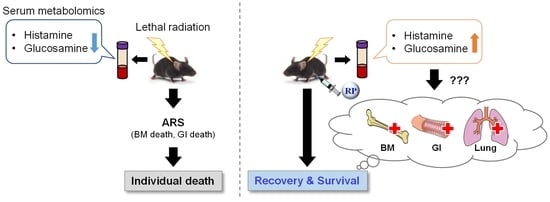
An Analysis of the Serum Metabolomic Profile for the Radiomitigative Effect of the Thrombopoietin Receptor Agonist Romiplostim in Lethally Whole-Body-Irradiated Mice
Abstract
:1. Introduction
2. Results
2.1. Effect of TBI on the Serum Metabolic Profile
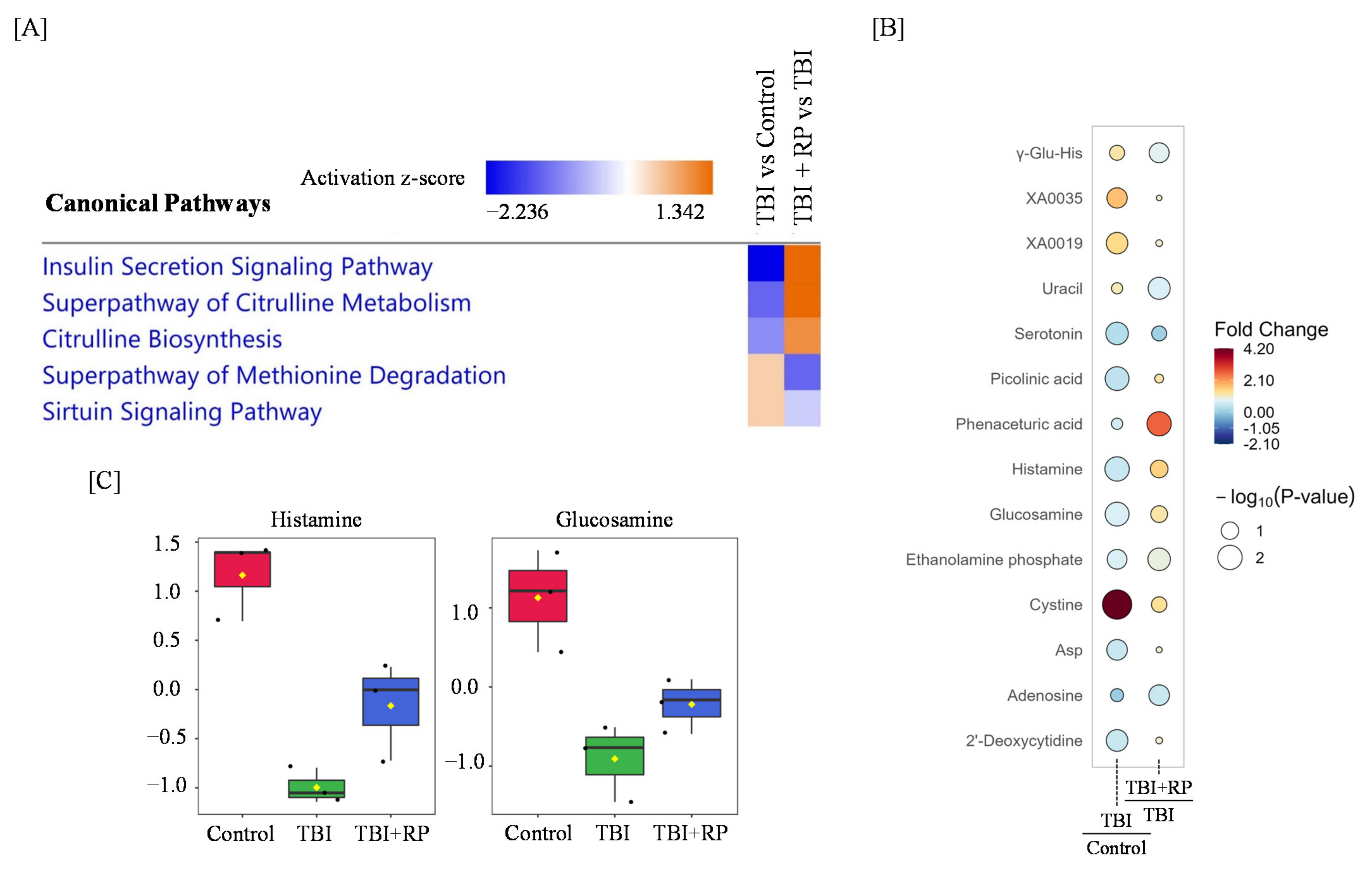
2.2. Effect of RP on the Serum Metabolic Profile
2.3. RP Attenuates Metabolic Changes in Response to TBI
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Exposure of Mice to a Lethal Dose of X-ray
4.3. Treatment with the Human Thrombopoietin-Mimetic c-mpl agonist RP
4.4. Sample Collection
4.5. Metabolome Analyses
4.6. Data Analyses Using Metaboanalyst and an IPA
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacVittie, T.J.; Bennett, A.W.; Farese, A.M.; Taylor-Howell, C.; Smith, C.P.; Gibbs, A.M.; Prado, K.; Jackson, W.I. The Effect of Radiation Dose and Variation in Neupogen® Initiation Schedule on the Mitigation of Myelosuppression during the Concomitant GI-ARS and H-ARS in a Nonhuman Primate Model of High-Dose Exposure with Marrow Sparing. Health Phys. 2015, 109, 427–439. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, A.L.; Maher, C.; Hick, J.L.; Hanfling, D.; Dainiak, N.; Chao, N.; Bader, J.L.; Coleman, C.N.; Weinstock, D.M. Radiation Injury after a Nuclear Detonation: Medical Consequences and the Need for Scarce Resources Allocation. Disaster Med. Public Health Prep. 2011, 5, S32–S44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.K.; Garcia, M.; Seed, T.M. A Review of Radiation Countermeasures Focusing on Injury-Specific Medicinals and Regulatory Approval Status: Part II. Countermeasures for Limited Indications, Internalized Radionuclides, Emesis, Late Effects, and Agents Demonstrating Efficacy in Large Animals with or without FDA IND Status. Int. J Radiat. Biol. 2017, 93, 870–884. [Google Scholar] [CrossRef]
- Singh, V.K.; Hanlon, B.K.; Santiago, P.T.; Seed, T.M. A Review of Radiation Countermeasures Focusing on Injury-Specific Medicinals and Regulatory Approval Status: Part III. Countermeasures under Early Stages of Development along with “standard of Care” Medicinal and Procedures Not Requiring Regulatory Approval for Use. Int. J. Radiat. Biol. 2017, 93, 885–906. [Google Scholar] [CrossRef]
- Kashiwakura, I.; Yamaguchi, M. Radioprotective/Mitigative Effects of Thrombopoietin Receptor Agonists. Radiat. Environ. Med. 2021, 10, 1–8. [Google Scholar]
- Kaushansky, K. Lineage-Specific Hematopoietic Growth Factors. N. Engl. J. Med. 2006, 354, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Kato, T. Thrombopoietin: Biology and Clinical Potentials. Int. J. Hematol. 1999, 70, 216–225. [Google Scholar] [PubMed]
