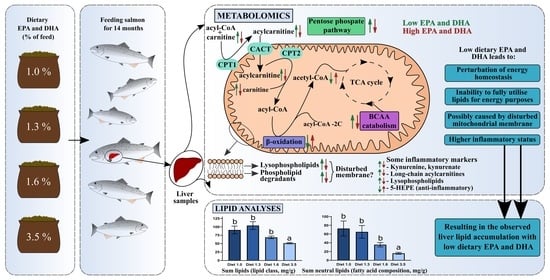
A Piece of the Puzzle—Possible Mechanisms for Why Low Dietary EPA and DHA Cause Hepatic Lipid Accumulation in Atlantic Salmon (Salmo salar)
Abstract
:1. Introduction
2. Results
2.1. Dietary Fatty Acids and Proximate Composition
2.2. Growth Is Higher and Hepatosomatic Index Is Lower with Higher Dietary EPA + DHA
2.3. Lipid Classes—Significantly More Fat in the Livers of Fish Given Diet 1.0 or 1.3 than Fish Given Diet 3.5
2.4. FA Composition in Neutral and Polar Lipids—Neutral Lipids Accumulate Oleic Acid When Dietary EPA + DHA Is Low
2.5. Metabolomics
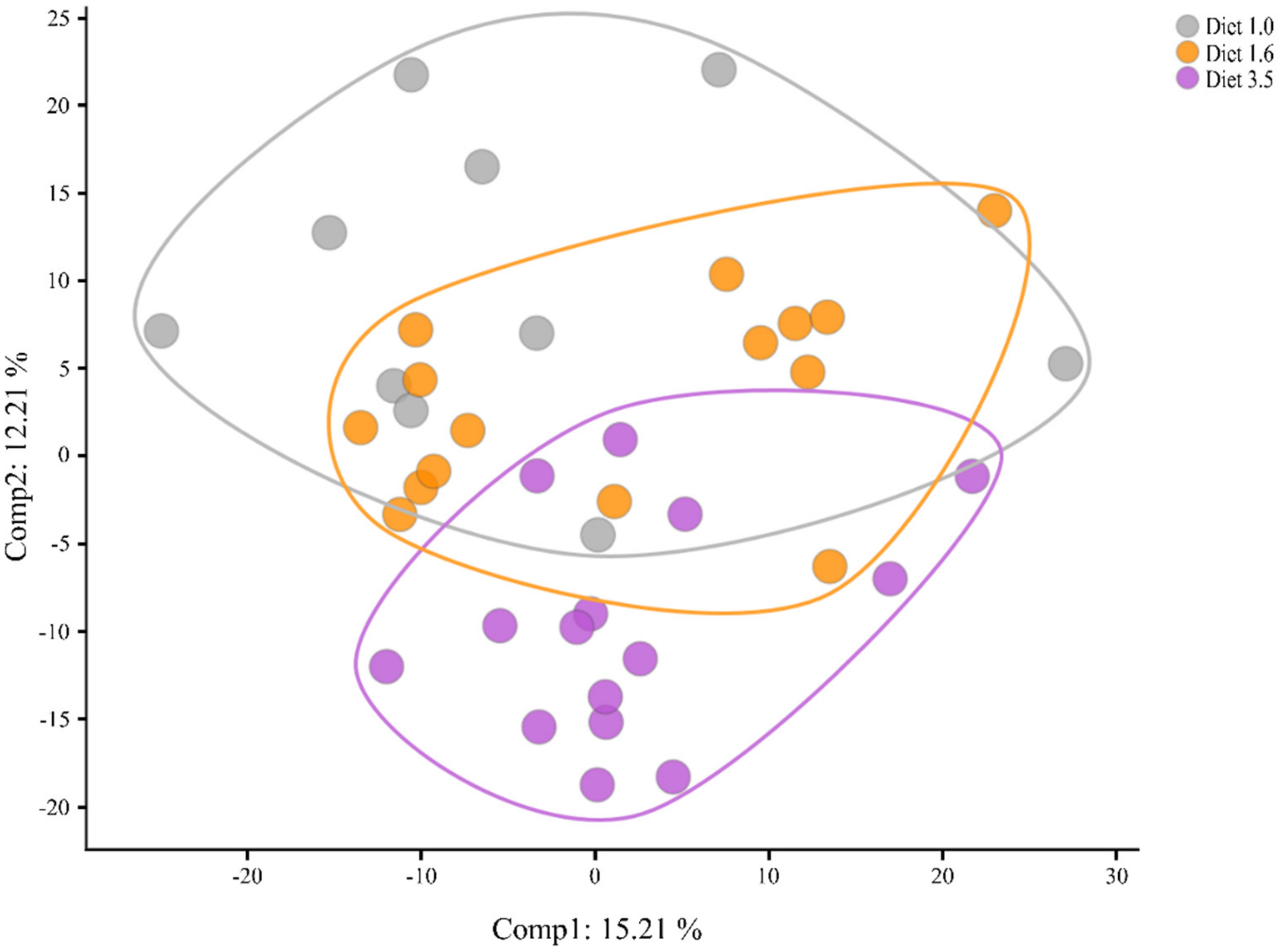
2.5.1. Differences between the Groups Based on Overall Metabolite Signature
2.5.2. Core Findings in the Metabolomics Data
2.5.3. The TCA Cycle, Pentose Phosphate Pathway, Creatine Metabolism and BCAA Metabolism Have Altered Activity When Decreasing the Dietary Content of EPA + DHA
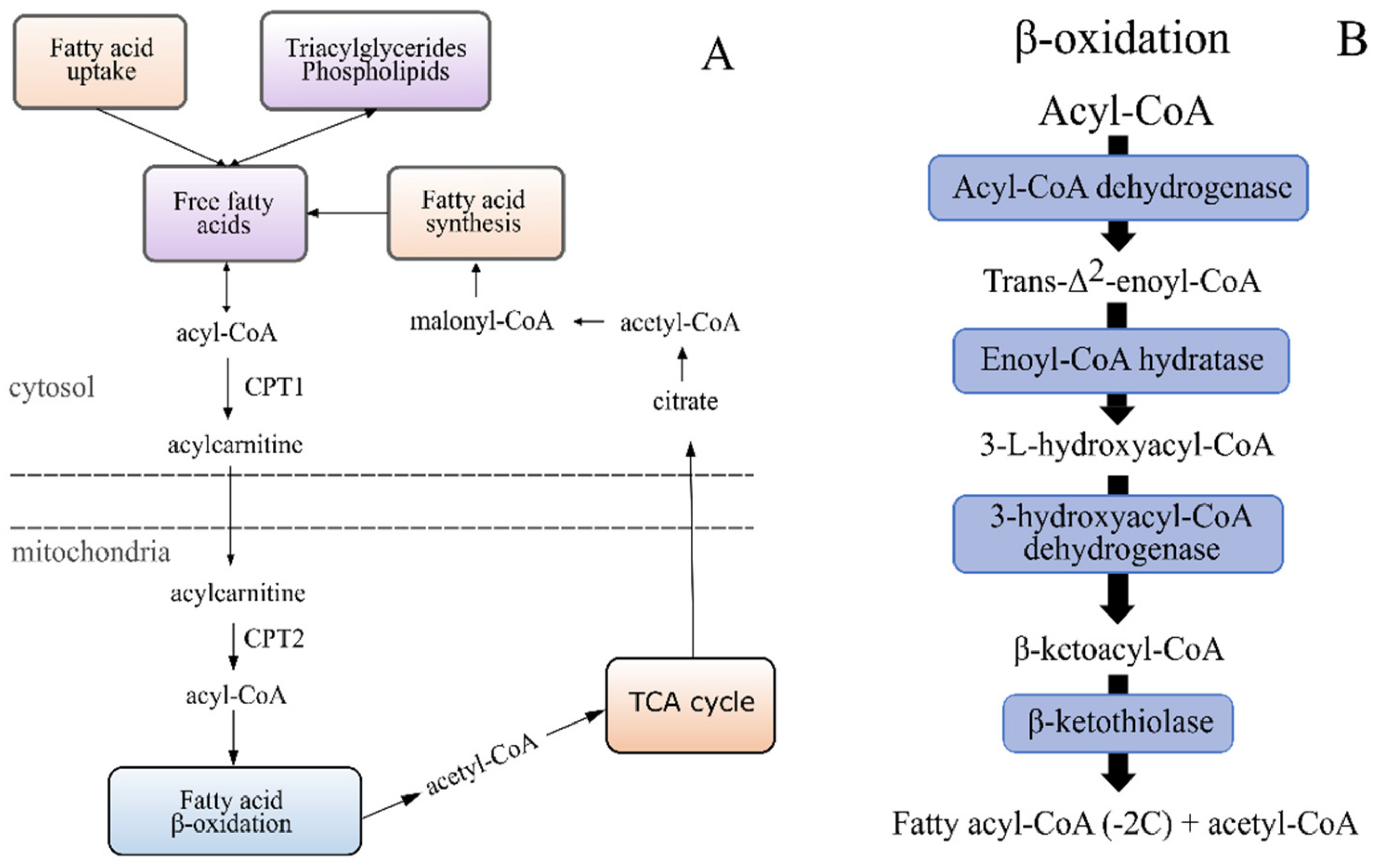
2.5.4. An increased Occurrence of Free Carnitine and Acyl Carnitines in the Lower EPA + DHA Dietary Groups Point to Issues with the Mitochondrial β-Oxidation Capacity
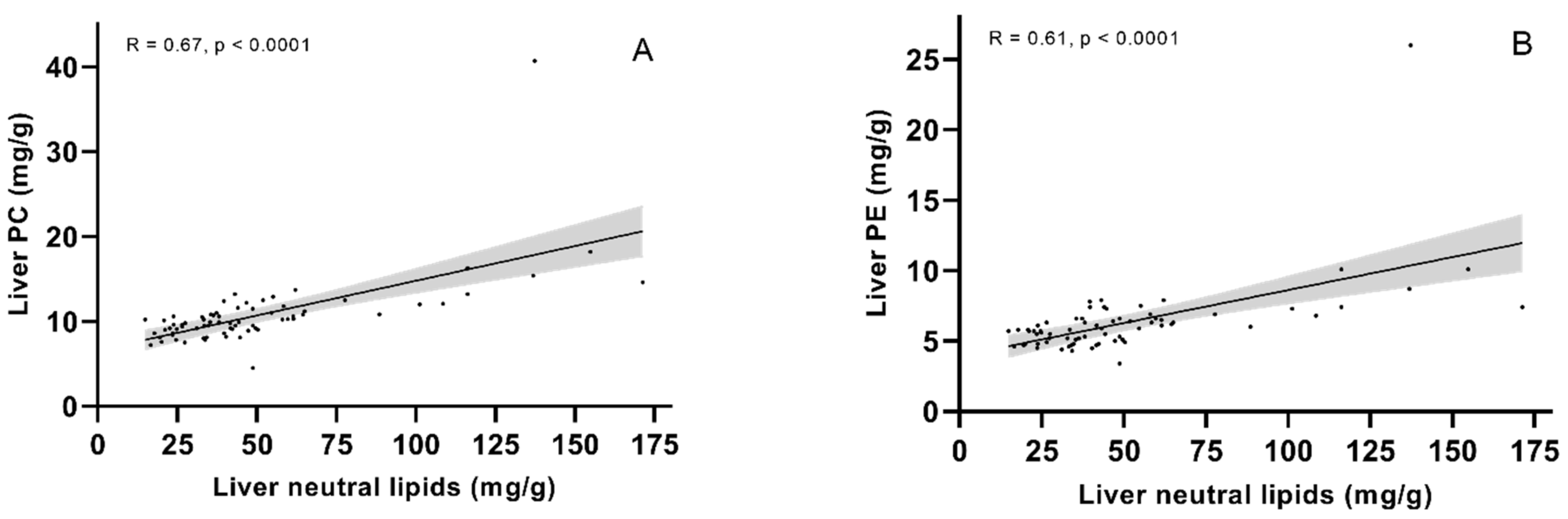
2.5.5. A Lower Dietary EPA + DHA Leads to Altered Phospholipid Metabolism, with Higher Levels of Phospholipid Degradants and Lysophospholipids
2.5.6. Alterations in Eicosanoid and Tryptophan Metabolites Levels When Changing Dietary EPA and DHA
2.5.7. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Diets and Fish Trial
4.2. Sampling
4.3. Lipid Analyses
4.3.1. Lipid Class
4.3.2. Fatty Acid Composition
4.4. Gene Expression
4.5. Metabolomics
4.6. Calculations
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Polar Lipids | Neutral Lipids | |||||||
|---|---|---|---|---|---|---|---|---|
| Diet 1.0 | Diet 1.3 | Diet 1.6 | Diet 3.5 | Diet 1.0 | Diet 1.3 | Diet 1.6 | Diet | |
| 14:0 | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.2 ± 0.0 b | 0.5 ± 0.4 | 0.5 ± 0.4 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| 16:0 | 2.4 ± 0.3 a | 2.3 ± 0.3 a | 2.3 ± 0.3 a | 2.7 ± 0.2 b | 2.3 ± 1.5 | 2.3 ± 1.9 | 1.3 ± 0.5 | 0.9 ± 0.3 |
| 18:0 | 1.3 ± 0.2 | 1.2 ± 0.2 | 1.3 ± 0.1 | 1.4 ± 0.1 | 2.1 ± 1.6 b | 1.8 ± 1.3 b | 0.9 ± 0.5 ab | 0.5 ± 0.2 a |
| 20:0 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.01 | 0.14 ± 0.14 b | 0.11 ± 0.07 b | 0.06 ± 0.03 b | 0.02 ± 0.00 a |
| Sum SFA | 3.9 ± 0.5 | 3.7 ± 0.4 | 3.8 ± 0.4 | 4.3 ± 0.3 | 5.3 ± 3.5 b | 4.9 ± 3.8 b | 2.7 ± 1.2 ab | 1.7 ± 0.7 a |
| 16:1n-9 | 0.07 ± 0.01 b | 0.06 ± 0.01 b | 0.06 ± 0.00 b | 0.06 ± 0.00 a | 0.3 ± 0.2 b | 0.3 ± 0.2 b | 0.2 ± 0.1 b | 0.1 ± 0.0 a |
| 16:1n-7 | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.2 ± 0.0 b | 0.9 ± 0.7 | 0.9 ± 0.8 | 0.5 ± 0.3 | 0.4 ± 0.2 |
| 18:1n-11 | 0.02 ± 0.00 a | 0.01 ± 0.00 a | 0.02 ± 0.00 a | 0.07 ± 0.02 b | 0.03 ± 0.02 b | 0.03 ± 0.01 b | 0.03 ± 0.01 b | 0.1 ± 0.01 a |
| 18:1n-9 | 4.5 ± 0.5 b | 4.0 ± 0.6 b | 3.7 ± 0.4 b | 2.7 ± 0.3 a | 39.1 ± 32.4 b | 34.5 ± 26.6 b | 18.4 ± 9.3 b | 6.5 ± 3.2 a |
| 18:1n-7 | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.5 ± 0.0 | 2.5 ± 2.0 b | 2.2 ± 1.6 b | 1.3 ± 0.6 ab | 0.7 ± 0.3 a |
| 20:1n-11 | <0.1 | <0.1 | <0.1 | <0.1 | 0.05 ± 0.04 | 0.04 ± 0.03 | 0.04 ± 0.02 | 0.06 ± 0.04 |
| 20:1n-9 | 0.5 ± 0.1 ab | 0.4 ± 0.1 a | 0.5 ± 0.1 ab | 0.6 ± 0.1 b | 4.0 ± 3.4 b | 3.5 ± 2.7 b | 2.0 ± 0.9 b | 0.9 ± 0.5 a |
| 20:1n-7 | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.02 ± 0.01 a | 0.03 ± 0.00 b | 0.10 ± 0.09 b | 0.09 ± 0.07 b | 0.05 ± 0.03 ab | 0.03 ± 0.02 a |
| 22:1n-11 | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.02 ± 0.00 b | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 |
| 22:1n-9 | <0.1 | <0.1 | <0.1 | <0.1 | 0.2 ± 0.2 b | 0.2 ± 0.1 b | 0.1 ± 0.1 ab | 0.1 ± 0.0 a |
| 24:1n-9 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.1 ± 0.1 b | 0.1 ± 0.1 b | 0.1 ± 0.0 b | 0.1 ± 0.0 a |
| Sum MUFA | 5.7 ± 0.5 b | 5.0 ± 0.7 ab | 4.8 ± 0.5 ab | 4.1 ± 0.4 a | 47.6 ± 39.2 b | 42.1 ± 32.4 b | 22.7 ± 11.3 b | 9.1 ± 4.4 a |
| 18:2n-6 | 2.3 ± 0.3 b | 2.2 ± 0.4 b | 1.9 ± 0.3 b | 1.1 ± 0.1 a | 11.0 ± 10.2 b | 9.4 ± 7.1 b | 5.0 ± 2.6 b | 1.8 ± 0.8 a |
| 18:3n-6 | <0.1 | <0.1 | <0.1 | <0.1 | 0.10 ± 0.07 c | 0.05 ± 0.03 bc | 0.02 ± 0.01 ab | 0.01 ± 0.0 a |
| 20:2n-6 | 0.5 ± 0.1 ab | 0.5 ± 0.1 ab | 0.5 ± 0.1 b | 0.4 ± 0.1 a | 1.7 ± 1.5 b | 1.6 ± 1.3 b | 0.9 ± 0.5 b | 0.4 ± 0.2 a |
| 20:3n-6 | 0.5 ± 0.1 c | 0.4 ± 0.1 b | 0.3 ± 0.1 b | 0.1 ± 0.0 a | 0.52 ± 0.46 c | 0.31 ± 0.22 bc | 0.09 ± 0.15 b | 0.04 ± 0.02 a |
| 20:4n-6 (ARA) | 0.7 ± 0.1 a | 0.6 ± 0.1 a | 0.7 ± 0.1 a | 0.8 ± 0.1 b | 0.5 ± 0.4 ab | 0.6 ± 0.5 b | 0.4 ± 0.2 ab | 0.2 ± 0.1 a |
| 22:5n-6 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.00 | <0.1 | <0.1 | <0.1 | <0.1 |
| Sum n-6 | 4.1 ± 0.3 b | 3.7 ± 0.5 b | 3.4 ± 0.4 b | 2.5 ± 0.1 a | 13.9 ± 12.6 b | 12.0 ± 9.2 b | 6.5 ± 3.3 b | 1.1 ± 2.4 a |
