Genetic Polymorphism in Angiotensinogen and Its Association with Cardiometabolic Diseases
Abstract
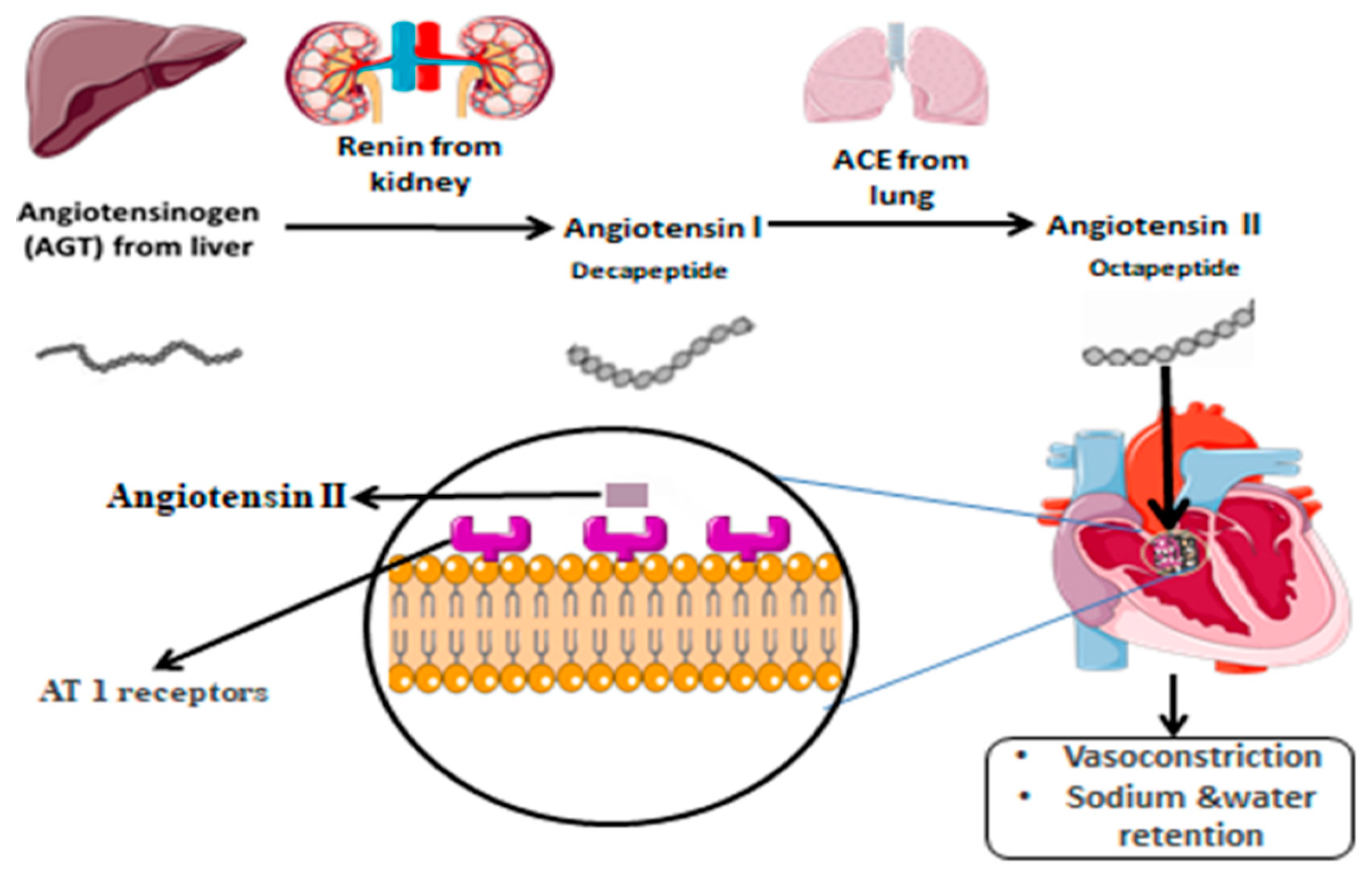
:1. Introduction
2. AGT Polymorphism and Associated Diseases
2.1. High Altitude Polycythemia
2.2. Pre-Eclampsia
2.3. Obesity
2.4. Cardiovascular Diseases
2.5. Diabetic Nephropathy
2.6. Hashimoto’s Thyroiditis
2.7. Essential Hypertension
2.8. Liver Cirrhosis
3. Discussion
4. Conclusions
Funding
Conflicts of Interest
References
- Cooke, J.N.; Bostrom, M.A.; Hicks, P.J.; Ng, M.C.; Hellwege, J.N.; Comeau, M.E.; Divers, J.; Langefeld, C.D.; Freedman, B.I.; Bowden, D.W. Polymorphisms in MYH9 are associated with diabetic nephropathy in European Americans. Nephrol. Dial. Transplant. 2012, 27, 1505–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, S.; Essawi, M. Genetic polymorphism studies in humans. Middle East J. Med. Genet. 2012, 1, 57–63. [Google Scholar] [CrossRef]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.; Thormann, A.; Flicek, P.; Cunningham, F. The ensembl variant effect predictor. Genome Biol. 2016, 17, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaina, S.; Pérez-Luque, E.L.; Lund, G. Genetics talks to epigenetics? The interplay between sequence variants and chromatin structure. Curr. Genom. 2010, 11, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L. The complex language of chromatin regulation during transcription. Nature 2007, 447, 407–412. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef] [Green Version]
- Guedes, M.J.; Nicolaou, N.; Patel, P.C. Genetic distance and the difference in new firm entry between countries. J. Evol. Econ. 2019, 29, 973–1016. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Y.; Wang, H.; Wang, H.; Zhang, Y.Y. Myocardial Infarction and AGTp.Thr174Met Polymorphism: A Meta-Analysis of 7657 Subjects. Cardiovasc. Ther. 2021, 2021, 6667934. [Google Scholar] [CrossRef]
- Takei, Y.; Joss, J.M.; Kloas, W.; Rankin, J.C. Identification of angiotensin I in several vertebrate species: Its structural and functional evolution. Gen. Comp. Endocrinol. 2004, 135, 286–292. [Google Scholar] [CrossRef]
- Lu, H.; Cassis, L.A.; Vander Kooi, C.W.; Daugherty, A. Structure and functions of angiotensinogen. Hypertens. Res. 2016, 39, 492–500. [Google Scholar] [CrossRef]
- Purkait, P.; Halder, K.; Thakur, S.; Ghosh Roy, A.; Raychaudhuri, P.; Bhattacharya, S.; Sarkar, B.N.; Naidu, J.M. Association of angiotensinogen gene SNPs and haplotypes with risk of hypertension in eastern Indian population. Clin. Hypertens. 2017, 23, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef] [PubMed]
- Rianto, F.; Hoang, T.; Revoori, R.; Sparks, M.A. Angiotensin receptors in the kidney and vasculature in hypertension and kidney disease. Mol. Cell. Endocrinol. 2021, 529, 111259. [Google Scholar] [CrossRef]
- Shahvaisizadeh, F.; Movafagh, A.; Omrani, M.D.; Vaisi-Raygani, A.; Rahimi, Z.; Rahimi, Z. The −20 and −217 Promoter Variants Dominate Differential Angiotensinogen Haplotype Regulation in Angiotensinogen-Expressing Cells. Hypertension 2007, 49, 631–639. [Google Scholar]
- Nakajima, T.; Inoue, I.; Cheng, T.; Lalouel, J.M. Molecular cloning and functional analysis of a factor that binds to the proximal promoter of human angiotensinogen. J. Hum. Genet. 2002, 47, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, R.; Du, Y.Y.; Zhang, Y.Z.; Chen, Q.H.; Zhao, L.S.; Li, L. Association between G-217A polymorphism in the AGT gene and essential hypertension: A meta-analysis. Genet. Mol. Res. 2015, 14, 5527–5534. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, V.; Lagou, E.; Mihas, C.; Vasiliki, G.; Katsiki, N.; Kollia, A.; Triposkiadis, F.; Degiannis, D.; Mavrogeni, S.; Kolovou, G. Angiotensinogen (AGT) M235T, AGT T174M and Angiotensin-1-Converting Enzyme (ACE) I/D Gene Polymorphisms in Essential Hypertension: Effects on Ramipril Efficacy. Open Cardiovasc. Med. J. 2015, 9, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Molina-Molina, M.; Xaubet, A.; Li, X.; Abdul-Hafez, A.; Friderici, K.; Jernigan, K.; Fu, W.; Ding, Q.; Pereda, J.; Serrano-Mollar, A.; et al. Angiotensinogen gene G-6A polymorphism influences idiopathic pulmonary fibrosis disease progression. Eur. Respir. J. 2008, 32, 1004–1008. [Google Scholar] [CrossRef] [Green Version]
- Sarzani, R.; Bordicchia, M.; Marcucci, P.; Minardi, D.; Muzzonigro, G.; Dessì-Fulgheri, P.; Rappelli, A. Angiotensinogen promoter variants influence gene expression in human kidney and visceral adipose tissue. J. Hum. Hypertens. 2010, 24, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Moreno, M.; Locia-Morales, D.; Peralta-Romero, J.; Sharma, T.; Meyre, D.; Cruz, M.; Flores-Alfaro, E.; Valladares-Salgado, A. AGT rs4762 is associated with diastolic blood pressure in Mexicans with diabetic nephropathy. J. Diabetes Its Complicat. 2021, 35, 107826. [Google Scholar] [CrossRef]