- Vadhan-Raj, S. Clinical Experience with Recombinant Human Thrombopoietin in Chemotherapy-Induced Thrombocytopenia. Semin. Hematol. 2000, 37, 28–34. [Google Scholar] [CrossRef]
- Hitchcock, I.S.; Kaushansky, K. Thrombopoietin from Beginning to End. Br. J. Haematol. 2014, 165, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B. The New Thrombopoietic Agenda: Impact on Leukemias and MDS. Best Pract. Res. Clin. Haematol. 2014, 27, 288–292. [Google Scholar] [CrossRef]
- Cines, D.B.; Wasser, J.; Rodeghiero, F.; Chong, B.H.; Steurer, M.; Provan, D.; Lyons, R.; Garcia-Chavez, J.; Carpenter, N.; Wang, X.; et al. Safety and Efficacy of Romiplostim in Splenectomized and Nonsplenectomized Patients with Primary Immune Thrombocytopenia. Haematologica 2017, 102, 1342–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virk, Z.M.; Kuter, D.J.; Al-Samkari, H. An Evaluation of Avatrombopag for the Treatment of Thrombocytopenia. Expert Opin. Pharmacother. 2021, 22, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Hirouchi, T.; Yoshioka, H.; Watanabe, J.; Kashiwakura, I. Diverse Functions of the Thrombopoietin Receptor Agonist Romiplostim Rescue Individuals Exposed to Lethal Radiation. Free. Radic. Biol. Med. 2019, 136, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Chiba, A.; Kawabata, N.; Yamaguchi, M.; Tokonami, S.; Kashiwakura, I. Regulation of Antioxidant Stress-Responsive Transcription Factor Nrf2 Target Gene in the Reduction of Radiation Damage by the Thrombocytopenia Drug Romiplostim. Biol. Pharm. Bull. 2020, 43, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Emerging Applications of Metabolomics in Drug Discovery and Precision Medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Chen, H.H.; Tseng, Y.J.; Wang, S.Y.; Tsai, Y.S.; Chang, C.S.; Kuo, T.C.; Yao, W.J.; Shieh, C.C.; Wu, C.H.; Kuo, P.H. The Metabolome Profiling and Pathway Analysis in Metabolic Healthy and Abnormal Obesity. Int. J. Obes. 2015, 39, 1241–1248. [Google Scholar] [CrossRef]
- Leitner, M.; Fragner, L.; Danner, S.; Holeschofsky, N.; Leitner, K.; Tischler, S.; Doerfler, H.; Bachmann, G.; Sun, X.; Jaeger, W.; et al. Combined Metabolomic Analysis of Plasma and Urine Reveals AHBA, Tryptophan and Serotonin Metabolism as Potential Risk Factors in Gestational Diabetes Mellitus (GDM). Front. Mol. Biosci. 2017, 4, 84. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Ji, J.; Zhao, L.; Chen, M.; Shi, A.; Pan, L.; Huang, Y.; Zhang, H.; Dong, B.; Gao, H. Prediction and Diagnosis of Renal Cell Carcinoma Using Nuclear Magnetic Resonance-Based Serum Metabolomics and Self-Organizing Maps. Oncotarget 2016, 7, 59189–59198. [Google Scholar] [CrossRef] [Green Version]