| 18:3n-3 | 0.6 ± 0.1 b | 0.5 ± 0.1 b | 0.5 ± 0.1 b | 0.3 ± 0.1 a | 3.4 ± 2.9 b | 3.3 ± 2.6 b | 1.9 ± 0.9 b | 0.7 ± 0.3 a |
| 18:4n-3 | 0.03 ± 0.0 b | 0.02 ± 0.0 ab | 0.02 ± 0.02 b | 0.01 ± 0.00 b | 0.16 ± 0.11 b | 0.11 ± 0.07 b | 0.06 ± 0.03 ab | 0.03 ± 0.01 a |
| 20:4n-3 | 0.3 ± 0.0 b | 0.2 ± 0.0 b | 0.2 ± 0.0 ab | 0.2 ± 0.0 a | 0.4 ± 0.4 | 0.4 ± 0.4 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| 20:5n-3 (EPA) | 1.9 ± 0.3 a | 2.0 ± 0.2 a | 2.1 ± 0.2 a | 2.6 ± 0.2 b | 0.5 ± 0.2 | 0.6 ± 0.4 | 0.4 ± 0.1 | 0.6 ± 0.2 |
| 21:5n-3 | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.03 ± 0.00 b | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.02 ± 0.01 | 0.03 ± 0.01 |
| 22:5n-3 | 0.6 ± 0.1 a | 0.6 ± 0.1 a | 0.6 ± 0.1 a | 0.8 ± 0.2 b | 0.1 ± 0.1 a | 0.1 ± 0.1 a | 0.1 ± 0.0 a | 0.3 ± 0.1 b |
| 22:6n-3 (DHA) | 4.1 ± 0.6 a | 4.4 ± 0.4 a | 4.8 ± 0.4 a | 5.9 ± 0.3 b | 0.4 ± 0.1 a | 0.5 ± 0.2 a | 0.4 ± 0.1 ab | 0.6 ± 0.2 b |
| 24:5n-3 | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.01 a | 0.04 ± 0.04 b | 0.03 ± 0.02 a | 0.04 ± 0.06 ab | 0.03 ± 0.02 a | 0.06 ± 0.02 b |
| 24:6n-3 | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | <0.1 | <0.1 | <0.1 | <0.1 |
| EPA + DHA | 6.1 ± 0.8 a | 6.3 ± 0.6 a | 6.9 ± 0.6 a | 8.4 ± 0.5 b | 0.9 ± 0.4 | 1.1 ± 0.6 | 0.9 ± 0.1 | 1.2 ± 0.3 |
| Sum n-3 | 7.7 ± 0.9 a | 7.7 ± 0.8 a | 8.2 ± 0.8 ab | 9.7 ± 0.4 b | 5.0 ± 3.7 | 5.1 ± 3.8 | 3.2 ± 1.2 | 2.5 ± 0.9 |
| Sum PUFA | 11.8 ± 1.1 | 11.5 ± 1.3 | 11.7 ± 1.2 | 12.3 ± 0.5 | 19.0 ± 16.3 b | 17.1 ± 13.0 b | 9.7 ± 4.6 ab | 4.9 ± 2.0 a |
| n6/n3 | 0.6 ± 0.1 c | 0.5 ± 0.0 bc | 0.4 ± 0.0 ab | 0.3 ± 0.0 a | 2.5 ± 0.6 c | 2.3 ± 0.2 bc | 1.9 ± 0.3 ab | 1.0 ± 0.2 a |
| n3/n6 | 1.8 ± 0.2 a | 2.1 ± 0.1 ab | 2.4 ± 0.2 b | 3.8 ± 0.2 c | 0.5 ± 0.2 a | 0.4 ± 0.0 a | 0.6 ± 0.2 ab | 1.1 ± 0.3 b |
| Sum fat | 21.6 ± 1.6 | 20.5 ± 2.4 | 20.8 ± 2.0 | 21.3 ± 1.0 | 72.6 ± 59.3 b | 65.0 ± 49.7 b | 35.6 ± 17.3 b | 16.1 ± 7.2 a |
| Polar Lipids | Neutral Lipids | |||||||
|---|---|---|---|---|---|---|---|---|
| Diet 1.0 | Diet 1.3 | Diet 1.6 | Diet 3.5 | Diet 1.0 | Diet 1.3 | Diet 1.6 | Diet 3.5 | |
| 14:0 | 0.4 ± 0.1 | 0.5 ± 0.0 | 0.6 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 a | 0.8 ± 0.1 a | 0.9 ± 0.1 a | 1.5 ± 0.2 b |
| 16:0 | 10.9 ± 0.9 a | 11.0 ± 0.6 a | 11.2 ± 0.7 a | 12.5 ± 1.0 b | 3.8 ± 1.2 a | 3.6 ± 0.4 a | 3.8 ± 0.7 a | 6.1 ± 1.1 b |
| 18:0 | 6.2 ± 0.7 | 6.1 ± 0.4 | 6.1 ± 0.4 | 6.3 ± 0.6 | 3.0 ± 0.6 | 2.8 ± 0.4 | 2.6 ± 0.6 | 2.9 ± 0.6 |
| 20:0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.0 b | 0.2 ± 0.0 b | 0.2 ± 0.0 b | 0.1 ± 0.0 a |
| Sum SFA | 18.1 ± 1.2 a | 18.1 ± 0.7 a | 18.5 ± 0.8 a | 20.5 ± 1.1 b | 8.0 ± 1.5 a | 7.6 ± 0.7 a | 7.7 ± 1.1 a | 11.1 ± 1.7 b |
| 16:1n-9 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.0 | 1.3 ± 0.3 a | 1.3 ± 0.2 a | 1.4 ± 0.1 a | 2.6 ± 0.2 b |
| 16:1n-7 | 0.5 ± 0.1 a | 0.5 ± 0.1 a | 0.5 ± 0.0 a | 0.8 ± 0.1 b | 1.3 ± 0.3 a | 1.3 ± 0.2 a | 1.4 ± 0.1 a | 2.6 ± 0.2 b |
| 18:1n-11 | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.4 ± 0.1 b | <0.1 | <0.1 | <0.1 | 0.1 ± 0.1 |
| 18:1n-9 | 20.9 ± 2.7 c | 19.4 ± 0.9 bc | 17.9 ± 1.0 ab | 12.4 ± 1.3 a | 52.9 ± 3.1 c | 52.9 ± 0.6 bc | 50.9 ± 2.6 b | 39.4 ± 2.8 a |
| 18:1n-7 | 2.0 ± 0.2 a | 2.0 ± 0.1 a | 2.0 ± 0.1 a | 2.3 ± 0.1 b | 3.4 ± 0.2 a | 3.5 ± 0.2 a | 3.6 ± 0.1 a | 4.2 ± 0.2 b |
| 20:1n-11 | <LOQ | <LOQ | <LOQ | <LOQ | <0.1 | <0.1 | <0.1 | <0.1 |
| 20:1n-9 | 2.2 ± 0.3 a | 2.1 ± 0.3 a | 2.3 ± 0.4 ab | 2.6 ± 0.6 b | 5.3 ± 0.7 | 5.4 ± 0.4 | 5.5 ± 0.4 | 5.4 ± 0.8 |
| 20:1n-7 | <0.1 | <0.1 | <0.1 | <0.1 | 0.1 ± 0.0 a | 0.1 ± 0.0 ab | 0.2 ± 0.1 ab | 0.2 ± 0.0 b |
| 22:1n-11 | <0.1 | <0.1 | <0.1 | <0.1 | 0.2 ± 0.1 a | 0.2 ± 0.0 a | 0.2 ± 0.1 a | 0.8 ± 0.3 b |
| 22:1n-9 | <0.1 | <0.1 | <0.1 | <0.1 | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| 24:1n-9 | <0.1 | <0.1 | <0.1 | <0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Sum MUFA | 26.3 ± 2.9 c | 24.6 ± 1.1 bc | 23.3 ± 1.0 ab | 19.3 ± 1.6 a | 64.4 ± 3.6 bc | 64.6 ± 0.5 b | 63.1 ± 2.8 ab | 55.1 ± 3.9 a |
| 18:2n-6 | 10.9 ± 1.1 c | 10.5 ± 0.7 bc | 9.2 ± 0.6 b | 5.3 ± 0.5 a | 14.4 ± 1.2 b | 14.4 ± 0.6 b | 13.9 ± 0.8 b | 10.9 ± 0.9 a |
| 18:3n-6 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| 20:2n-6 | 2.1 ± 0.3 ab | 2.3 ± 0.3 b | 2.3 ± 0.3 b | 1.8 ± 0.3 a | 2.3 ± 0.3 | 2.4 ± 0.3 | 2.5 ± 0.2 | 2.3 ± 0.2 |
| 20:3n-6 | 2.5 ± 0.4 d | 1.7 ± 0.3 c | 1.2 ± 0.2 b | 0.4 ± 0.1 a | 0.7 ± 0.2 c | 0.5 ± 0.1 b | 0.4 ± 0.1 b | 0.3 ± 0.1 a |
| 20:4n-6 (ARA) | 3.1 ± 0.4 a | 3.1 ± 0.2 a | 3.3 ± 0.2 ab | 3.9 ± 0.3 b | 0.8 ± 0.1 a | 0.9 ± 0.1 ab | 1.1 ± 0.2 b | 1.5 ± 0.2 c |
| 22:5n-6 | 0.3 ± 0.1 a | 0.4 ± 0.0 ab | 0.4 ± 0.0 b | 0.4 ± 0.0 b | <0.1 | <0.1 | <0.1 | <0.1 |
| Sum n-6 | 19.2 ± 0.9 d | 18.1 ± 0.7 c | 16.6 ± 0.8 b | 12.0 ± 0.4 a | 18.4 ± 1.4 b | 18.3 ± 0.8 b | 18.0 ± 0.8 b | 15.1 ± 0.9 a |
| 18:3n-3 | 2.6 ± 0.5 b | 2.6 ±0.3 b | 2.5 ± 0.3 b | 1.4 ± 0.4 a | 4.6 ± 0.4 ab | 5.0 ± 0.4 bc | 5.2 ± 0.3 c | 4.2 ± 0.6 a |