- Dickson, M.E.; Sigmund, C.D. Genetic Basis of Hypertension. Hypertension 2006, 48, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mohana, V.U.; Swapna, N.; Surender, R.S.; Vishnupriya, S.; Padma, T. Gender-related association of AGT gene variants (M235T and T174M) with essential hypertension—A case-control study. Clin. Exp. Hypertens. 2012, 34, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lanz, J.R.; Pereira, A.C.; Lemos, P.A.; Martinez, E.; Krieger, J.E. Angiotensinogen M235T polymorphism is associated with coronary artery disease severity. Clin. Chim. Acta 2005, 362, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, R.; Hu, S.; Rong, J. Gene polymorphism associated with angiotensinogen (M235T), endothelial lipase (584C/T) and susceptibility to coronary artery disease: A meta-analysis. Biosci. Rep. 2020, 40, BSR20201414. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, P.; Zak, I.; Wita, K. The M235T polymorphism of the AGT gene modifies the risk of coronary artery disease associated with the presence of hypercholesterolemia. Eur. J. Epidemiol. 2008, 23, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Ma, L.; Zhang, Z.; Li, Y.; Hao, M.; Zhao, Z.; Zhao, Y.; Liu, F.; Liu, L.; Luo, X.; et al. Associations of high-altitude polycythemia with polymorphisms in PIK3CD and COL4A3 in Tibetan populations. Hum. Genom. 2018, 12, 37. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhang, Y.; Zhang, Z.; Zhao, Y.; Fan, X.; Ma, L.; Zhang, Y.; He, H.; Kang, L. Associations of high altitude polycythemia with polymorphisms in EPHA2 and AGT in Chinese Han and Tibetan populations. Oncotarget 2017, 8, 53234–53243. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Ishida, J.; Imagawa, S.; Saito, T.; Suzuki, N.; Matsuoka, T.; Sugaya, T.; Tanimoto, K.; Yokoo, T.; Olmeda, O.; et al. Enhanced erythropoiesis mediated by activation of the renin-angiotensin system via angiotensin II type 1a receptor. FASEB J. 2005, 19, 2023–2025. [Google Scholar] [CrossRef] [Green Version]
- Vlahakos, D.V.; Marathias, K.P.; Madias, N.E. The Role of the Renin-Angiotensin System in the Regulation of Erythropoiesis. Am. J. Kidney Dis. 2010, 56, 558–565. [Google Scholar] [CrossRef]
- Duley, L. The Global Impact of Pre-Eclampsia and Eclampsia. In Seminars in Perinatology; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Aggarwal, S.; Dimri, N.; Tandon, I.; Agarwal, S. Preeclampsia in North Indian women: The contribution of genetic polymorphisms. J. Obstet. Gynaecol. Res. 2011, 37, 1335–1341. [Google Scholar] [CrossRef]
- Steegers, E.A.; Von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Ohtsu, H.; Suzuki, H.; Shirai, H.; Frank, G.D.; Eguchi, S. Angiotensin II signal transduction through the AT1 receptor: Novel insights into mechanisms and pathophysiology. Clin. Sci. 2007, 112, 417–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistry, H.D.; Kurlak, L.O.; Broughton Pipkin, F. The placental renin–angiotensin system and oxidative stress in pre-eclampsia. Placenta 2013, 34, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.X.; Peng, W.J.; Li, Z.W.; Zhang, C.H.; Di, H.H.; Shen, X.P.; Zhu, J.F.; Yan, W.R. The Gene Variants of Maternal/Fetal Renin-Angiotensin System in Preeclampsia: A Hybrid Case-Parent/Mother-Control Study. Sci. Rep. 2017, 7, 5087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, G.; Li, Y.; Gao, J.; Wang, W.; Wang, H.; Bai, G. Associations between AGT, MTHFR, and VEGF gene polymorphisms and preeclampsia in the Chinese population. Placenta 2022, 118, 38–45. [Google Scholar] [CrossRef]
- Lin, R.; Lei, Y.; Yuan, Z.; Ju, H.; Li, D. Angiotensinogen Gene M235T and T174M Polymorphisms and Susceptibility of Pre-Eclampsia: A Meta-Analysis. Ann. Hum. Genet. 2012, 76, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Zhao, J.; Yi, J.; Luan, Y.; Wang, Q. Association Between Gene Polymorphisms on Chromosome 1 and Susceptibility to Pre-Eclampsia: An Updated Meta-Analysis. Med. Sci. Monit. 2016, 22, 2202–2214. [Google Scholar] [CrossRef] [Green Version]
- Roberts, C.B.; Rom, L.; Moodley, J.; Pegoraro, R.J. Hypertension-related gene polymorphisms in pre-eclampsia, eclampsia and gestational hypertension in Black South African women. J. Hypertens. 2004, 22, 945–948. [Google Scholar] [CrossRef]