- Pannkuk, E.L.; Fornace, A.J.; Laiakis, E.C. Metabolomic Applications in Radiation Biodosimetry: Exploring Radiation Effects through Small Molecules. Int. J. Radiat. Biol. 2017, 93, 1151–1176. [Google Scholar] [CrossRef]
- Jelonek, K.; Pietrowska, M.; Widlak, P. Systemic Effects of Ionizing Radiation at the Proteome and Metabolome Levels in the Blood of Cancer Patients Treated with Radiotherapy: The Influence of Inflammation and Radiation Toxicity. Int. J. Radiat. Biol. 2017, 93, 683–696. [Google Scholar] [CrossRef]
- Tokuhisa, H.; Koichi, I.; Manabu, N.; Satoru, M.; Hironori, Y.; Mitsuru, C.; Masaharu, H.; Akira, N.; Junya, I.; Masaru, Y.; et al. Mitigative Effects of a Combination of Multiple Pharmaceutical Drugs on the Survival of Mice Exposed to Lethal Ionizing Radiation. Curr. Pharm. Biotechnol. 2016, 17, 190–199. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hirouchi, T.; Yokoyama, K.; Nishiyama, A.; Murakami, S.; Kashiwakura, I. The Thrombopoietin Mimetic Romiplostim Leads to the Complete Rescue of Mice Exposed to Lethal Ionizing Radiation. Sci. Rep. 2018, 8, 10659. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, J.D.; Herrington, J.D. Treatment Options for Chronic Refractory Idiopathic Thrombocytopenic Purpura in Adults: Focus on Romiplostim and Eltrombopag. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010, 30, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Want, E.; Masson, P. Processing and Analysis of GC/LC-MS-Based Metabolomics Data. Methods Mol. Biol. 2011, 708, 277–298. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Laiakis, E.C.; Girgis, M.; Dowd, S.E.; Dhungana, S.; Nishita, D.; Bujold, K.; Bakke, J.; Gahagen, J.; Authier, S.; et al. Temporal Effects on Radiation Responses in Nonhuman Primates: Identification of Biofluid Small Molecule Signatures by Gas Chromatography–Mass Spectrometry Metabolomics. Metabolites 2019, 9, 98. [Google Scholar] [CrossRef] [Green Version]
- Broin, P.Ó.; Vaitheesvaran, B.; Saha, S.; Hartil, K.; Chen, E.I.; Goldman, D.; Fleming, W.H.; Kurland, I.J.; Guha, C.; Golden, A. Intestinal Microbiota-Derived Metabolomic Blood Plasma Markers for Prior Radiation Injury. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Kurland, I.J.; Broin, P.Ó.; Golden, A.; Su, G.; Meng, F.; Liu, L.; Mohney, R.; Kulkarni, S.; Guha, C. Integrative Metabolic Signatures for Hepatic Radiation Injury. PLoS ONE 2015, 10, e0124795. [Google Scholar] [CrossRef] [Green Version]