| 18:4n-3 | At LOQ | At LOQ | At LOQ | At LOQ | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.1 |
| 20:4n-3 | 1.3 ± 0.2 c | 1.2 ± 0.2 ab | 1.1 ± 0.1 b | 0.8 ± 0.1 a | 0.7 ± 0.3 a | 0.6 ± 0.1 a | 0.7 ± 0.1 a | 1.2 ± 0.1 b |
| 20:5n-3 (EPA) | 8.9 ± 0.8 a | 9.5 ± 0.6 ab | 10.1 ± 0.6 b | 12.0 ± 0.7 c | 1.0 ± 0.8 a | 1.0 ± 0.3 a | 1.5 ± 0.9 ab | 3.8 ± 0.8 b |
| 21:5n-3 | At LOQ | At LOQ | At LOQ | At LOQ | <0.1 | <0.1 | <0.1 | <0.1 |
| 22:5n-3 | 2.8 ± 0.3 a | 2.8 ± 0.2 a | 2.9 ± 0.2 a | 3.7 ± 0.7 b | 0.2 ± 0.3 a | 0.2 ± 0.0 a | 0.3 ± 0.2 a | 1.7 ± 0.4 b |
| 22:6n-3 (DHA) | 19.2 ± 2.0 a | 21.3 ± 1.1 ab | 23.0 ± 1.1 b | 27.5 ± 1.1 c | 1.1 ± 1.5 a | 1.0 ± 0.4 a | 1.6 ± 1.2 a | 4.6 ± 1.8 b |
| 24:5n-3 | 0.1 ± 0.0 a | 0.1 ± 0.0 ab | 0.1 ± 0.0 ab | 0.2 ± 0.0 b | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.4 ± 0.1 b |
| 24:6n-3 | 0.2 ± 0.0 b | 0.2 ± 0.0 b | 0.1 ± 0.1 ab | 0.1 ± 0.0 a | <0.1 | <0.1 | <0.1 | <0.1 |
| EPA + DHA | 28.1 ± 2.6 a | 30.8 ± 1.0 ab | 33.1 ± 1.2 bc | 39. 5 ± 1.6 c | 2.1 ± 2.3 a | 2.0 ± 0.7 a | 3.1 ± 2.1 a | 8.4 ± 2.5 b |
| Sum n-3 | 35.4 ± 2.3 a | 37.9 ± 1.0 ab | 39.9 ± 1.1 bc | 45.7 ± 0.9 c | 7.9 ± 2.9 a | 8.1 ± 0.6 ab | 9.7 ± 2.4 b | 16.4 ± 2.9 bc |
| Sum PUFA | 54.6 ± 1.7 a | 56.0 ± 0.7 ab | 56.6 ± 1.0 bc | 57.7 ± 0.9 c | 26.3 ± 2.8 a | 26.5 ± 2.4 a | 27.7 ± 2.4 ab | 31.5 ± 2.5 b |
| n6/n3 | 0.6 ± 0.1 c | 0.5 ± 0.0 bc | 0.4 ± 0.0 ab | 0.3 ± 0.0 a | 2.5 ± 0.6 c | 2.3 ± 0.2 bc | 1.9 ± 0.3 ab | 1.0 ± 0.2 a |
| n3/n6 | 1.8 ± 0.2 a | 2.1 ± 0.1 ab | 2.4 ± 0.2 b | 3.8 ± 0.2 c | 0.5 ± 0.2 a | 0.4 ± 0.0 a | 0.6 ± 0.2 ab | 1.1 ± 0.3 b * |
| Sum fat | 21.6 ±1.6 | 20.4 ± 2.4 | 20.8 ± 2.0 | 21.3 ± 1.0 | 72.6 ± 59.3 b | 65.0 ± 49.7 b | 35.6 ± 17.3 b | 16.1 ± 7.1 a |
| Pathway | Biochemical Name | Fold Change | ||
|---|---|---|---|---|
| Diet 1.0/Diet 3.5 | Diet 1.6/Diet 3.5 | Diet 1.0/Diet 1.6 | ||
| Fatty Acid Metabolism (Acyl Carnitine, Short Chain) | acetylcarnitine (C2) | 1.25 | 1.43 | 0.87 |
| isocaproylcarnitine | 0.53 | 0.83 | 0.64 | |
| Fatty Acid Metabolism (Acyl Carnitine, Medium Chain) | hexanoylcarnitine (C6) | 1.75 | 1.87 | 0.94 |
| cis-3,4-methyleneheptanoylcarnitine | 0.39 | 0.96 | 0.40 | |
| decanoylcarnitine (C10) | 1.65 | 1.52 | 1.09 | |
| laurylcarnitine (C12) | 1.73 | 1.52 | 1.14 | |
| Fatty Acid Metabolism (Acyl Carnitine, Long Chain Saturated) | palmitoylcarnitine (C16) | 0.88 | 1.42 | 0.62 |
| margaroylcarnitine (C17) | 0.64 | 1.17 | 0.55 | |
| stearoylcarnitine (C18) | 1.21 | 1.69 | 0.72 | |
| arachidoylcarnitine (C20) | 1.25 | 1.46 | 0.86 | |
| behenoylcarnitine (C22) | 1.47 | 1.39 | 1.06 | |
| lignoceroylcarnitine (C24) | 1.11 | 1.22 | 0.91 | |
| Acid Metabolism (Acyl Carnitine, Monounsaturated) | palmitoleoylcarnitine (C16:1) | 0.62 | 0.98 | 0.63 |
| oleoylcarnitine (C18:1) | 1.14 | 2.03 | 0.56 | |
| eicosenoylcarnitine (C20:1) | 1.18 | 1.89 | 0.62 | |
| erucoylcarnitine (C22:1) | 0.62 | 0.71 | 0.87 | |
| ximenoylcarnitine (C26:1) | 0.64 | 0.79 | 0.81 | |
| Fatty Acid Metabolism (Acyl Carnitine, Polyunsaturated) | linoleoylcarnitine (C18:2) | 1.12 | 1.75 | 0.64 |
| linolenoylcarnitine (C18:3) | 0.97 | 1.46 | 0.67 | |
| dihomo-linoleoylcarnitine (C20:2) | 1.19 | 1.99 | 0.60 | |
| arachidonoylcarnitine (C20:4) | 0.87 | 1.11 | 0.78 | |
| dihomo-linolenoylcarnitine (C20:3n3 or 6) | 2.48 | 2.56 | 0.97 | |
| docosadienoylcarnitine (C22:2) | 1.12 | 1.61 | 0.70 | |
| docosapentaenoylcarnitine (C22:5n3) | 0.64 | 1.01 | 0.63 | |
| docosahexaenoylcarnitine (C22:6) | 0.94 | 1.21 | 0.77 | |
| Fatty Acid Metabolism (Acyl Carnitine, Hydroxy) | (S)-3-hydroxybutyrylcarnitine | 1.16 | 1.37 | 0.84 |
| 3-hydroxyhexanoylcarnitine (1) | 1.36 | 1.29 | 1.05 | |
| 3-hydroxyoctanoylcarnitine (1) | 1.75 | 1.58 | 1.11 | |
| 3-hydroxyoctanoylcarnitine (2) | 1.54 | 1.47 | 1.05 | |
| 3-hydroxydecanoylcarnitine | 2.36 | 1.75 | 1.35 | |
| 3-hydroxypalmitoylcarnitine | 1.54 | 1.47 | 1.05 | |
| 3-hydroxyoleoylcarnitine | 1.58 | 1.88 | 0.84 | |
| Pathway | Biochemical Name | Fold Change | ||
|---|---|---|---|---|
| Diet 1.0/Diet 3.5 | Diet 1.6/Diet 3.5 | Diet 1.0/Diet 1.6 | ||
| Phospholipid metabolism | glycerophosphoethanolamine | 1.46 | 1.39 | 1.05 |
| glycerophosphoserine | 1.25 | 1.35 | 0.93 | |
| glycerophosphoinositol | 1.34 | 1.18 | 1.14 | |
| Lysophospholipid | 1-palmitoyl-GPC (16:0) | 1.06 | 0.96 | 1.10 |
| 2-palmitoyl-GPC (16:0) | 1.62 | 1.03 | 1.57 | |
| 1-palmitoleoyl-GPC (16:1) | 0.71 | 0.72 | 0.99 | |
| 2-palmitoleoyl-GPC (16:1) | 0.65 | 0.73 | 0.88 | |
| 1-stearoyl-GPC (18:0) | 1.41 | 1.31 | 1.08 | |
| 1-oleoyl-GPC (18:1) | 1.42 | 1.38 | 1.03 | |
| 1-linoleoyl-GPC (18:2) | 2.09 | 1.80 | 1.16 | |
| 1-linolenoyl-GPC (18:3) | 2.01 | 1.91 | 1.05 | |
| 1-dihomo-linolenoyl-GPC (20:3n3 or 6) | 3.30 | 1.98 | 1.67 | |
| 2-dihomo-linolenoyl-GPC (20:3n3 or 6) | 4.62 | 2.85 | 1.62 | |
| 1-omega-arachidonoyl-GPC (20:4n3) | 1.42 | 1.21 | 1.17 | |
| 1-arachidonoyl-GPC (20:4n6) | 0.81 | 0.72 | 1.13 | |