- Knyrim, E.; Muetze, S.; Eggermann, T.; Rudnik-Schoeneborn, S.; Lindt, R.; Ortlepp, J.R.; Rath, W.; Zerres, K. Genetic Analysis of the Angiotensinogen Gene in Pre-Eclampsia: Study of German Women and Review of the Literature. Gynecol. Obstet. Investig. 2008, 66, 203–208. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef]
- Giacchetti, G.; Faloia, E.; Sardu, C.; Camilloni, M.A.; Mariniello, B.; Gatti, C.; Garrapa, G.G.M.; Guerrieri, M.; Mantero, F. Gene expression of angiotensinogen in adipose tissue of obese patients. Int. J. Obes. 2000, 24, S142–S143. [Google Scholar] [CrossRef] [PubMed]
- Marta, P.; Tomasz, F.; Jan, K.; Przemysław, A.; Jacek, C.; Irena, P. Angiotensinogen gene M235T and T174M polymorphisms in patients with morbid obesity and type 2 diabetes mellitus. J. Diabetes Metab. 2015, 6, 479. [Google Scholar]
- Umemura, S.; Nyui, N.; Tamura, K.; Hibi, K.; Yamaguchi, S.; Nakamaru, M.; Ishigami, T.; Yabana, M.; Kihara, M.; Inoue, S.; et al. Plasma angiotensinogen concentrations in obese patients. Am. J. Hypertens. 1997, 10, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Giacchetti, G.; Faloia, E.; Mariniello, B.; Sardu, C.; Gatti, C.; Camilloni, M.A.; Guerrieri, M.; Mantero, F. Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am. J. Hypertens. 2002, 15, 381–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massiéra, F.; Bloch-Faure, M.; Ceiler, D.; Murakami, K.; Fukamizu, A.; Gasc, J.M.; Quignard-Boulangé, A.; Negrel, R.; Ailhaud, G.; Seydoux, J.; et al. Adipose angiotensinogen is involved in adipose tissue 548 growth and blood pressure regulation. FASEB J. 2001, 15, 2727–2729. [Google Scholar] [CrossRef] [PubMed]
- Prat-Larquemin, L.; Oppert, J.M.; Clément, K.; Hainault, I.; Basdevant, A.; Guy-Grand, B.; Quignard-Boulangé, A. Adipose angiotensinogen secretion, blood pressure, and AGT M235T polymorphism in obese patients. Obes. Res. 2004, 12, 556–561. [Google Scholar] [CrossRef]
- Khamlaoui, W.; Mehri, S.; Hammami, S.; Elosua, R.; Hammami, M. Association of angiotensin-converting enzyme insertion/deletion (ACE I/D) and angiotensinogen (AGT M235T) polymorphisms with the risk of obesity in a Tunisian population. J. Renin-Angiotensin-Aldosterone Syst. 2020, 21, 1470320320907820. [Google Scholar] [CrossRef]
- Farmer, J.A.; Torre-Amione, G. The renin angiotensin system as a risk factor for coronary artery disease. Curr. Atheroscler. Rep. 2001, 3, 117–124. [Google Scholar] [CrossRef]
- Al-Najai, M.; Muiya, P.; Tahir, A.I.; Elhawari, S.; Gueco, D.; Andres, E.; Mazhar, N.; Altassan, N.; Alshahid, M.; Dzimiri, N. Association of the angiotensinogen gene polymorphism with atherosclerosis and its risk traits in the Saudi population. BMC Cardiovasc. Disord. 2013, 13, 17. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Singh, V.P.; Baker, K.M. The intracellular renin–angiotensin system: Implications in cardiovascular remodeling. Curr. Opin. Nephrol. Hypertens. 2008, 17, 168–173. [Google Scholar] [CrossRef]
- Schlüter, K.-D.; Wenzel, S. Angiotensin II: A hormone involved in and contributing to pro-hypertrophic cardiac networks and target of anti-hypertrophic cross-talks. Pharmacol. Ther. 2008, 119, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Raygan, F.; Karimian, M.; Rezaeian, A.; Bahmani, B.; Behjati, M. Angiotensinogen-M235T as a risk factor for myocardial infarction in Asian populations: A genetic association study and a bioinformatics approach. Croat. Med. J. 2016, 57, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Sivitskaya, L.N.; Kushniarevich, E.I.; Danilenko, N.G.; Novogrodski, T.A.; Davydenko, O.G. Gene polymorphism of the renin-angiotensin system in six ethnogeographic regions of Belarus. Russ. J. Genet. 2008, 44, 609–616. [Google Scholar] [CrossRef]
- Xu, M.Q.; Ye, Z.; Hu, F.B.; He, L. Quantitative assessment of the effect of angiotensinogen gene polymorphisms on the risk of coronary heart disease. Circulation 2007, 116, 1356–1366. [Google Scholar] [CrossRef]
- Sethi, A.A.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Angiotensinogen gene polymorphism, plasma angiotensinogen, and risk of hypertension and ischemic heart disease: A meta-analysis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1269–1275. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos Soares, D.; Pinto, G.H.; Lopes, A.; Caetano, D.S.L.; Nascimento, T.G.; Andrades, M.E.; Clausell, N.; Rohde, L.E.P.; Leitão, S.A.T.; Biolo, A. Cardiac hypertrophy in mice submitted to a swimming protocol: Influence of training volume and intensity on myocardial renin-angiotensin system. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2019, 316, R776–R782. [Google Scholar] [CrossRef]