- Cheema, A.K.; Suman, S.; Kaur, P.; Singh, R.; Fornace, A.J.; Datta, K. Long-Term Differential Changes in Mouse Intestinal Metabolomics after γ and Heavy Ion Radiation Exposure. PLoS ONE 2014, 9, e87079. [Google Scholar] [CrossRef]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-Omics Analyses of Radiation Survivors Identify Radioprotective Microbes and Metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef]
- Medina, V.A.; Croci, M.; Carabajal, E.; Bergoc, R.M.; Rivera, E.S. Histamine Protects Bone Marrow against Cellular Damage Induced by Ionising Radiation. Int. J. Radiat. Biol. 2010, 86, 283–290. [Google Scholar] [CrossRef]
- Langendorff, H.; Melching, H.J.; Ladner, H.A. 5-Hydroxytryptamine as a Radiation Protective Substance in Animals. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1959, 1, 24–27. [Google Scholar] [CrossRef]
- Vasin, M.V.; Ushakov, I.B. Comparative Efficacy and the Window of Radioprotection for Adrenergic and Serotoninergic Agents and Aminothiols in Experiments with Small and Large Animals. J. Radiat. Res. 2015, 56, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyburski, J.B.; Patterson, A.D.; Krausz, K.W.; Slavík, J.; Fornace, A.J.; Gonzalez, F.J.; Idle, J.R. Radiation Metabolomics. 2. Dose- and Time-Dependent Urinary Excretion of Deaminated Purines and Pyrimidines after Sublethal Gamma-Radiation Exposure in Mice. Radiat. Res. 2009, 172, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Medina, V.A.; Croci, M.; Mohamad, N.A.; Massari, N.; Garbarino, G.; Cricco, G.P.; Núñez, M.A.; Martín, G.A.; Crescenti, E.J.V.; Bergoc, R.M.; et al. Mechanisms Underlying the Radioprotective Effect of Histamine on Small Intestine. Int. J. Radiat. Biol. 2007, 83, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Carabajal, E.; Massari, N.; Croci, M.; Martinel Lamas, D.J.; Prestifilippo, J.P.; Bergoc, R.M.; Rivera, E.S.; Medina, V.A. Radioprotective Potential of Histamine on Rat Small Intestine and Uterus. Eur. J. Histochem. 2012, 56, e48. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Ma, N.; Liang, Y.; Liu, J.; Zhang, P.; Han, Y.; Chen, W.; Du, L.; Qu, B. Glucosamine Protects against Radiation-Induced Lung Injury via Inhibition of Epithelial-Mesenchymal Transition. J. Cell. Mol. Med. 2020, 24, 11018–11023. [Google Scholar] [CrossRef] [PubMed]
- Patt, H.M.; Clark, J.W.; Vogel, H.H. Comparative Protective Effect of Cysteine against Fat Neutron and Gamma Irradiation in Mice. Proc. Soc. Exp. Biol. Med. 1953, 84, 189–193. [Google Scholar] [CrossRef]