| 1-eicosapentaenoyl-GPC (20:5) | 0.70 | 0.76 | 0.93 | |
| 1-lignoceroyl-GPC (24:0) | 1.81 | 1.77 | 1.02 | |
| 1-palmitoyl-GPE (16:0) | 0.88 | 0.87 | 1.01 | |
| 1-stearoyl-GPE (18:0) | 1.18 | 1.17 | 1.01 | |
| 2-stearoyl-GPE (18:0) | 1.28 | 0.98 | 1.31 | |
| 1-oleoyl-GPE (18:1) | 1.19 | 1.17 | 1.02 | |
| 1-linoleoyl-GPE (18:2) | 1.43 | 1.26 | 1.13 | |
| 2-dihomo-linolenoyl-GPE (20:3n3 or 6) | 4.20 | 2.34 | 1.79 | |
| 1-dihomo-linolenoyl-GPE (20:3n3 or 6) | 2.36 | 1.67 | 1.41 | |
| 1-omega-arachidonoyl-GPE (20:4n3) | 1.24 | 1.06 | 1.17 | |
| 1-arachidonoyl-GPE (20:4n6) | 1.07 | 0.86 | 1.25 | |
| 1-eicosapentaenoyl-GPE (20:5) | 1.27 | 1.10 | 1.16 | |
| 1-palmitoyl-GPS (16:0) | 0.96 | 1.04 | 0.92 | |
| 1-stearoyl-GPS (18:0) | 1.63 | 2.06 | 0.79 | |
| 1-oleoyl-GPS (18:1) | 1.20 | 1.38 | 0.86 | |
| 1-palmitoyl-GPG (16:0) | 1.08 | 0.65 | 1.66 | |
| 1-palmitoyl-GPI (16:0) | 1.27 | 0.90 | 1.41 | |
| 1-stearoyl-GPI (18:0) | 1.42 | 1.05 | 1.35 | |
| 1-oleoyl-GPI (18:1) | 2.01 | 1.26 | 1.59 | |
| 1-linoleoyl-GPI (18:2) | 2.56 | 1.60 | 1.60 | |
| 1-arachidonoyl-GPI (20:4) | 1.00 | 0.81 | 1.24 | |
References
- Ruyter, B.; Sissener, N.H.; Ostbye, T.K.; Simon, C.J.; Krasnov, A.; Bou, M.; Sanden, M.; Nichols, P.D.; Lutfi, E.; Berge, G.M. n-3 Canola oil effectively replaces Fish oil as a new safe dietary source of DHA in feed for juvenile Atlantic salmon. Br. J. Nutr. 2019, 122, 1329–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bou, M.; Berge, G.E.; Baeverfjord, G.; Sigholt, T.; Østbye, T.-K.; Ruyter, B. Low levels of very-long-chain n-3 PUFA in Atlantic salmon (Salmo salar) diet reduce Fish robustness under challenging conditions in sea cages. J. Nutr. Sci. 2017, 6, e32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanden, M.; Liland, N.S.; Saele, O.; Rosenlund, G.; Du, S.; Torstensen, B.E.; Stubhaug, I.; Ruyter, B.; Sissener, N.H. Minor lipid metabolic perturbations in the liver of Atlantic salmon (Salmo salar L.) caused by suboptimal dietary content of nutrients from Fish oil. Fish Physiol. Biochem. 2016, 42, 1463–1480. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R.; Bell, J.G.; Dick, J.R.; Crampton, V.O. Effects of dietary vegetable oil on Atlantic salmon hepatocyte fatty acid desaturation and liver fatty acid compositions. Lipids 2003, 38, 723–732. [Google Scholar] [CrossRef]
- Sissener, N.H.; Torstensen, B.E.; Owen, M.A.G.; Liland, N.S.; Stubhaug, I.; Rosenlund, G. Temperature modulates liver lipid accumulation in Atlantic salmon (Salmo salar L.) fed low dietary levels of long-chain n-3 fatty acids. Aquac. Nutr. 2017, 23, 865–878. [Google Scholar] [CrossRef]
- Alvheim, A.R.; Torstensen, B.E.; Lin, Y.H.; Lillefosse, H.H.; Lock, E.-J.; Mdsen, L.; Hibbeln, J.R.; Malde, M.K. Dietary linoleic acid elevates endogenous 2-arachidonoylglycerol and anandamide in Atlantic salmon (Salmo salar L.) and mice, and induces weight gain and inflammation in mice. Br. J. Nutr. 2013, 109, 1508–1517. [Google Scholar] [CrossRef] [Green Version]
- Ruyter, B.; Moya-Falcón, C.; Rosenlund, G.; Vegusdal, A. Fat content and morphology of liver and intestine of Atlantic salmon (Salmo salar): Effects of temperature and dietary soybean oil. Aquaculture 2006, 252, 441–452. [Google Scholar] [CrossRef]
- Liland, N.S.; Espe, M.; Rosenlund, G.; Waagbø, R.; Hjelle, J.I.; Lie, Ø.; Fontanillas, R.; Torstensen, B.E. High levels of dietary phytosterols affect lipid metabolism and increase liver and plasma TAG in Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2013, 110, 1958–1967. [Google Scholar] [CrossRef] [Green Version]
- Torstensen, B.E.; Espe, M.; Stubhaug, I.; Lie, O. Dietary plant proteins and vegetable oil blends increase adiposity and plasma lipids in Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2011, 106, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Bransden, M.P.; Carter, C.G.; Nichols, P.D. Replacement of Fish oil with sunflower oil in feeds for Atlantic salmon (Salmo salar L.): Effect on growth performance, tissue fatty acid composition and disease resistance. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 611–625. [Google Scholar] [CrossRef]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Bang, K.B.; Cho, Y.K. Comorbidities and Metabolic Derangement of NAFLD. J. Lifestyle Med. 2015, 5, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Dessen, J.E.; Østbye, T.K.; Ruyter, B.; Bou, M.; Thomassen, M.S.; Rørvik, K.A. Sudden increased mortality in large seemingly healthy farmed Atlantic salmon (Salmo salar L.) was associated with environmental and dietary changes. J. Appl. Aquac. 2021, 33, 165–182. [Google Scholar] [CrossRef] [Green Version]
- Mørkøre, T.; Åsli, M.; Dessen, J.E.; Sanden, K.W.; Bjerke, M.; Hoås, K.G.; Rørvik, K.A. Tekstur Og Fett I Laksefilet. Nofima Rep. 38/2012. 2013, 1, 1–71. Available online: https://nofima.com/bc_tax_pubtype/report/ (accessed on 16 January 2022).