- Wang, X.; Ye, Y.; Gong, H.; Wu, J.; Yuan, J.; Wang, S.; Yin, P.; Ding, Z.; Kang, L.; Jiang, Q.; et al. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang (1–7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. J. Mol. Cell. Cardiol. 2016, 97, 180–190. [Google Scholar] [CrossRef]
- Lyu, L.; Wang, H.; Li, B.; Qin, Q.; Qi, L.; Nagarkatti, M.; Nagarkatti, P.; Janicki, J.S.; Wang, X.L.; Cui, T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J. Mol. Cell. Cardiol. 2015, 89, 268–279. [Google Scholar] [CrossRef] [Green Version]
- Pugliese, G. Updating the natural history of diabetic nephropathy. Acta Diabetol. 2014, 51, 905–915. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Cravedi, P.; Remuzzi, G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat. Rev. Nephrol. 2010, 6, 319–330. [Google Scholar] [CrossRef]
- Sparks, M.A.; Crowley, S.D.; Gurley, S.B.; Mirotsou, M.; Coffman, T.M. Classical renin-angiotensin system in kidney physiology. Compr. Physiol. 2014, 4, 1201. [Google Scholar] [PubMed] [Green Version]
- Roscioni, S.S.; Heerspink, H.J.L.; De Zeeuw, D. The effect of RAAS blockade on the progression of diabetic nephropathy. Nat. Rev. Nephrol. 2014, 10, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Giacchetti, G.; Sechi, L.A.; Rilli, S.; Carey, R.M. The renin–angiotensin–aldosterone system, glucose metabolism and diabetes. Trends Endocrinol. Metab. 2005, 16, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Chawla, T.; Sharma, D.; Singh, A. Role of the renin angiotensin system in diabetic nephropathy. World J. Diabetes 2010, 1, 141–145. [Google Scholar] [CrossRef]
- Rogus, J.J.; Moczulski, D.; Freire, M.B.S.; Yang, Y.; Warram, J.H.; Krolewski, A.S. Diabetic Nephropathy Is Associated With AGT Polymorphism T235. Hypertension 1998, 31, 627–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tien, K.J.; Hsiao, J.Y.; Hsu, S.C.; Liang, H.T.; Lin, S.R.; Chen, H.C.; Hsieh, M.C. Gender-Dependent Effect of ACE I/D and AGT M235T Polymorphisms on the Progression of Urinary Albumin Excretion in Taiwanese with Type 2 Diabetes. Am. J. Nephrol. 2009, 29, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Makuc, J.; Šeruga, M.; Završnik, M.; Cilenšek, I.; Petrovič, D. Angiotensinogen (AGT) gene missense polymorphisms (rs699 and rs4762) and diabetic nephropathy in Caucasians with type 2 diabetes mellitus. Bosn. J. Basic Med. Sci. 2017, 17, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, Z. The Role of Renin Angiotensin Aldosterone System Genes in Diabetic Nephropathy. Can. J. Diabetes 2016, 40, 178–183. [Google Scholar] [CrossRef]
- Stasiołek, M.; Lewiński, A. Lokalny układ renina-angiotensyna-nowy łącznik pomiędzy mózgiem a tarczycą? Folia Med. Lodz. 2011, 2, 245–283. [Google Scholar]
- Platten, M.; Youssef, S.; Hur, E.M.; Ho, P.P.; Han, M.H.; Lanz, T.V.; Phillips, L.K.; Goldstein, M.J.; Bhat, R.; Raine, C.S.; et al. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1-and TH17-mediated autoimmunity. Proc. Natl. Acad. Sci. USA 2009, 106, 14948–14953. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowska-Durczyńska, K.; Pacholczyk, M.; Zygmunt, A.; Krawczyk-Rusiecka, K.; Ferenc, T.; Lewiński, A. Angiotensinogen gene T174M polymorphism is related to Hashimoto’s thyroiditis. Neuro Endocrinol. Lett. 2019, 39, 579–585. [Google Scholar] [PubMed]
- Smith, K.R.; Mehta, S. Comparative risk assessments for indoor air pollution in India. J. Environ. Stud. Policy 2002, 5, 71. [Google Scholar]
- Lifton, R.P.; Gharavi, A.G.; Geller, D.S. Molecular mechanisms of human hypertension. Cell 2001, 104, 545–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavoie, J.L.; Sigmund, C.D. Minireview: Overview of the renin-angiotensin system—An endocrine and paracrine system. Endocrinology 2003, 144, 2179–2183. [Google Scholar] [CrossRef]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef]
- Kooffreh, M.E.; Anumudu, C.I. Increased plasma angiotensinogen levels and its association with the M235T gene polymorphism and hypertension in Calabar and Uyo cities, Nigeria. Asian J. Biomed. Pharm. Sci. 2014, 3, 15–18. [Google Scholar]
- Yuan, J.; Tang, W.; Chun, Y.; Ying, H.; Yang, Y.; Xiao, C. Angiotensinogen T174M and M235T variants and hypertension in the Hani and Yi minority groups of China. Biochem. Genet. 2009, 47, 344–350. [Google Scholar] [CrossRef]
- Zeng, R.; Wang, Q.P.; Fang, M.X.; Zhuang, J.; Fan, R.X. Association of A-20C polymorphism in the angiotensinogen gene with essential hypertension: A meta-analysis. Genet. Mol. Res. 2015, 14, 12984–12992. [Google Scholar] [CrossRef]