- Mirkovic, N.; Voehringer, D.W.; Story, M.D.; McConkey, D.J.; McDonnell, T.J.; Meyn, R.E. Resistance to Radiation-Induced Apoptosis in Bcl-2-Expressing Cells Is Reversed by Depleting Cellular Thiols. Oncogene 1997, 15, 1461–1470. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y.; Bowen, B.P.; Nguyen, D.H.; Parsa, S.; Huang, Y.; Mao, J.H.; Northen, T.R. Low-Dose Ionizing Radiation-Induced Blood Plasma Metabolic Response in a Diverse Genetic Mouse Population. Radiat. Res. 2012, 178, 551–555. [Google Scholar] [CrossRef]
- Vlachodimitropoulou, E.; Chen, Y.L.; Garbowski, M.; Koonyosying, P.; Psaila, B.; Sola-Visner, M.; Cooper, N.; Hider, R.; Porter, J. Eltrombopag: A Powerful Chelator of Cellular or Extracellular Iron (III) Alone or Combined with a Second Chelator. Blood 2017, 130, 1923–1933. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Yoshida, Y.; Suda, M.; Minamino, T. DNA Damage Response and Metabolic Disease. Cell Metab. 2014, 20, 967–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nylander, V.; Ingerslev, L.R.; Andersen, E.; Fabre, O.; Garde, C.; Rasmussen, M.; Citirikkaya, K.; Bæk, J.; Christensen, G.L.; Aznar, M.; et al. Ionizing Radiation Potentiates High-Fat Diet-Induced Insulin Resistance and Reprograms Skeletal Muscle and Adipose Progenitor Cells. Diabetes 2016, 65, 3573–3584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windmueller, H.G. Glutamine Utilization by the Small Intestine. Adv. Enzymol. Relat. Areas Mol. Biol. 1982, 53, 201–237. [Google Scholar] [CrossRef] [PubMed]
- Onal, C.; Kotek, A.; Unal, B.; Arslan, G.; Yavuz, A.; Topkan, E.; Yavuz, M. Plasma Citrulline Levels Predict Intestinal Toxicity in Patients Treated with Pelvic Radiotherapy. Acta Oncol. 2011, 50, 1167–1174. [Google Scholar] [CrossRef]
- Lutgens, L.C.H.W.; Deutz, N.; Granzier-Peeters, M.; Beets-Tan, R.; De Ruysscher, D.; Gueulette, J.; Cleutjens, J.; Berger, M.; Wouters, B.; von Meyenfeldt, M.; et al. Plasma Citrulline Concentration: A Surrogate End Point for Radiation-Induced Mucosal Atrophy of the Small Bowel. A Feasibility Study in 23 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 275–285. [Google Scholar] [CrossRef]
- Jones, J.W.; Tudor, G.; Li, F.; Tong, Y.; Katz, B.; Farese, A.M.; MacVittie, T.J.; Booth, C.; Kane, M.A. Citrulline as a Biomarker in the Murine Total-Body Irradiation Model: Correlation of Circulating and Tissue Citrulline to Small Intestine Epithelial Histopathology. Health Phys. 2015, 109, 452–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.W.; Bennett, A.; Carter, C.L.; Tudor, G.; Hankey, K.G.; Farese, A.M.; Booth, C.; MacVittie, T.J.; Kane, M.A. Citrulline