- De Castro, G.S.; Calder, P.C. Non-alcoholic fatty liver disease and its treatment with n-3 polyunsaturated fatty acids. Clin. Nutr. 2018, 37, 37–55. [Google Scholar] [CrossRef] [Green Version]
- Suzuki-Kemuriyama, N.; Matsuzaka, T.; Kuba, M.; Ohno, H.; Han, S.I.; Takeuchi, Y.; Isaka, M.; Kobayashi, K.; Iwasaki, H.; Yatoh, S.; et al. Different Effects of Eicosapentaenoic and Docosahexaenoic Acids on Atherogenic High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice. PLoS ONE 2016, 11, e0157580. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Zahradka, P.; Cordero-Monroy, L.; Wright, B.; Taylor, C.G. Dietary Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) Operate by Different Mechanisms to Modulate Hepatic Steatosis and Hyperinsulemia in fa/fa Zucker Rats. Nutrients 2019, 11, 917. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Hall, J.R.; Caballero-Solares, A.; Eslamloo, K.; Taylor, R.G.; Parrish, C.C.; Rise, M.L. Liver Transcriptome Profiling Reveals That Dietary DHA and EPA Levels Influence Suites of Genes Involved in Metabolism, Redox Homeostasis, and Immune Function in Atlantic Salmon (Salmo salar). Mar. Biotechnol. 2020, 22, 263–284. [Google Scholar] [CrossRef]
- Katan, T.; Caballero-Solares, A.; Taylor, R.G.; Rise, M.L.; Parrish, C.C. Effect of plant-based diets with varying ratios of ω6 to ω3 fatty acids on growth performance, tissue composition, fatty acid biosynthesis and lipid-related gene expression in Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 30, 290–304. [Google Scholar] [CrossRef]
- Stubhaug, I.; Lie, Ø.; Torstensen, B.E. Fatty acid productive value and b-oxidation capacity in Atlantic salmon (Salmo salar L.) fed on different lipid sources along the whole growth period. Aquacult. Nutr. 2007, 13, 145–155. [Google Scholar] [CrossRef]
- Stubhaug, I.; Lie, O.; Torstensen, B.E. β-Oxidation capacity in liver increases during parr-smolt transformation of Atlantic salmon fed vegetable oil and Fish oil. J. Fish Biol. 2006, 69, 504–517. [Google Scholar] [CrossRef]
- Jordal, A.E.; Torstensen, B.E.; Tsoi, S.; Tocher, D.R.; Lall, S.P.; Douglas, S.E. Dietary rapeseed oil affects the expression of genes involved in hepatic lipid metabolism in Atlantic salmon (Salmo salar L.). J. Nutr. 2005, 135, 2355–2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vegusdal, A.; Gjoen, T.; Berge, R.K.; Thomassen, M.S.; Ruyter, B. Effect of 18:1n-9, 20:5n-3, and 22:6n-3 on lipid accumulation and secretion by Atlantic salmon hepatocytes. Lipids 2005, 40, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Kjær, M.A.; Vegusdal, A.; Gjøen, T.; Rustan, A.C.; Todorčević, M.; Ruyter, B. Effect of rapeseed oil and dietary n-3 fatty acids on triacylglycerol synthesis and secretion in Atlantic salmon hepatocytes. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2008, 1781, 112–122. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Berge, G.M.; Mørkøre, T.; Krasnov, A.; Baeverfjord, G.; Ytrestøyl, T.; Carlehög, M.; Sweetman, J.; Ruyter, B. Microalgal Schizochytrium limacinum Biomass Improves Growth and Filet Quality When Used Long-Term as a Replacement for Fish Oil, in Modern Salmon Diets. Front. Mar. Sci. 2020, 7, 57. [Google Scholar] [CrossRef]
- Kjær, M.A.; Ruyter, B.; Berge, G.M.; Sun, Y.; Ostbye, T.K. Regulation of the Omega-3 Fatty Acid Biosynthetic Pathway in Atlantic Salmon Hepatocytes. PLoS ONE 2016, 11, e0168230. [Google Scholar] [CrossRef]
- Frøyland, L.; Madsen, L.; Eckhoff, K.; Lie, Ø.; Berge, R.K. Carnitine palmitoyltransferase I, carnitine palmitoyltransferase II, and acy-CoA oxidase activities in Atlantic salmon (Salmon salar). Lipids 1998, 33, 923–930. [Google Scholar] [CrossRef]
- Stubhaug, I.; Froyland, L.; Torstensen, B.E. Beta-Oxidation capacity of red and white muscle and liver in Atlantic salmon (Salmo salar L.)-effects of increasing dietary rapeseed oil and olive oil to replace capelin oil. Lipids 2005, 40, 39–47. [Google Scholar] [CrossRef]
- Jordal, A.-E.O.; Lie, Ø.; Torstensen, B.E. Complete replacement of dietary Fish oil with a vegetable oil blend affect liver lipid and plasma lipoprotein levels in Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2007, 13, 114–130. [Google Scholar] [CrossRef]
- Roques, S.; Deborde, C.; Richard, N.; Skiba-Cassy, S.; Moing, A.; Fauconneau, B. Metabolomics and Fish nutrition: A review in the context of sustainable feed development. Rev. Aquac. 2020, 12, 261–282. [Google Scholar] [CrossRef] [Green Version]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Sissener, N.H.; Waagbø, R.; Rosenlund, G.; Tvenning, L.; Susort, S.; Lea, T.B.; Oaland, Ø.; Chen, L.; Breck, O. Reduced n-3 long chain fatty acid levels in feed for Atlantic salmon (Salmo salar L.) do not reduce growth, robustness or product quality through an entire full scale commercial production cycle in seawater. Aquaculture 2016, 464, 236–245. [Google Scholar] [CrossRef]
- Aas, T.S.; Ytrestøyl, T.; Åsgård, T. Utilization of feed resources in the production of Atlantic salmon (Salmo salar) in Norway: An update for 2016. Aquac. Rep. 2019, 15, 100216. [Google Scholar] [CrossRef]
- Lutfi, E.; Berge, G.; Baeverfjord, G.; Sigholt, T.; Bou, M.; Larsson, T.; Mørkøre, T.; Evensen, Ø.; Sissener, N.H.; Rosenlund, G.; et al. Increasing dietary levels of the omega-3 long-chain polyunsaturated fatty acids, EPA and DHA, improves the growth, welfare, robustness, and fillet quality of Atlantic salmon in sea cages. Br. J. Nutr. 2022; accepted. [Google Scholar]
- Rudy, M.D.; Kainz, M.J.; Graeve, M.; Colombo, S.M.; Arts, M.T. Handling and Storage Procedures Have Variable Effects on Fatty Acid Content in Fish es with Different Lipid Quantities. PLoS ONE 2016, 11, e0160497. [Google Scholar] [CrossRef] [Green Version]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.G.; Reily, M.D.; Lehman-Mckeeman, L.D.; Vaillancourt, R.R.; Cherrington, N.J. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids 2015, 47, 603–615. [Google Scholar] [CrossRef]
- Sissener, N.H.; Torstensen, B.E.; Ruyter, B.; Østbye, T.-K.; Waagbø, R.; Jørgensen, S.M.; Hatlen, B.; Liland, N.S.; Ytteborg, E.; Sæle, Ø.; et al. Oppdatering av Utredningen: Effekter av Endret Fettsyresammensetning i fôr Til Laks Relatert Til Fiskens Helse, Velferd og Robusthet. “Fett for Fiskehelse 2016”; NIFES, Nofima: Bergen, Norway, 2016. [Google Scholar]
- Sissener, N.H.; Liland, N.S.; Holen, E.; Stubhaug, I.; Torstensen, B.E.; Rosenlund, G. Phytosterols are not involved in the development of fatty liver in plant oil fed Atlantic salmon (Salmo salar) at high or low water temperature. Aquaculture 2017, 480, 123–134. [Google Scholar] [CrossRef]
- Mørkøre, T.; Moreno, H.M.; Borderias, J.; Larsson, T.; Hellberg, H.; Hatlen, B.; Romarheim, O.H.; Ruyter, B.; Lazado, C.C.; Jimenez-Guerrero, R.; et al. Dietary inclusion of Antarctic krill meal during the finishing feed period improves health and fillet quality of Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2020, 124, 418–431. [Google Scholar] [CrossRef] [Green Version]
- Tacon, A.G.J. Nutritional Fish Pathology. Morphological Signs of Nutrient Deficiency and Toxicity in Farmed Fish. In FAO Fish Technical Paper No. 330; FAO: Rome, Italy, 1992. [Google Scholar]