- Tran, T.T.; Mai, T.P.; Tran, H.C.B.; Le, L.H.G.; Vu, H.A.; Tran, T.K.; Hoang, S.V.; Chau, H.N.; Do, M.D. Association between AGT M235T and left ventricular mass in Vietnamese patients diagnosed with essential hypertension. Front. Cardiovasc. Med. 2021, 8, 608948. [Google Scholar] [CrossRef]
- Dhanachandra Singh, K.; Jajodia, A.; Kaur, H.; Kukreti, R.; Karthikeyan, M. Gender Specific Association of RAS Gene Polymorphism with Essential Hypertension: A Case-Control Study. BioMed Res. Int. 2014, 2014, 538053. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.L.; Mo, Y.P.; He, Y.S.; Yang, F.; Xu, Y.; Li, C.C.; Wang, J.; Reng, H.M.; Long, L. Correlation between renin-angiotensin system gene polymorphisms and essential hypertension in the Chinese Yi ethnic group. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 975–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramu, P.; Umamaheswaran, G.; Shewade, D.G.; Swaminathan, R.P.; Dutta, T.K.; Balachander, J.; Adithana, C. Candidate Gene Polymorphisms of Renin Angiotensin System and Essential Hypertension in a South Indian Tamilian Population. Int. J. Hum. Genet. 2011, 11, 31–40. [Google Scholar] [CrossRef]
- Li, Y.-Y. Lack of association of A-6G polymorphism of AGT gene with essential hypertension in the Chinese population. J. Cardiovasc. Med. 2012, 13, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, J.; Berg, T. The etiology, diagnosis and prevention of liver cirrhosis: Part 1 of a series on liver cirrhosis. Dtsch. Arztebl. Int. 2013, 110, 85–91. [Google Scholar]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Desmet, V.J.; Roskams, T. Cirrhosis reversal: A duel between dogma and myth. J. Hepatol. 2004, 40, 860–867. [Google Scholar] [CrossRef]
- Lu, P.; Liu, H.; Yin, H.; Yang, L. Expression of angiotensinogen during hepatic fibrogenesis and its effect on hepatic stellate cells. Med. Sci. Monit. 2011, 17, Br248–Br256. [Google Scholar] [CrossRef] [Green Version]
- Friedman, S.L. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat. Clin. Pract. Gastroenterol. Hepatol. 2004, 1, 98–105. [Google Scholar] [CrossRef]
- Yoshiji, H.; Kuriyama, S.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Yanase, K.; Namisaki, T.; Yamazaki, M.; Tsujinoue, H.; Imazu, H.; et al. Angiotensin-II induces the tissue inhibitor of metalloproteinases-1 through the protein kinase-C signaling pathway in rat liver fibrosis development. Hepatol. Res. 2003, 27, 51–56. [Google Scholar] [CrossRef]
- Cavaliere, A.F.; Perelli, F.; Zaami, S.; Piergentili, R.; Mattei, A.; Vizzielli, G.; Scambia, G.; Straface, G.; Restaino, S.; Signore, F. Towards Personalized Medicine: Non-Coding RNAs and Endometrial Cancer. Healthcare 2021, 9, 965. [Google Scholar] [CrossRef]
- Anaya, J.M.; Duarte-Rey, C.; Sarmiento-Monroy, J.C.; Bardey, D.; Castiblanco, J.; Rojas-Villarraga, A. Personalized medicine. Closing the gap between knowledge and clinical practice. Autoimmun. Rev. 2016, 15, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Piergentili, R.; Zaami, S.; Cavaliere, A.F.; Signore, F.; Scambia, G.; Mattei, A.; Marinelli, E.; Gulia, C.; Perelli, F. Non-Coding RNAs as Prognostic Markers for Endometrial Cancer. Int. J. Mol. Sci. 2021, 22, 3151. [Google Scholar] [CrossRef] [PubMed]
- Peneş, N.O.; Weber, B.; Păun, S.D. Role of genetic polymorphism in nutritional supplementation therapy in personalized medicine. Rom. J. MorpholEmbryol. 2017, 58, 53–58. [Google Scholar]
- Tavakolpour, S. Towards personalized medicine for patients with autoimmune diseases: Opportunities and challenges. Immunol. Lett. 2017, 190, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, Y. Association between angiotensinogen T174M polymorphism and the risk of diabetic nephropathy: A meta-analysis. J. Renin. Angiotensin Aldosterone Syst. 2019, 20, 1470320318823927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kravets, Y.B.; Yurchenko, Y.V.; Freidin, M.B.; Samoilova, Y.G.; Solodilova, Y.A. Relationship of the I/D-polymorphism of the ACE gene and the T174M-polymorphism of the AGT gene to diabetic microangiopathies in children and adolescents. Probl. Endokrinol. (Mosk.) 2006, 52, 3–6. [Google Scholar] [PubMed]
- Hu, P.Y.; Wang, Y.W.; Pang, X.H.; Wang, H.W. T174M polymorphism in the angiotensinogen gene and risk of myocardial infarction: A meta-analysis. Genet. Mol. Res. 2015, 14, 3767–3774. [Google Scholar] [CrossRef]
- Ou, Z.; Chen, H.; Liu, G.; Li, C.; Lin, S.; Lin, J. Association between angiotensinogen T174M polymorphism and ischemic stroke: A meta-analysis. J. Res. Med. Sci. 2015, 20, 619–623. [Google Scholar]
