google Scholar] [CrossRef]
- Syed, G.H.; Tang, H.; Khan, M.; Hassanein, T.; Liu, J.; Siddiqui, A. Hepatitis C virus stimulates low-density lipoprotein receptor expression to facilitate viral propagation. J. Virol. 2014, 88, 2519–2529. [Google Scholar] [CrossRef] [Green Version]
- Albecka, A.; Belouzard, S.; Op de Beeck, A.; Descamps, V.; Goueslain, L.; Bertrand-Michel, J.; Tercé, F.; Duverlie, G.; Rouillé, Y.; Dubuisson, J. Role of low-density lipoprotein receptor in the hepatitis C virus life cycle. Hepatology 2012, 55, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Labonté, P.; Begley, S.; Guévin, C.; Asselin, M.C.; Nassoury, N.; Mayer, G.; Prat, A.; Seidah, N.G. PCSK9 impedes hepatitis C virus infection in vitro and modulates liver CD81 expression. Hepatology 2009, 50, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.T.; Blanchet, M.; Seidah, N.G.; Labonté, P. Plasma Membrane Tetraspanin CD81 Complexes with Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) and Low-Density Lipoprotein Receptor (LDLR), and Its Levels Are Reduced by PCSK9. J. Biol. Chem. 2015, 290, 23385–23400. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, A.; Gusarova, V.; Stahl, N.; Gurnett-Bander, A.; Kyratsous, C.A. Alirocumab, a Therapeutic Human Antibody to PCSK9, Does Not Affect CD81 Levels or Hepatitis C Virus Entry and Replication into Hepatocytes. PLoS ONE 2016, 11, e0154498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Simmons, C.P.; Farrar, J.J.; Nguyen, V.V.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef]
- Soto-Acosta, R.; Bautista-Carbajal, P.; Cervantes-Salazar, M.; Angel-Ambrocio, A.H.; Del Angel, R.M. DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: A potential antiviral target. PLoS Pathog. 2017, 13, e1006257. [Google Scholar] [CrossRef]
- Bryan-Marrugo, O.L.; Arellanos-Soto, D.; Rojas-Martinez, A.; Barrera-Saldaña, H.; Ramos-Jimenez, J.; Vidaltamayo, R.; Rivas-Estilla, A.M. The anti-dengue virus properties of statins may be associated with alterations in the cellular antiviral profile expression. Mol. Med. Rep. 2016, 14, 2155–2163. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Gutierrez, M.; Correa-Londoño, L.A.; Castellanos, J.E.; Gallego-Gómez, J.C.; Osorio, J.E. Lovastatin delays infection and increases survival rates in AG129 mice infected with dengue virus serotype 2. PLoS ONE 2014, 9, e87412. [Google Scholar]
- Whitehorn, J.; Nguyen, C.V.V.; Khanh, L.P.; Kien, D.T.H.; Quyen, N.T.H.; Tran, N.T.T.; Hang, N.T.; Truong, N.T.; Hue Tai, L.T.; Cam Huong, N.T.; et al. Lovastatin for the Treatment of Adult Patients with Dengue: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2016, 62, 468–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, E.S.; Tan, H.C.; Le, D.H.T.; Huynh, T.T.; Wills, B.; Seidah, N.G.; Ooi, E.E.; Yacoub, S. Dengue virus induces PCSK9 expression to alter antiviral responses and disease outcomes. J. Clin. Investig. 2020, 130, 5223–5234. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://covid19.who.int/ (accessed on 15 January 2022).