- Caballero, M.J.; Obach, A.; Rosenlund, G.; Montero, D.; Gisvold, M.; Izquierdo, M. Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture 2002, 214, 57–62. [Google Scholar] [CrossRef]
- Espe, M.; Xie, S.; Chen, S.; Pedro, A.; Holen, E. Development of a fatty liver model using oleic acid in primary liver cells isolated from Atlantic salmon and the prevention of lipid accumulation using metformin. Aquacult. Nutr. 2019, 25, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Eynaudi, A.; Díaz-Castro, F.; Bórquez, J.C.; Bravo-Sagua, R.; Parra, V.; Troncoso, R. Differential Effects of Oleic and Palmitic Acids on Lipid Droplet-Mitochondria Interaction in the Hepatic Cell Line HepG2. Front. Nutr. 2021, 8, 775382. [Google Scholar] [CrossRef] [PubMed]
- Hollie, N.I.; Cash, J.G.; Matlib, M.A.; Wortman, M.; Basford, J.E.; Abplanalp, W.; Hui, D.Y. Micromolar changes in lysophosphatidylcholine concentration cause minor effects on mitochondrial permeability but major alterations in function. Biochim. Biophys. Acta 2014, 1841, 888–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labonte, E.D.; Pfluger, P.T.; Cash, J.G.; Kuhel, D.G.; Roja, J.C.; Magness, D.P.; Jandacek, R.J.; Tschop, M.H.; Hui, D.Y. Postprandial lysophospholipid suppresses hepatic fatty acid oxidation: The molecular link between group 1B phospholipase A2 and diet-induced obesity. FASEB J. 2010, 24, 2516–2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Priore, P.; Stanca, E.; Gnoni, G.V.; Siculella, L. Dietary fat types differently modulate the activity and expression of mitochondrial carnitine/acylcarnitine translocase in rat liver. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 1341–1349. [Google Scholar] [CrossRef]
- Tian, J.-J.; Lu, R.-H.; Ji, H.; Sun, J.; Li, C.; Liu, P.; Lei, C.-X.; Chen, L.-Q.; Du, Z.-Y. Comparative analysis of the hepatopancreas transcriptome of grass carp (Ctenopharyngodon idellus) fed with lard oil and Fish oil diets. Gene 2015, 565, 192–200. [Google Scholar] [CrossRef]
- Turchini, G.M.; Mentasti, T.; Froyland, L.; Orban, E.; Caprino, F.; Moretti, V.M.; Valfre, F. Effects of alternative dietary lipid sources on performance, tissue chemical composition, mitochondrial fatty acid oxidation capabilities and sensory characteristics in brown trout (Salmo trutta L.). Aquaculture 2003, 225, 251–267. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Visser, G.; Ferdinandusse, S.; Vaz, F.M.; Houtkooper, R.H. Mitochondrial Fatty Acid Oxidation Disorders: Laboratory Diagnosis, Pathogenesis, and the Complicated Route to Treatment. J. Lipid Atheroscler. 2020, 9, 313–333. [Google Scholar] [CrossRef]
- Ruiz-Sala, P.; Peña-Quintana, L. Biochemical Markers for the Diagnosis of Mitochondrial Fatty Acid Oxidation Diseases. J. Clin. Med. 2021, 10, 4855. [Google Scholar] [CrossRef]
- Torstensen, B.E.; Stubhaug, I. β-oxidation of 18:3n-3 in Atlantic Salmon (Salmo salar L.) hepatocytes treated with different fatty acids. Lipids 2004, 39, 153–160. [Google Scholar] [CrossRef]
- Kjær, M.A.; Todorcevic, M.; Torstensen, B.E.; Vegusdal, A.; Ruyter, B. Dietary n-3 HUFA affects mitochondrial fatty acid beta-oxidation capacity and susceptibility to oxidative stress in Atlantic salmon. Lipids 2008, 43, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Závorka, L.; Crespel, A.; Dawson, N.J.; Papatheodoulou, M.; Killen, S.S.; Kainz, M.J. Climate change-induced deprivation of dietary essential fatty acids can reduce growth and mitochondrial efficiency of wild juvenile salmon. Funct. Ecol. 2021, 35, 1960–1971. [Google Scholar] [CrossRef]
- Salin, K.; Villasevil, E.M.; Anderson, G.J.; Lamarre, S.G.; Melanson, C.A.; Mccarthy, I.; Selman, C.; Metcalfe, N.B. Differences in mitochondrial efficiency explain individual variation in growth performance. Proc. Biol. Sci. 2019, 286, 20191466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hundal, B.K.; Liland, N.S.; Rosenlund, G.; Höglund, E.; Araujo, P.; Stubhaug, I.; Sissener, N.H. Increasing the dietary n-6/n-3 ratio alters the hepatic eicosanoid production after acute stress in Atlantic salmon (Salmo salar). Aquaculture 2021, 534, 736272. [Google Scholar] [CrossRef]
- Sissener, N.H.; Araujo, P.; Sæle, Ø.; Rosenlund, G.; Stubhaug, I.; Sanden, M. Dietary 18:2n-6 affects EPA (20:5n-3) and ARA (20:4n-6) content in cell membranes and eicosanoid production in Atlantic salmon (Salmo salar L.). Aquaculture 2020, 522, 735098. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Yao, L.; Zhang, X.; Zhang, X.; Ye, C.; Jiang, H.; He, J.; Zhu, Y.; Ai, D. Hydroxyeicosapentaenoic acids and epoxyeicosatetraenoic acids attenuate early occurrence of nonalcoholic fatty liver disease. Br. J. Pharmacol. 2017, 174, 2358–2372. [Google Scholar] [CrossRef]
- Duan, J.; Song, Y.; Zhang, X.; Wang, C. Effect of ω-3 Polyunsaturated Fatty Acids-Derived Bioactive Lipids on Metabolic Disorders. Front. Physiol. 2021, 12, 646491. [Google Scholar] [CrossRef]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed. Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef]
- Liu, Y.; Sen, S.; Wannaiampikul, S.; Palanivel, R.; Hoo, R.L.; Isserlin, R.; Bader, G.D.; Tungtrongchitr, R.; Deshaies, Y.; Xu, A.; et al. Metabolomic profiling in liver of adiponectin-knockout mice uncovers lysophospholipid metabolism as an important target of adiponectin action. Biochem. J. 2015, 469, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Todorcevic, M.; Kjaer, M.A.; Djakovic, N.; Vegusdal, A.; Torstensen, B.E.; Ruyter, B. N-3 HUFAs affect fat deposition, susceptibility to oxidative stress, and apoptosis in Atlantic salmon visceral adipose tissue. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 152, 135–143. [Google Scholar] [CrossRef]
- Todorčević, M.; Vegusdal, A.; Gjøen, T.; Sundvold, H.; Torstensen, B.E.; Kjær, M.A.; Ruyter, B. Changes in fatty acids metabolism during differentiation of Atlantic salmon preadipocytes; Effects of n-3 and n-9 fatty acids. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2008, 1781, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Bou, M.; Torgersen, J.S.; Ostbye, T.K.; Ruyter, B.; Wang, X.; Skugor, S.; Kristiansen, I.O.; Todorcevic, M. DHA Modulates Immune Response and Mitochondrial Function of Atlantic Salmon Adipocytes after LPS Treatment. Int. J. Mol. Sci. 2020, 21, 4101. [Google Scholar] [CrossRef] [PubMed]
- Mccoin, C.S.; Knotts, T.A.; Adams, S.H. Acylcarnitines-old actors auditioning for new roles in metabolic physiology. Nat. Rev. Endocrinol. 2015, 11, 617–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; Mccoin, C.S.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.H.; Hwang, D.H. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1378–E1387. [Google Scholar] [CrossRef] [Green Version]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan metabolic pathways and brain serotonergic activity: A compartative review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Dantzer, R. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression: Preclinical Approaches. Curr. Top. Behav. Neurosci. 2017, 31, 117–138. [Google Scholar] [CrossRef]