- Stetskaia, T.A.; Bushueva, O.I.; Bulgakova, I.V.; Vialykh, E.K.; Shuteeva, T.V.; Biriukov, A.E.; Ivanov, V.P.; Polonikov, A.V. Association of T174M polymorphism of the angiotensinogen gene with the higher risk of cerebral stroke in women. Ter. Arkh. 2014, 86, 66–71. [Google Scholar] [CrossRef]
- Phillips, M.I.; de Oliveira, E.M. Brain renin angiotensin in disease. J. Mol. Med. 2008, 86, 715–722. [Google Scholar] [CrossRef]
- Wang, W.Z. Association between T174M polymorphism in the angiotensinogen gene and risk of coronary artery disease: A meta-analysis. J. Geriatr. Cardiol. 2013, 10, 59–65. [Google Scholar] [PubMed]
- Li, X.; Li, Q.; Wang, Y.; Li, Y.; Ye, M.; Ren, J.; Wang, Z. AGT gene polymorphisms (M235T, T174M) are associated with coronary heart disease in a Chinese population. J. Renin. Angiotensin Aldosterone Syst. 2013, 14, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Kuken, B.; Yang, Y.; Liu, Z.; He, P.; Wulasihan, M. Relationship between M235T and T174M polymorphisms in angiotensin gene and atrial fibrillation in Uyghur and Han populations of Xinjiang, China. Int. J. Clin. Exp. Pathol. 2020, 13, 2065–2074. [Google Scholar] [PubMed]
- Tsai, C.T.; Lai, L.P.; Lin, J.L.; Chiang, F.T.; Hwang, J.J.; Ritchie, M.D.; Moore, J.H.; Hsu, K.L.; Tseng, C.D.; Liau, C.S.; et al. Renin-angiotensin system gene polymorphisms and atrial fibrillation. Circulation 2004, 109, 1640–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zitouni, H.; Ben Ali Gannoum, M.; Raguema, N.; Maleh, W.; Zouari, I.; Faleh, R.E.; Guibourdenche, J.; Almawi, W.Y.; Mahjoub, T. Contribution of angiotensinogen M235T and T174M gene variants and haplotypes to preeclampsia and its severity in (North African) Tunisians. J. Renin. Angiotensin Aldosterone Syst. 2018, 19, 1470320317753924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardemann, A.; Stricker, J.; Humme, J.; Nguyen, Q.D.; Katz, N.; Philipp, M.; Tillmanns, H.; Hehrlein, F.W.; Haberbosch, W. Angiotensinogen T174M and M235T gene polymorphisms are associated with the extent of coronary atherosclerosis. Atherosclerosis 1999, 145, 309–314. [Google Scholar] [CrossRef]
- Husain, K.; Hernandez, W.; Ansari, R.A.; Ferder, L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J. Biol. Chem. 2015, 6, 209. [Google Scholar] [CrossRef]
- Fajar, J.K.; Pikir, B.S.; Sidarta, E.P.; Saka, P.N.B.; Akbar, R.R.; Tamara, F.; Mayasari, E.D.; Gunawan, A.; Heriansyah, T. The genes polymorphism of angiotensinogen (AGT) M235T and AGT T174M in patients with essential hypertension: A meta-analysis. Gene Rep. 2019, 16, 100421. [Google Scholar] [CrossRef]
- Ni, S.; Zhang, Y.; Deng, Y.; Gong, Y.; Huang, J.; Bai, Y.; Zhou, R. AGT M235T polymorphism contributes to risk of preeclampsia: Evidence from a meta-analysis. J. Renin. Angiotensin Aldosterone Syst. 2012, 13, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Wen, M.; Mi, L.; Hu, C.J.; Zhang, Y.; Wang, J.T.; Tang, L. Associations between angiotensinogen M235T polymorphisms and the risk of diabetic nephropathy: A meta-analysis. Diabetes Res. Clin. Pract. 2018, 142, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Saud, C.G.M.; Reis, A.F.D.; Dias, A.M.D.C.; Cardoso, R.N.; Carneiro, A.C.K.V.; Souza, L.P.D.; Fonseca, A.B.M.; Ribeiro, G.S.; Faria, C.A.C.D. AGT*M235T polymorphism in acute ischemic cardiac dysfunction: The gisca project. Arq. Bras. Cardiol. 2010, 95, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imen, T.; Grissa, M.H.; Boubaker, H.; Beltaief, K.; Messous, S.; Tounsi, N.; Slimani, A.; Bouida, W.; Boukef, R.; Slimene, M.N.; et al. AGT M235t polymorphism and heart failure in a cohort of Tunisian population: Diagnostic and prognostic value. Int. J. Clin. Exp. Med. 2015, 8, 16346–16351. [Google Scholar] [PubMed]
- Zhen, Z.; Gao, L.; Wang, Q.; Chen, X.; Na, J.; Xu, X.; Yuan, Y. Angiotensinogen M235T polymorphism and susceptibility to hypertrophic cardiomyopathy in Asian population: A meta-analysis. J. Renin. Angiotensin Aldosterone Syst. 2020, 21, 1470320320978100. [Google Scholar] [CrossRef]
- Liang, B.; Qin, L.; Wei, H.; Yan, Y.; Su, L.; Wu, G.; Tan, J.; Gu, L. AGT M235T polymorphisms and ischemic stroke risk: A meta-analysis. J. Neurol. Sci. 2013, 331, 118–125. [Google Scholar] [CrossRef]