as a Biomarker in the Non-Human Primate Total- and Partial-Body Irradiation Models: Correlation of Circulating Citrulline to Acute and Prolonged Gastrointestinal Injury. Health Phys. 2015, 109, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Herbers, A.H.; Feuth, T.; Donnelly, J.P.; Blijlevens, N.M. Citrulline-Based Assessment Score: First Choice for Measuring and Monitoring Intestinal Failure after High-Dose Chemotherapy. Ann. Oncol. 2010, 21, 1706–1711. [Google Scholar] [CrossRef]
- Lutgens, L.C.H.W.; Blijlevens, N.M.A.; Deutz, N.E.P.; Donnelly, J.P.; Lambin, P.; de Pauw, B.E. Monitoring Myeloablative Therapy-Induced Small Bowel Toxicity by Serum Citrulline Concentration: A Comparison with Sugar Permeability Tests. Cancer 2005, 103, 191–199. [Google Scholar] [CrossRef]
- Derikx, J.P.M.; Blijlevens, N.M.A.; Donnelly, J.P.; Fujii, H.; Kanda, T.; van Bijnen, A.A.; Heineman, E.; Buurman, W.A. Loss of Enterocyte Mass Is Accompanied by Diminished Turnover of Enterocytes after Myeloablative Therapy in Haematopoietic Stem-Cell Transplant Recipients. Ann. Oncol. 2009, 20, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Simile, M.M.; Latte, G.; Feo, C.F.; Feo, F.; Calvisi, D.F.; Pascale, R.M. Alterations of Methionine Metabolism in Hepatocarcinogenesis: The Emergent Role of Glycine N-Methyltransferase in Liver Injury. Ann. Gastroenterol. 2018, 31, 552–560. [Google Scholar] [CrossRef] [PubMed]
- LoBianco, F.V.; Krager, K.J.; Carter, G.S.; Alam, S.; Yuan, Y.; Lavoie, E.G.; Dranoff, J.A.; Aykin-Burns, N. The Role of Sirtuin 3 in Radiation-Induced Long-Term Persistent Liver Injury. Antioxidants 2020, 9, E409. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Yamaguchi, M.; Tatara, Y.; Kashiwakura, I. Proteomic Changes by Radio-Mitigative Thrombopoietin Receptor Agonist Romiplostim in the Blood of Mice Exposed to Lethal Total-Body Irradiation. Int. J. Radiat. Biol. 2020, 96, 1125–1134. [Google Scholar] [CrossRef]
- Lok, S.; Kaushansky, K.; Holly, R.D.; Kuijper, J.L.; Lofton-Day, C.E.; Oort, P.J.; Grant, F.J.; Heipel, M.D.; Burkhead, S.K.; Kramer, J.M. Cloning and Expression of Murine Thrombopoietin CDNA and Stimulation of Platelet Production In Vivo. Nature 1994, 369, 565–568. [Google Scholar] [CrossRef]
- Mouthon, M.A.; Van der Meeren, A.; Vandamme, M.; Squiban, C.; Gaugler, M.H. Thrombopoietin Protects Mice from Mortality and Myelosuppression Following High-Dose Irradiation: Importance of Time Scheduling. Can. J. Physiol. Pharmacol. 2002, 80, 717–721. [Google Scholar] [CrossRef]