- Rørvik, K.A.; Dehli, A.; Thomassen, M.; Ruyter, B.; Steien, S.H.; Salte, R. Synergistic effects of dietary iron and omega-3 fatty acid levels on survival of farmed Atlantic salmon, Salmo salar L.; during natural outbreaks of furunculosis and cold water vibriosis. J. Fish Dis. 2003, 26, 477–485. [Google Scholar] [CrossRef]
- Hatlen, B.; Jørgensen, S.M.; Timmerhaus, G.; Krasnov, A.; Bou, M.; Ruyter, B.; Evensen, Ø. Styrt Fettsyresammensetning i fôr for å Forebygge Utbrudd av Viktige Virussykdommer i Laks; Nofima: Bergen, Norway, 2016; p. 48. [Google Scholar]
- Martinez-Rubio, L.; Evensen, O.; Krasnov, A.; Jorgensen, S.M.; Wadsworth, S.; Ruohonen, K.; Vecino, J.L.G.; Tocher, D.R. Effects of functional feeds on the lipid composition, transcriptomic responses and pathology in heart of Atlantic salmon (Salmo salar L.) before and after experimental challenge with Piscine Myocarditis Virus (PMCV). BMC Genom. 2014, 15, 462. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Rubio, L.; Morais, S.; Evensen, Ø.; Wadsworth, S.; Vecino, J.G.; Ruohonen, K.; Bell, J.G.; Tocher, D.R. Effect of functional feeds on fatty acid and eicosanoid metabolism in liver and head kidney of Atlantic salmon (Salmo salar L.) with experimentally induced heart and skeletal muscle inflammation. Fish Shellfish Immunol. 2013, 34, 1533–1545. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Rubio, L.; Morais, S.; Evensen, O.; Wadsworth, S.; Ruohonen, K.; Vecino, J.L.; Bell, J.G.; Tocher, D.R. Functional feeds reduce heart inflammation and pathology in Atlantic Salmon (Salmo salar L.) following experimental challenge with Atlantic salmon reovirus (ASRV). PLoS ONE 2012, 7, e40266. [Google Scholar] [CrossRef] [Green Version]
- Henderson, R.J.; Tocher, D.R. Thin-layer chromatography. In Lipid Analysis A Practical Approach; Hamilton, R.J., Hamilton, S., Eds.; IRL Press: Oxford, UK, 1992; pp. 65–111. [Google Scholar]
- Sissener, N.H.; Torstensen, B.E.; Stubhaug, I.; Rosenlund, G. Long-term feeding of Atlantic salmon in seawater with low dietary long-chain n-3 fatty acids affects tissue status of the brain, retina and erythrocytes. Br. J. Nutr. 2016, 115, 1919–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, A.M.; Dehaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, Nontargeted Ultrahigh Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry Platform for the Identification and Relative Quantification of the Small-Molecule Complement of Biological Systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
- Reitman, Z.J.; Jin, G.; Karoly, E.D.; Spasojevic, I.; Yang, J.; Kinzler, K.W.; He, Y.; Bigner, D.D.; Vogelstein, B.; Yan, H. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc. Natl. Acad. Sci. USA 2011, 108, 3270–3275. [Google Scholar] [CrossRef] [Green Version]
- Dehaven, C.D.; Evans, A.M.; Dai, H.; Lawton, K.A. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J. Cheminform. 2010, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Dietary EPA + DHA Levels (%) | ||||
|---|---|---|---|---|
| 1.0 | 1.3 | 1.6 | 3.5 | |
| PROXIMATE COMPOSITION | ||||
| Moisture (%) | 6.0 | 6.0 | 6.0 | 6.0 |
| Energy–crude (MJ/Kg) | 26.2 | 26.2 | 26.2 | 26.1 |
| Protein–crude (%) | 33.4 | 33.4 | 33.4 | 33.5 |
| Fat–crude (%) | 38.7 | 38.7 | 38.6 | 38.4 |
| Ash (%) | 4.4 | 4.4 | 4.4 | 4.4 |
| FATTY ACIDS | ||||
| 14:0 | 0.9 | 1.1 | 1.4 | 2.8 |
| 16:0 | 7.0 | 7.2 | 7.5 | 9.6 |
| 18:0 | 2.8 | 2.7 | 2.7 | 3.0 |
| 20:0 | 0.7 | 0.6 | 0.6 | 0.5 |
| ΣSFA 1 | 12.6 | 13.1 | 13.3 | 17.0 |
| 16:1 n-7 | 1.0 | 1.3 | 1.8 | 3.6 |
| 18:1 n-7 | 2.8 | 3.0 | 3.0 | 3.2 |
| 18:1 n-9 | 46.5 | 45.8 | 43.8 | 33.0 |
| 20:1 n-9 | 1.6 | 1.9 | 2.4 | 4.2 |
| 20:1 n-11 | 0.3 | 0.4 | 0.5 | 1.1 |
| 22:1 n-9 | 0.2 | 0.2 | 0.2 | 0.3 |
| 22:1 n-11 | 0.6 | 0.9 | 1.3 | 3.1 |
| 24:1 n-9 | 0.2 | 0.2 | 0.2 | 0.2 |
| Σ MUFA 2 | 53.2 | 53.7 | 53.1 | 48.8 |
| 16:2 n-6 | 0.1 | 0.1 | 0.2 | 0.3 |
| 18:2 n-6 | 20.0 | 18.7 | 16.9 | 12.5 |
| 18:3 n-3 | 8.1 | 8.0 | 7.7 | 5.1 |
| 20:4 n-3 | 0.1 | 0.2 | 0.3 | 0.7 |
| 20:5 n-3 | 1.5 | 2.0 | 2.4 | 5.1 |
| 22:5 n-3 | 0.2 | 0.3 | 0.3 | 0.7 |
| 22:6 n-3 | 1.4 | 1.8 | 2.3 | 5.0 |
| EPA + DHA | 2.8 | 3.8 | 4.7 | 10.2 |
| ΣPUFA | 31.6 | 31.4 | 30.4 | 30.1 |
| Σn-3 3 | 11.5 | 12.5 | 13.3 | 17.2 |
| Σn-6 4 | 20.2 | 19.0 | 17.2 | 13.1 |
| n-6/n-3 | 1.8 | 1.5 | 1.3 | 0.8 |
| TFA mg/g feed | 354.4 | 356.5 | 357.7 | 362.6 |
| Diet 1.0 | Diet 1.3 | Diet 1.6 | Diet 3.5 | |
|---|---|---|---|---|
| Initial weight (g) | 275.4 ± 1.4 | 275.2 ± 2.9 | 276.4 ± 0.6 | 277.1 ± 0.7 |
| Final weight (g) | 4748.5 ± 33.4 a | 4938.0 ± 85.6 a | 4963.6 ± 68.4 a | 5364.6 ± 56.9 b |
| SGR | 0.68 ± 0.0003 a | 0.69 ± 0.005 a | 0.69 ± 0.003 a | 0.70 ± 0.002 b |
| TGC | 3.1 ± 0.016 a | 3.1 ± 0.031 a | 3.1 ± 0.023 a | 3.3 ± 0.017 b |
| HSI | 1.15 ± 0.02 c | 1.12 ± 0.02 bc | 1.08 ± 0.02 ab | 1.05 ± 0.02 a |
| Diet 1.0 | Diet 1.3 | Diet 1.6 | Diet 3.5 | |
|---|---|---|---|---|
| PC | 12.0 ± 7.6 ab | 11.6 ± 2.7 b | 10.1 ± 1.2 ab | 9.1 ± 1.1 a |
| PS | 1.5 ± 0.9 | 1.4 ± 0.3 | 1.5 ± 0.4 | 1.4 ± 0.2 |
| PI | 2.4 ± 1.5 | 2.2 ± 0.5 | 2.1 ± 0.4 | 2.2 ± 0.5 |
| PA/CL/PG | 1.2 ± 0.6 | 1.1 ± 0.4 | 1.3 ± 0.3 | 1.1 ± 0.2 |
| PE | 7.2 ± 4.9 ab | 6.8 ± 1.4 b | 5.8 ± 0.9 ab | 5.3 ± 0.6 a |
| Sum polar lipids | 32.3 ± 19.1 ab | 30.7 ± 5.9 b | 27.5 ± 4.2 ab | 24.6 ± 2.4 a |
| Sum neutral lipids | 58.4 ± 32.2 b | 73.2 ± 44.7 b | 41.2 ± 12.6 b | 26.9 ± 7.0 a |
| Sum lipids | 90.8 ± 48.6 b | 103.8 ± 48.7 b | 68.7 ± 15.5 ab | 51.9 ± 8.8 a |
| Diet 1.0 | Diet 1.3 | Diet 1.6 | Diet 3.5 | |
|---|---|---|---|---|
| 16:0 | 10.9 ± 0.9 a | 11.0 ± 0.6 a | 11.2 ± 0.7 a | 12.5 ± 1.0 b |
| 18:0 | 6.2 ± 0.7 | 6.1 ± 0.4 | 6.1 ± 0.4 | 6.3 ± 0.6 |
| Sum SFA * | 18.1 ± 1.2 a | 18.1 ± 0.7 a | 18.5 ± 0.8 a | 20.5 ± 1.1 b |
| 16:1n-7 | 0.5 ± 0.1 a | 0.5 ± 0.1 a | 0.5 ± 0.0 a | 0.8 ± 0.1 b |
| 18:1n-9 | 20.9 ± 2.7 c | 19.4 ± 0.9 bc | 17.9 ± 1.0 ab | 12.4 ± 1.3 a |
| 18:1n-7 | 2.0 ± 0.2 a | 2.0 ± 0.1 a | 2.0 ± 0.1 a | 2.3 ± 0.1 b |
| 20:1n-9 | 2.2 ± 0.3 a | 2.1 ± 0.3 a | 2.3 ± 0.4 ab | 2.6 ± 0.6 b |
| Sum MUFA ** | 26.3 ± 2.9 c | 24.6 ± 1.1 bc | 23.3 ± 1.0 ab | 19.3 ± 1.6 a |
| 18:2n-6 | 10.9 ± 1.1 c | 10.5 ± 0.7 bc | 9.2 ± 0.6 b | 5.3 ± 0.5 a |
| 20:2n-6 | 2.1 ± 0.3 ab | 2.3 ± 0.3 b | 2.3 ± 0.3 b | 1.8 ± 0.3 a |
| 20:3n-6 | 2.5 ± 0.4 d | 1.7 ± 0.3 c | 1.2 ± 0.2 b | 0.4 ± 0.1 a |
| 20:4n-6 (ARA) | 3.1 ± 0.4 a | 3.1 ± 0.2 a | 3.3 ± 0.2 ab | 3.9 ± 0.3 b |
| Sum n-6 *** | 19.2 ± 0.9 d | 18.1 ± 0.7 c | 16.6 ± 0.8 b | 12.0 ± 0.4 a |
| 18:3n-3 | 2.6 ± 0.5 b | 2.6 ±0.3 b | 2.5 ± 0.3 b | 1.4 ± 0.4 a |
| 20:4n-3 | 1.3 ± 0.2 c | 1.2 ± 0.2 bc | 1.1 ± 0.1 b | 0.8 ± 0.1 a |
| 20:5n-3 (EPA) | 8.9 ± 0.8 a | 9.5 ± 0.6 ab | 10.1 ± 0.6 b | 12.0 ± 0.7 c |
| 22:5n-3 | 2.8 ± 0.3 a | 2.8 ± 0.2 a | 2.9 ± 0.2 a | 3.7 ± 0.7 b |
| 22:6n-3 (DHA) | 19.2 ± 2.0 a | 21.3 ± 1.1 ab | 23.0 ± 1.1 b | 27.5 ± 1.1 c |
| EPA + DHA | 28.1 ± 2.6 a | 30.8 ± 1.0 ab | 33.1 ± 1.2 bc | 39. 5 ± 1.6 c |
| Sum n-3 **** | 35.4 ± 2.3 a | 37.9 ± 1.0 ab | 39.9 ± 1.1 bc | 45.7 ± 0.9 c |
| Sum PUFA | 54.6 ± 1.7 a | 56.0 ± 0.7 ab | 56.6 ± 1.0 bc | 57.7 ± 0.9 c |
| n6/n3 | 0.6 ± 0.1 c | 0.5 ± 0.0 bc | 0.4 ± 0.0 ab | 0.3 ± 0.0 a |
| TFA | 21.6 ±1.6 | 20.4 ± 2.4 | 20.8 ± 2.0 | 21.3 ± 1.0 |
| Diet 1.0 | Diet 1.3 | Diet 1.6 | Diet 3.5 | |
|---|---|---|---|---|
| 16:0 | 2.3 ± 1.5 | 2.3 ± 1.9 | 1.3 ± 0.5 | 0.9 ± 0.3 |
| 18:0 | 2.1 ± 1.6 b | 1.8 ± 1.3 b | 0.9 ± 0.5 ab | 0.5 ± 0.2 a |