- Zafarmand, M.H.; Van Der Schouw, Y.T.; Grobbee, D.E.; De Leeuw, P.W.; Bots, M.L. The M235T polymorphism in the AGT gene and CHD risk: Evidence of a Hardy-Weinberg equilibrium violation and publication bias in a meta-analysis. PLoS ONE 2008, 3, e2533. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Pan, Y. The M235T polymorphism in the angiotensinogen gene and myocardial infarction risk: A meta-analysis. J. Renin. Angiotensin Aldosterone Syst. 2014, 15, 294–300. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Quignard-Boulangé, A. Role of adipose tissue renin–angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011, 79, 162–168. [Google Scholar] [CrossRef]
- López-León, S.; Janssens, A.C.J.; Tiemeier, H.; Hofman, A.; Aulchenko, Y.S.; Snijders, P.J.; Claes, S.; Oostra, B.A.; van Duijn, C.M. Angiotensinogen M235T polymorphism and symptoms of depression in a population-based study and a family-based study. Psychiatr. Genet. 2008, 18, 162–166. [Google Scholar] [CrossRef]
- Taylor, W.D.; Benjamin, S.; McQuoid, D.R.; Payne, M.E.; Krishnan, R.R.; MacFall, J.R.; Ashley-Koch, A. AGTR1 gene variation: Association with depression and frontotemporal morphology. Psychiatry Res. 2012, 202, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Xi, B.; Shen, Y.; Yan, Y.; Mi, J. Association of polymorphisms in the AGT gene with essential hypertension in the Chinese population. J. Renin. Angiotensin Aldosterone Syst. 2012, 13, 282–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.J.; Chiang, F.T.; Jiang, J.R.; Hsu, K.L.; Chern, T.H.; Tseng, Y.Z. The G-217A variant of the angiotensinogen gene affects basal transcription and is associated with hypertension in a Taiwanese population. J. Hypertens. 2003, 21, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Q.; Hu, Y.; Xu, F. Studies on the association of angiotensinogen polymorphisms and hypoxia acclimatization. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2016, 32, 314–318. [Google Scholar]
- Alaee, E.; Mirahmadi, M.; Ghasemi, M.; Kashani, E.; Attar, M.; Shahbazi, M. Association study of M235T and A-6G polymorphisms in angiotensinogen gene with risk of developing preeclampsia in Iranian population. Ann. Hum. Genet. 2019, 83, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Chaves, F.J.; Giner, V.; Corella, D.; Pascual, J.; Marin, P.; Armengod, M.E.; Redon, J. Body weight changes and the A-6G polymorphism of the angiotensinogen gene. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1173–1178. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Wei, H.; Song, S.; Li, G.; Song, C. Polymorphisms in the promoter region of the angiotensinogen gene are associated with liver cirrhosis in patients with chronic hepatitis B. J. Gastroenterol. Hepatol. 2006, 21, 1488–1491. [Google Scholar] [CrossRef]
- Saidi, S.; Mallat, S.G.; Almawi, W.Y.; Mahjoub, T. Association between renin-angiotensin-aldosterone system genotypes and haplotypes and risk of ischemic stroke of atherosclerotic etiology. Acta Neurol. Scand. 2009, 119, 356–363. [Google Scholar] [CrossRef]
- Gorący, I.; Gorący, A.; Kaczmarczyk, M.; Rosik, J.; Lewandowska, K.; Ciechanowicz, A. The Genetic Variants in the Renin-Angiotensin System and the Risk of Heart Failure in Polish Patients. Genes 2022, 13, 1257. [Google Scholar] [CrossRef]
- Mtiraoui, N.; Ezzidi, I.; Turki, A.; Chaieb, M.; Mahjoub, T.; Almawi, W.Y. Renin-angiotensin-aldosterone system genotypes and haplotypes affect the susceptibility to nephropathy in type 2 diabetes patients. J. Renin. Angiotensin Aldosterone Syst. 2011, 12, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Niu, W.; Qi, Y.; Cen, W.; Cui, C.; Zhuoma, C.; Cai, D.; Zhou, W.; Qiu, C. Genetic polymorphisms of angiotensinogen and essential hypertension in a Tibetan population. Hypertens. Res. 2007, 30, 1129–1137. [Google Scholar] [CrossRef]
- Cooper Worobey, C.; Fisher, N.D.; Cox, D.; Forman, J.P.; Curhan, G.C. Genetic polymorphisms and the risk of accelerated renal function decline in women. PLoS ONE 2009, 4, e4787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Lin, J.; Wang, C.; Yang, M.; Lv, H.; Yang, M.; Xu, B.; Chen, X.; Jiang, J. The relationship among angiotensinogen genes polymorphisms and hs-CRP and coronary artery disease. J. Clin. Lab. Anal. 2019, 33, e22881. [Google Scholar] [CrossRef] [PubMed]
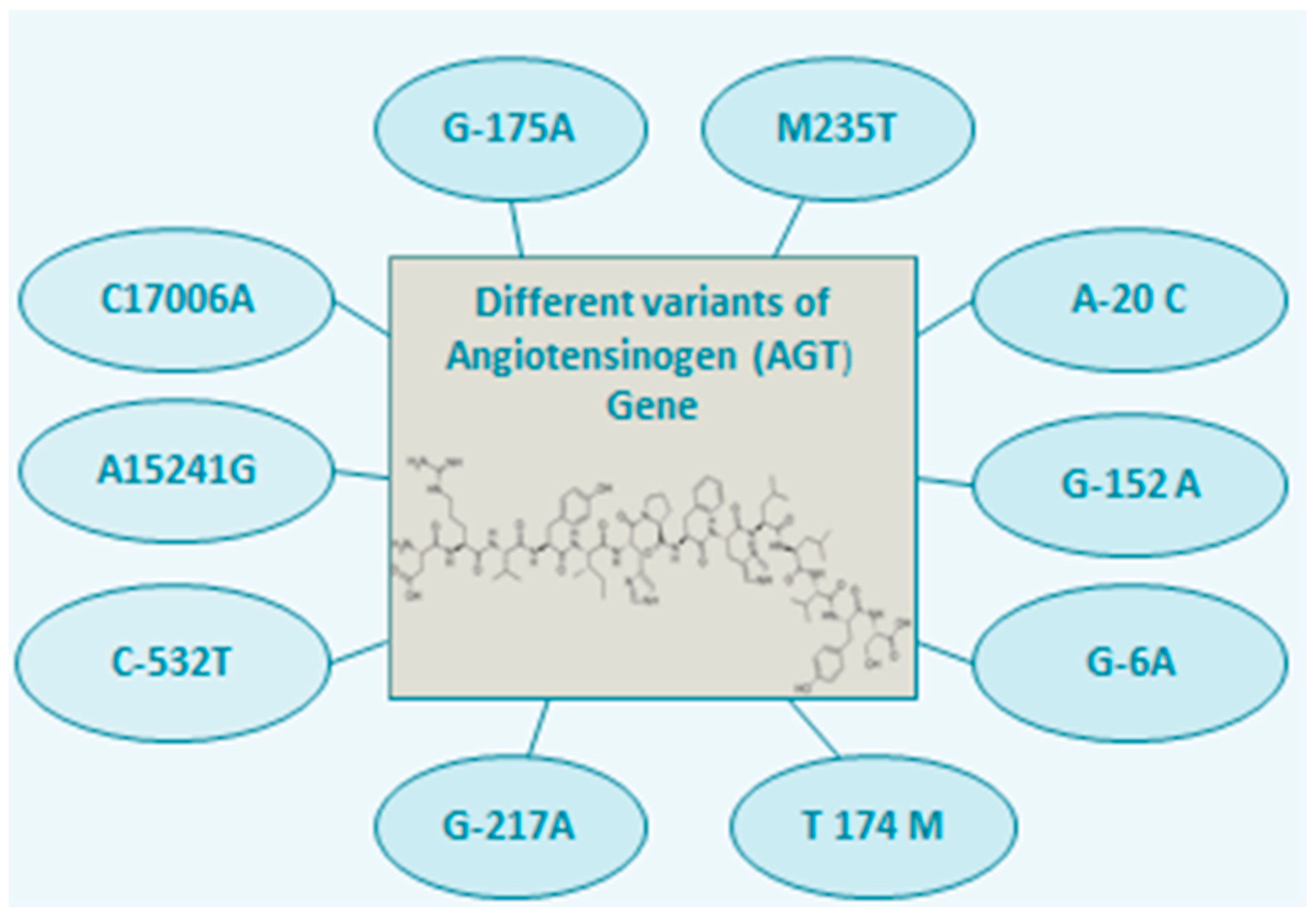
| Gene Variant | Associated Diseases | Mechanism (Due to High Serum Level of AGT) | Ref. |
|---|---|---|---|
| AGT T174M | Diabetic Nephropathy | Increased peripheral and renal resistance, oxidative stress and endothelial dysfunction | [96] |
| Diabetic Microangiopathy | Altered cell matrix, vasoconstriction and impaired vascular tone regulation | [97] | |
| Myocardial Infarction | Cardiac fibrosis, collagen synthesis and modified growth of cardiac fibroblast led to the development of atherosclerosis | [98] | |