- Mehra, M.R.; Desai, S.S.; Kuy, S.; Henry, T.D.; Patel, A.N. Retraction: Cardiovascular Disease, Drug Therapy, and Mortality in COVID-19. N. Engl. J. Med. 2020, 382, 2582. [Google Scholar] [CrossRef]
- Barkas, F.; Milionis, H.; Anastasiou, G.; Liberopoulos, E. Statins and PCSK9 inhibitors: What is their role in coronavirus disease 2019? Med. Hypotheses 2021, 146, 110452. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Nägele, M.P.; Haubner, B.; Tanner, F.C.; Ruschitzka, F.; Flammer, A.J. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis 2020, 314, 58–62. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Bayazeed, B.; Hook, G.; Johnson, A.; Cronin, J.; Baron, A.D. Endothelial dysfunction is associated with cholesterol levels in the high normal range in humans. Circulation 1997, 96, 3287–3293. [Google Scholar] [CrossRef]
- Leucker, T.M.; Gerstenblith, G.; Schär, M.; Brown, T.T.; Jones, S.R.; Afework, Y.; Weiss, R.G.; Hays, A.G. Evolocumab, a PCSK9-Monoclonal Antibody, Rapidly Reverses Coronary Artery Endothelial Dysfunction in People Living with HIV and People with Dyslipidemia. J. Am. Heart Assoc. 2020, 9, e016263. [Google Scholar] [CrossRef]
- Available online: https://www.acc.org/latest-in-cardiology/articles/2020/10/02/12/59/are-pcsk9-inhibitors-the-next-front-line-therapies-to-improve-vascular-dysfunction (accessed on 15 January 2022).
- Kastelein, J.J.; Ginsberg, H.N.; Langslet, G.; Hovingh, G.K.; Ceska, R.; Dufour, R.; Blom, D.; Civeira, F.; Krempf, M.; Lorenzato, C.; et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur. Heart J. 2015, 36, 2996–3003. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Xiao, J.; Ji, J.; Chen, L. Association of lipid-lowering drugs with COVID-19 outcomes from a Mendelian randomization study. elife 2021, 10, e73873. [Google Scholar] [CrossRef] [PubMed]
- Mbikay, M.; Mayne, J.; Seidah, N.G.; Chrétien, M. Of PCSK9, cholesterol homeostasis and parasitic infections: Possible survival benefits of loss-of-function PCSK9 genetic polymorphisms. Med. Hypotheses 2007, 69, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Arama, C.; Diarra, I.; Kouriba, B.; Sirois, F.; Fedoryak, O.; Thera, M.A.; Coulibaly, D.; Lyke, K.E.; Plowe, C.V.; Chrétien, M.; et al. Malaria severity: Possible influence of the E670G PCSK9 