| Sum SFA * | 5.3 ± 3.5 b | 4.9 ± 3.8 b | 2.7 ± 1.2 ab | 1.7 ± 0.7 a |
| 16:1n-7 | 0.9 ± 0.7 | 0.9 ± 0.8 | 0.5 ± 0.3 | 0.4 ± 0.2 |
| 18:1n-9 | 39.1 ± 32.4 b | 34.5 ± 26.6 b | 18.4 ± 9.3 b | 6.5 ± 3.2 a |
| 18:1n-7 | 2.5 ± 2.0 b | 2.2 ± 1.6 b | 1.3 ± 0.6 ab | 0.7 ± 0.3 a |
| 20:1n-9 | 4.0 ± 3.4 b | 3.5 ± 2.7 b | 2.0 ± 0.9 b | 0.9 ± 0.5 a |
| Sum MUFA ** | 47.6 ± 39.2 b | 42.1 ± 32.4 b | 22.7 ± 11.3 b | 9.1 ± 4.4 a |
| 18:2n-6 | 11.0 ± 10.2 b | 9.4 ± 7.1 b | 5.0 ± 2.6 b | 1.8 ± 0.8 a |
| 20:2n-6 | 1.7 ± 1.5 b | 1.6 ± 1.3 b | 0.9 ± 0.5 b | 0.4 ± 0.2 a |
| 20:3n-6 | 0.52 ± 0.46 c | 0.31 ± 0.22 bc | 0.09 ± 0.15 b | 0.04 ± 0.02 a |
| 20:4n-6 (ARA) | 0.5 ± 0.4 ab | 0.6 ± 0.5 b | 0.4 ± 0.2 ab | 0.2 ± 0.1 a |
| Sum n-6 *** | 13.9 ± 12.6 b | 12.0 ± 9.2 b | 6.5 ± 3.3 b | 2.4 ± 1.1 a |
| 18:3n-3 | 3.4 ± 2.9 b | 3.3 ± 2.6 b | 1.9 ± 0.9 b | 0.7 ± 0.3 a |
| 20:4n-3 | 0.4 ± 0.4 | 0.4 ± 0.4 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| 20:5n-3 (EPA) | 0.5 ± 0.2 | 0.6 ± 0.4 | 0.4 ± 0.1 | 0.6 ± 0.2 |
| 22:5n-3 | 0.1 ± 0.1 a | 0.1 ± 0.1 a | 0.1 ± 0.0 a | 0.3 ± 0.1 b |
| 22:6n-3 (DHA) | 0.4 ± 0.1 a | 0.5 ± 0.2 a | 0.4 ± 0.1 ab | 0.6 ± 0.2 b |
| EPA + DHA | 0.9 ± 0.4 | 1.1 ± 0.6 | 0.9 ± 0.1 | 1.2 ± 0.3 |
| Sum n-3 **** | 5.0 ± 3.7 | 5.1 ± 3.8 | 3.2 ± 1.2 | 2.5 ± 0.9 |
| Sum PUFA | 19.0 ± 16.3 b | 17.1 ± 13.0 b | 9.7 ± 4.6 ab | 4.9 ± 2.0 a |
| n6/n3 | 2.5 ± 0.6 c | 2.3 ± 0.2 bc | 1.9 ± 0.3 ab | 1.0 ± 0.2 a |
| TFA | 72.6 ± 59.3 b | 65.0 ± 49.7 b | 35.6 ± 17.3 b | 16.1 ± 7.2 a |
| Pathway | Metabolites | Core Finding | |
|---|---|---|---|
| Energy production | Tricarboxylic acid cycle (TCA) | Decreased citrate, cis- aconitate and isocitrate and increased succinate with lower dietary EPA and DHA. | Indicates less use of FA oxidation as input into TCA cycle, and then a shift to using BCAA, PPP and creatine instead when salmon is fed a lower dietary EPA and DHA. |
| Pentose Phosphate Pathway | Increased ribulose-5-phosphate (intermediate in PPP) and galactonate (feeds into the PPP) in fish fed less EPA and DHA. | ||
| BCAA metabolism | Increased levels of BCAA, dipeptides containing BCAA (protein degradants), and acyl carnitines involved in BCAA metabolism in fish given lower dietary EPA and DHA. | ||
| Creatine metabolism | Higher levels of these metabolites with lower dietary EPA and DHA | ||
| Transport of FA into mitochondria | Carnitine metabolism | Increased levels of carnitine and deoxycarnitine in fish fed less EPA and DHA. | Suggests some dysfunction with the mitochondrial β-oxidation. |
| Acyl carnitines | Higher occurrence with lower dietary EPA and DHA, particularly fish fed diet 1.6. | ||
| Phospholipid metabolism | Phospholipid degradants | Higher content of PL degradants with lower dietary EPA and DHA. | Implies an altered PL metabolism with lowered dietary EPA and DHA. Can possibly disturb the mitochondrial membrane. |
| Lysophospholipids | More of almost all lysophospholipids in liver of fish given diets low in EPA and DHA. |
| Pathway | Biochemical Name | Fold Change | ||
|---|---|---|---|---|
| Diet 1.0/Diet 3.5 | Diet 1.6/Diet 3.5 | Diet 1.0/Diet 1.6 | ||
| TCA Cycle | citrate | 0.77 | 1.32 | 0.58 |
| aconitate [cis or trans] | 0.83 | 1.56 | 0.53 | |
| isocitrate | 0.64 | 1.09 | 0.59 | |
| succinate | 1.47 | 1.10 | 1.33 | |
| Fatty acid metabolism | acetyl-CoA | 0.93 | 0.90 | 1.03 |
| Pentose Phosphate Pathway | ribulose 5-phosphate | 1.83 | 0.97 | 1.89 |
| Fructose, Mannose and Galactose Metabolism | galactonate | 1.53 | 1.48 | 1.04 |
| Leucine, Isoleucine and Valine Metabolism | leucine | 1.10 | 1.01 | 1.09 |
| isoleucine | 1.09 | 1.02 | 1.06 | |
| valine | 1.18 | 1.06 | 1.11 | |
| glycylisoleucine | 1.56 | 1.05 | 1.48 | |
| Dipeptide | glycylleucine | 1.78 | 1.12 | 1.59 |
| glycylvaline | 2.13 | 1.23 | 1.74 | |
| isoleucylglycine | 5.03 | 1.21 | 4.14 | |
| lysylleucine | 2.73 | 1.23 | 2.23 | |
| prolylglycine | 2.00 | 1.31 | 1.53 | |
| valylglutamine | 3.75 | 1.31 | 2.86 | |
| valylglycine | 6.17 | 1.15 | 5.36 | |
| valylleucine | 3.40 | 1.20 | 2.84 | |
| leucylglutamine | 4.53 | 1.21 | 3.75 | |
| Fatty Acid Metabolism (also BCAA Metabolism) | butyrylcarnitine (C4) | 1.41 | 1.69 | 0.83 |
| propionylcarnitine (C3) | 1.48 | 1.33 | 1.12 | |
| Creatine Metabolism | guanidinoacetate | 2.16 | 1.07 | 2.01 |
| creatine | 1.06 | 1.20 | 0.89 | |
| creatinine | 1.28 | 1.17 | 1.09 | |
| Pathway | Biochemical Name | Fold Change | ||
|---|---|---|---|---|
| Diet 1.0/Diet 3.5 | Diet 1.6/Diet 3.5 | Diet 1.0/Diet 1.6 | ||
| Carnitine Metabolism | deoxycarnitine | 1.13 | 1.37 | 0.83 |
| carnitine | 1.23 | 1.32 | 0.93 | |
| Pathway | Biochemical Name | Fold Change | ||
|---|---|---|---|---|
| Diet 1.0/Diet 3.5 | Diet 1.6/Diet 3.5 | Diet 1.0/Diet 1.6 | ||
| Eicosanoid | 5-HEPE | 0.29 | 0.33 | 0.88 |
| Tryptophan metabolism | kynurenine | 1.70 | 2.10 | 0.81 |
| kynurenate | 1.03 | 1.90 | 0.54 | |
| Gene | Forward | Reverse | GenBank Accession Number | Efficiency |
|---|---|---|---|---|
| Reference genes | ||||
| arp | GAAAATCATCCAATTGCTGGATG | CTTCCCACGCAAGGACAGA | AY255630 | 84% |
| b-actin | CCAAAGCCAACAGGGAGAA | AGGGACAACACTGCCTGGAT | BG933897 | 109% |
| ef1ab | TGCCCCTCCAGGATGTCTAC | CACGGCCCACAGGTACTG | AF321836 | 111% |
| Target genes | ||||
| aco | CACTGCCAGGTGTGGTGGTA | GGAATTGTACGTTCTCCAATTTCA | DQ364432 | 97% |
| apob100 | TGCAGAGACCTTTAAGTTCATTCA | TGTGCAGTGGTTGCCTTGAC | gi:854619 | 124% |
| cact | GTTCGCTGTCTGCTTCTTCG | TACTTCACCTCCCCTTTGGC | BT044930.1 | 87% |
| cd36 | GGATGAACTCCCTGCATGTGA | TGAGGCCAAAGTACTCGTCGA | AY606034 | 113% |
| cpt1 | CTTTGGGAAGGGCCTGATC | CATGGACGCCTCGTACGTTA | AM230810 | 92% |
| cpt2 | TGCTCAGCTAGCGTTCCATATG | AGTGCTGCAGGACTCGTATGTG | BG934647 | 102% |
| ppara | TCTCCAGCCTGGACCTGAAC | GCCTCGTAGACGCCGTACTT | NM001123560 | 107% |
| plin2 | CCACTCTGCCTCGCAAATCT | GGGTAAAAGGGACCTACCAGC | XM_014206726.1 | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hundal, B.K.; Lutfi, E.; Sigholt, T.; Rosenlund, G.; Liland, N.S.; Glencross, B.; Sissener, N.H. A Piece of the Puzzle—Possible Mechanisms for Why Low Dietary EPA and DHA Cause Hepatic Lipid Accumulation in Atlantic Salmon (Salmo salar). Metabolites 2022, 12, 159. https://doi.org/10.3390/metabo12020159
Hundal BK, Lutfi E, Sigholt T, Rosenlund G, Liland NS, Glencross B, Sissener NH. A Piece of the Puzzle—Possible Mechanisms for Why Low Dietary EPA and DHA Cause Hepatic Lipid Accumulation in Atlantic Salmon (Salmo salar). Metabolites. 2022; 12(2):159. https://doi.org/10.3390/metabo12020159
Chicago/Turabian StyleHundal, Bjørg Kristine, Esmail Lutfi, Trygve Sigholt, Grethe Rosenlund, Nina Sylvia Liland, Brett Glencross, and Nini Hedberg Sissener. 2022. "A Piece of the Puzzle—Possible Mechanisms for Why Low Dietary EPA and DHA Cause Hepatic Lipid Accumulation in Atlantic Salmon (Salmo salar)" Metabolites 12, no. 2: 159. https://doi.org/10.3390/metabo12020159
APA StyleHundal, B. K., Lutfi, E., Sigholt, T., Rosenlund, G., Liland, N. S., Glencross, B., & Sissener, N. H. (2022). A Piece of the Puzzle—Possible Mechanisms for Why Low Dietary EPA and DHA Cause Hepatic Lipid Accumulation in Atlantic Salmon (Salmo salar). Metabolites, 12(2), 159. https://doi.org/10.3390/metabo12020159