- Inra, C.N.; Zhou, B.O.; Acar, M.; Murphy, M.M.; Richardson, J.; Zhao, Z.; Morrison, S.J. A Perisinusoidal Niche for Extramedullary Haematopoiesis in the Spleen. Nature 2015, 527, 466–471. [Google Scholar] [CrossRef]
- Ohashi, Y.; Hirayama, A.; Ishikawa, T.; Nakamura, S.; Shimizu, K.; Ueno, Y.; Tomita, M.; Soga, T. Depiction of Metabolome Changes in Histidine-Starved Escherichia Coli by CE-TOFMS. Mol. Biosyst. 2008, 4, 135–147. [Google Scholar] [CrossRef]
- Ooga, T.; Sato, H.; Nagashima, A.; Sasaki, K.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomic Anatomy of an Animal Model Revealing Homeostatic Imbalances in Dyslipidaemia. Mol. Biosyst. 2011, 7, 1217–1223. [Google Scholar] [CrossRef]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary Electrophoresis Mass Spectrometry-Based Saliva Metabolomics Identified Oral, Breast and Pancreatic Cancer-Specific Profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]

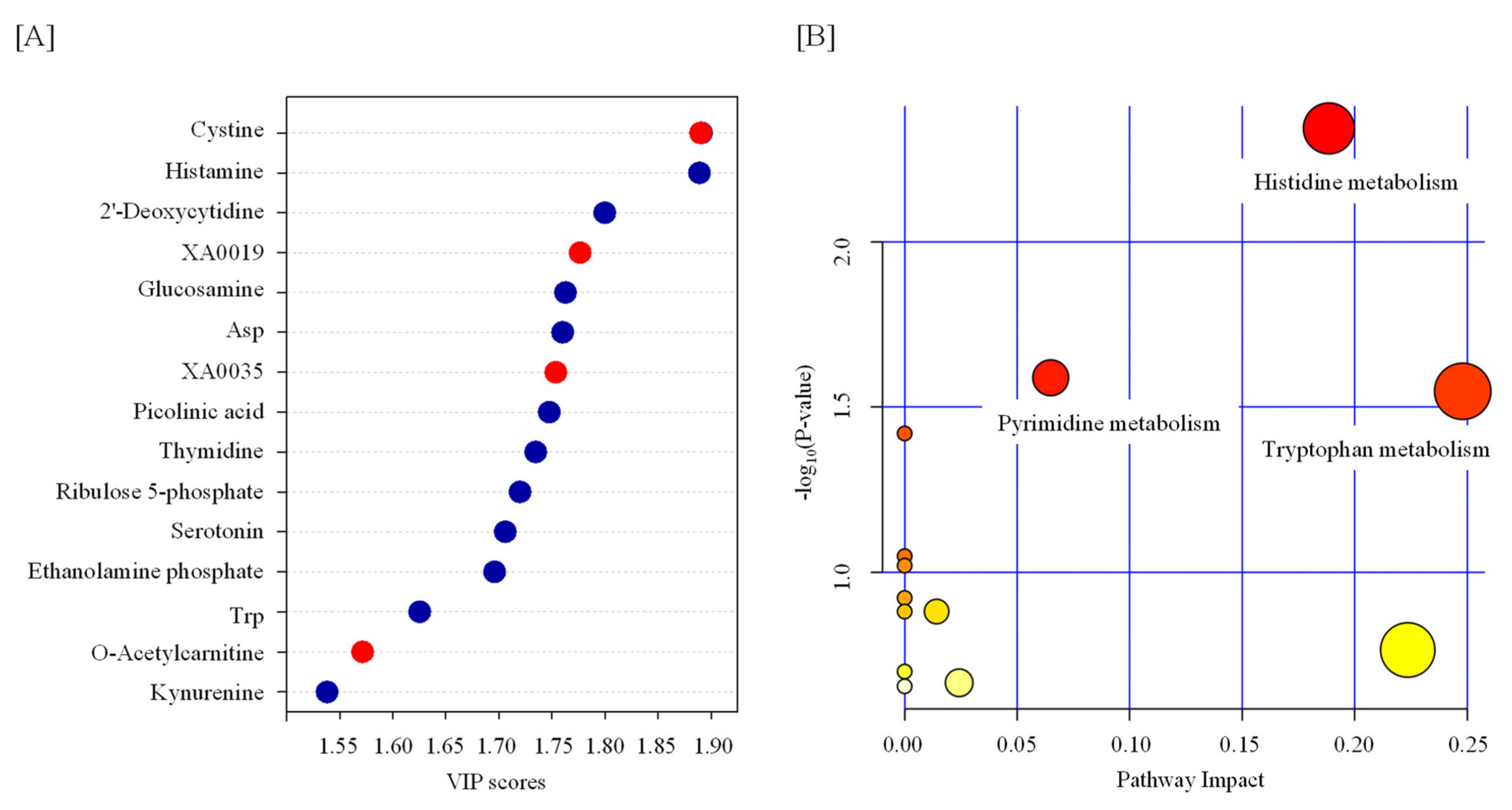

| Name | Fold Change | p Value |
|---|---|---|
| cystine | 4.2705 | 0.000991 |
| histamine | 0.4549 | 0.001072 |
| 2’-Deoxycytidine | 0.5 | 0.007763 |
| XA0019 | 1.3864 | 0.01047 |
| glucosamine | 0.68444 | 0.012282 |
| Asp | 0.5375 | 0.012634 |
| XA0035 | 1.7021 | 0.013546 |
| picolinic acid | 0.39647 | 0.014431 |
| thymidine | 0.50685 | 0.016358 |
| ribulose 5-phosphate | 0.23725 | 0.01874 |
| serotonin | 0.28095 | 0.021134 |
| ethanolamine phosphate | 0.6748 | 0.022919 |
| Trp | 0.70548 | 0.037526 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Yamaguchi, M.; Kashiwakura, I. An Analysis of the Serum Metabolomic Profile for the Radiomitigative Effect of the Thrombopoietin Receptor Agonist Romiplostim in Lethally Whole-Body-Irradiated Mice. Metabolites 2022, 12, 161. https://doi.org/10.3390/metabo12020161
Sato Y, Yamaguchi M, Kashiwakura I. An Analysis of the Serum Metabolomic Profile for the Radiomitigative Effect of the Thrombopoietin Receptor Agonist Romiplostim in Lethally Whole-Body-Irradiated Mice. Metabolites. 2022; 12(2):161. https://doi.org/10.3390/metabo12020161
Chicago/Turabian StyleSato, Yoshiaki, Masaru Yamaguchi, and Ikuo Kashiwakura. 2022. "An Analysis of the Serum Metabolomic Profile for the Radiomitigative Effect of the Thrombopoietin Receptor Agonist Romiplostim in Lethally Whole-Body-Irradiated Mice" Metabolites 12, no. 2: 161. https://doi.org/10.3390/metabo12020161
APA StyleSato, Y., Yamaguchi, M., & Kashiwakura, I. (2022). An Analysis of the Serum Metabolomic Profile for the Radiomitigative Effect of the Thrombopoietin Receptor Agonist Romiplostim in Lethally Whole-Body-Irradiated Mice. Metabolites, 12(2), 161. https://doi.org/10.3390/metabo12020161