polymorphism: A preliminary case-control study in Malian children. PLoS ONE 2018, 13, e0192850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedoryak, O.; Arama, C.; Diarra, I.; Kouriba, B.; Chrétien, M.; Mbikay, M. Association of the rs562556 PCSK9 Gene Polymorphism with Reduced Mortality in Severe Malaria among Malian Children. Can. J. Infect. Dis. Med. Microbiol. 2020, 9340480. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, Y.; Santos, R.D.; Al-Rasadi, K.; Rizzo, M. Targeting PCSK9 for therapeutic gains: Have we addressed all the concerns? Atherosclerosis 2016, 248, 62–75. [Google Scholar] [CrossRef] [Green Version]
- Shan, L.; Pang, L.; Zhang, R.; Murgolo, N.J.; Lan, H.; Hedrick, J.A. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem. Biophys. Res. Commun. 2008, 375, 69–73. [Google Scholar] [CrossRef]
- Du, F.; Hui, Y.; Zhang, M.; Linton, M.F.; Fazio, S.; Fan, D. Novel domain interaction regulates secretion of proprotein convertase subtilisin/kexin type 9 (PCSK9) protein. J. Biol. Chem. 2011, 286, 43054–43061. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Eigenbrot, C.; Zhou, L.; Shia, S.; Li, W.; Quan, C.; Tom, J.; Moran, P.; Di Lello, P.; Skelton, N.J. Identification of a small peptide that inhibits PCSK9 protein binding to the low-density lipoprotein receptor. J. Biol. Chem. 2014, 289, 942–955. [Google Scholar] [CrossRef] [Green Version]
- Graham, M.J.; Lemonidis, K.M.; Whipple, C.P.; Subramaniam, A.; Monia, B.P.; Crooke, S.T.; Crooke, R.M. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J. Lipid Res. 2007, 48, 763–767. [Google Scholar] [CrossRef] [Green Version]
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef]
- Walker, H.E.; Rizzo, M.; Fras, Z.; Jug, B.; Banach, M.; Penson, P.E. CRISPR Gene Editing in Lipid Disorders and Atherosclerosis: Mechanisms and Opportunities. Metabolites 2021, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Galabova, G.; Brunner, S.; Winsauer, G.; Juno, C.; Wanko, B.; Mairhofer, A.; Lührs, P.; Schneeberger, A.; von Bonin, A.; Mattner, F. Peptide-based anti-PCSK9 vaccines—An approach for long-term LDLc management. PLoS ONE 2014, 9, e114469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Pan, Y.; Yang, S.; Li, C.; Zhou, Y.; Wang, Y.; Chen, X.; Zhou, Z.; Liao, Y.; Qiu, Z. PCSK9Qβ-003 Vaccine Attenuates Atherosclerosis in Apolipoprotein E-Deficient Mice. Cardiovasc. Drugs Ther. 2021, 35, 141–151. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, Y.; Wu, H.; Chen, X.; Hu, X.; Zhang, H.; Zhou, Z.; Qiu, Z.; Liao, Y. A Therapeutic Peptide Vaccine against PCSK9. Sci. Rep. 2017, 7, 12534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
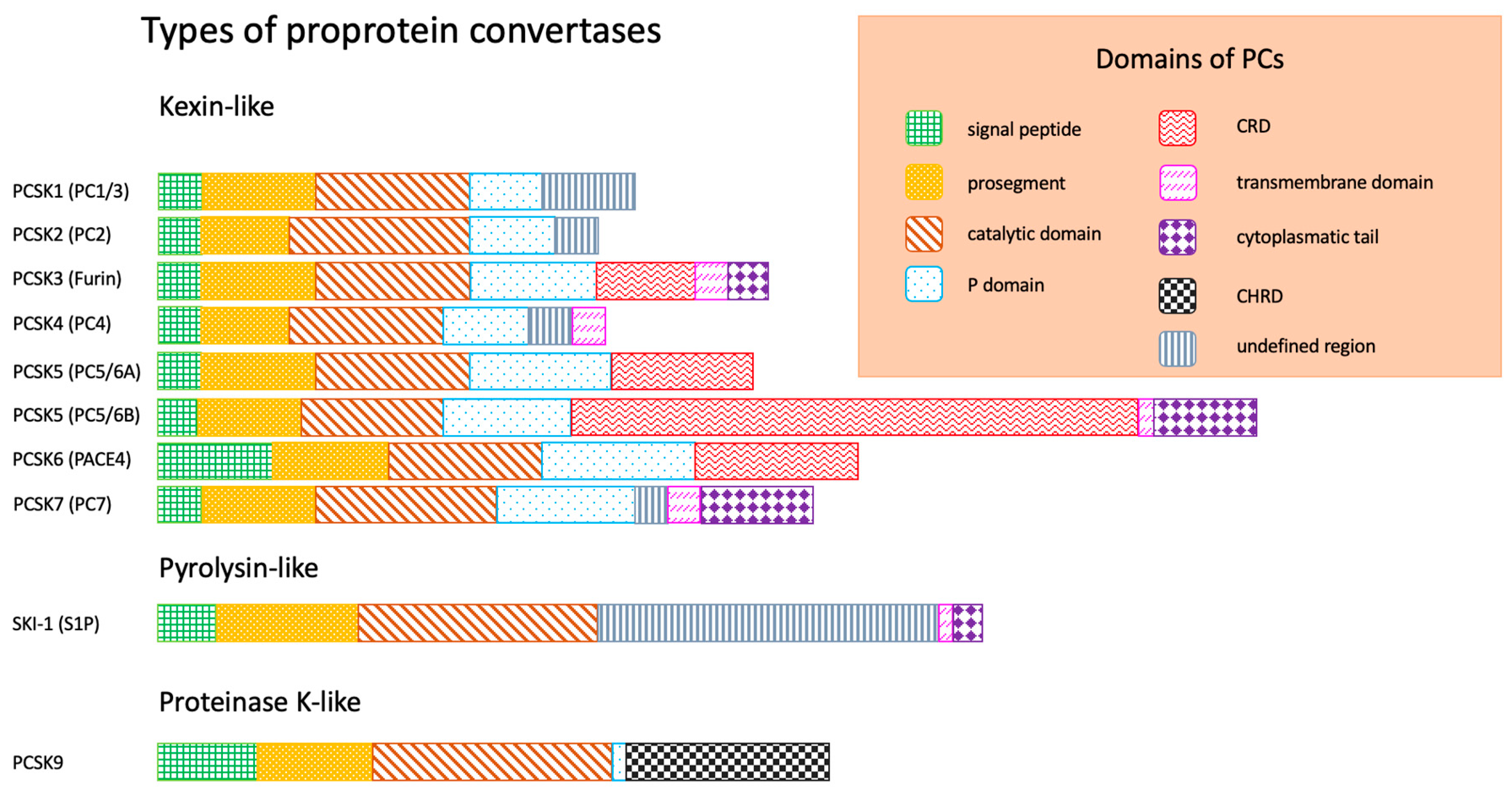
| Proprotein Convertase (PC) | Tissue Distribution | Function |
|---|---|---|
| PCSK1 (PC1/3) | Hypothalamus, pituitary, pancreas, thyroid gland, adrenal glands | Regulation of appetite; activation of insulin, glucagone, orexin, ghrelin |
| PCSK2 (PC2) | Central nervous system (CNS), pituitary, pancreas | Regulation of carbohydrate metabolism |
| PCSK3 (furin) | Ubiquitous | Regulation of embryogenesis, activation of growth factors and bacterial toxins (e.g., Clostridioides spp.), carcinogenesis |
| PCSK4 (PC4) | Germinal | Regulation of reproduction processes |
| PCSK5 (PC5/6) | Ubiquitous | Regulation of embryogenesis (e.g., CNS) |
| PCSK6 (PACE4) | ||
| PCSK7 (PC7) | Colon, spleen, liver | Regulation of lipid metabolism, atherogenesis |
| SKI-1 (S1P) | Ubiquitous | |
| PCSK9 | Liver, small intestine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maligłówka, M.; Kosowski, M.; Hachuła, M.; Cyrnek, M.; Bułdak, Ł.; Basiak, M.; Bołdys, A.; Machnik, G.; Bułdak, R.J.; Okopień, B. Insight into the Evolving Role of PCSK9. Metabolites 2022, 12, 256. https://doi.org/10.3390/metabo12030256
Maligłówka M, Kosowski M, Hachuła M, Cyrnek M, Bułdak Ł, Basiak M, Bołdys A, Machnik G, Bułdak RJ, Okopień B. Insight into the Evolving Role of PCSK9. Metabolites. 2022; 12(3):256. https://doi.org/10.3390/metabo12030256
Chicago/Turabian StyleMaligłówka, Mateusz, Michał Kosowski, Marcin Hachuła, Marcin Cyrnek, Łukasz Bułdak, Marcin Basiak, Aleksandra Bołdys, Grzegorz Machnik, Rafał Jakub Bułdak, and Bogusław Okopień. 2022. "Insight into the Evolving Role of PCSK9" Metabolites 12, no. 3: 256. https://doi.org/10.3390/metabo12030256
APA StyleMaligłówka, M., Kosowski, M., Hachuła, M., Cyrnek, M., Bułdak, Ł., Basiak, M., Bołdys, A., Machnik, G., Bułdak, R. J., & Okopień, B. (2022). Insight into the Evolving Role of PCSK9. Metabolites, 12(3), 256. https://doi.org/10.3390/metabo12030256