| Ischemic Stroke | Activation of vascular cell apoptosis, and vasoconstriction | [99] | |
| Hoshimoto’s Thyroiditis | Autoimmune neuroinflammation | [72] | |
| Cerebral Stroke | Neurogenic hypertension | [100,101] | |
| Coronary Artery Disease | Myocardial hypertrophy, vasoconstriction and fibrosis | [102,103] | |
| Arterial Fibrillation | Stimulation of mitogen-activated protein kinase pathway | [104,105] | |
| Pre-eclampsia | Vasoconstriction and dysregulation of salt and water homeostasis | [106,37] | |
| Coronary Atherosclerosis | Endothelial injury due to oxidative stress (oxidation of NADPH generates the oxidative species which further oxidizes the DNA, lipids and lipoproteins leading to endothelial damage) | [107,108] | |
| Hypertension | Vasoconstriction and increased vascular resistance | [22,109] | |
| AGT M235T | Pre-eclampsia | Vasoconstriction and dysregulation of salt and water homeostasis | [110] |
| Diabetic Nephropathy | Increased peripheral and renal resistance, proteinuria and oxidative stress lead to endothelial damage | [111] | |
| Acute Ischemic Cardiac Dysfunction | Modification of extracellular matrix and myocytes in the heart | [112] | |
| Heart Failure | Sodium retention, myocardial fibrosis and vasoconstriction | [113] | |
| Hypertrophic Cardiomyopathy | Angiotensin II exerts positive inotropic, apoptotic and hypertrophic effects | [114] | |
| Ischemic Stroke | Activation of vascular cell apoptosis, and vasoconstriction | [115] | |
| Chronic Heart Disease (CHD) | Increased vasoconstriction and sodium retention leads to hypertension which promotes the progression of CHD | [116] | |
| Myocardial Infarction | Cardiac fibrosis, collagen synthesis and modified growth of cardiac fibroblast led to the development of atherosclerosis | [117] | |
| Obesity and dyslipidemia | Upregulation of lipid synthesis through AT II receptors and downregulation of lipolysis through AT I receptors | [118,48] | |
| Depression | Increased plasma level of AGT produces increased corticotrophin releasing factor which stimulates the hypothalamic-pituitary-adrenal, regulation of norepinephrine, increased release of cytokines, and inhibition of neurogenesis process | [119,120] | |
| AGT G-217A | Essential Hypertension | Vasoconstriction and increased vascular resistance | [16,121,122] |
| Hypoxia acclimatization | Increased oxygen saturation | [123] | |
| AGT A-6G | Pre-eclampsia | Vasoconstriction and dysregulation of salt and water homeostasis | [124] |
| Obesity | Increased expression of AGT in adipose tissues which is thought to be associated with the development of these tissues. The metabolic alteration may also occur | [125] | |
| Liver Cirrhosis | AGT activates the Hepatic Stellate Cells (HSC) resulting in increasing the expression of Transforming Growth Factor β-1 (TGFβ-1) and Tissue inhibitor of Metallopeptidase 1(TIMP1) and reduces the degradation of collagen which leads to liver cirrhosis | [89,126] | |
| Ischemic stroke of atherosclerotic | Activation of vascular cell apoptosis, and vasoconstriction | [127] | |
| Heart failure | Modification in vascular structure, hemodynamic disturbances and left ventricular hypertrophy | [128] | |
| Diabetic nephropathy | Sclerosis of the glomerulus, increased cellular growth and proliferation leading to renal damage | [129] | |
| AGT A-20C | Essential hypertension | Vasoconstriction and increased vascular resistance | [80,130] |
| Decline in renal function | Reduction in glomerular filtration rate due to vasoconstriction and altered systolic and diastolic pressure | [131] | |
| Liver Cirrhosis | AGT activates the Hepatic Stellate Cells (HSC) resulting in increasing the expression of Transforming Growth Factor β-1 (TGFβ-1) and Tissue inhibitor of Metallopeptidase 1(TIMP1) and reduces the degradation of collagen which leads to liver cirrhosis | [89,125] | |
| AGT G152A | Coronary artery disease | Vasoconstriction, increased resistance in a coronary artery, the proliferation of myocardial cells, and stimulation of the sympathetic nervous system. | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, M.; Rehman, K.; Akash, M.S.H.; Suhail, S.; Kamal, S.; Imran, M.; Assiri, M.A. Genetic Polymorphism in Angiotensinogen and Its Association with Cardiometabolic Diseases. Metabolites 2022, 12, 1291. https://doi.org/10.3390/metabo12121291
Shahid M, Rehman K, Akash MSH, Suhail S, Kamal S, Imran M, Assiri MA. Genetic Polymorphism in Angiotensinogen and Its Association with Cardiometabolic Diseases. Metabolites. 2022; 12(12):1291. https://doi.org/10.3390/metabo12121291
Chicago/Turabian StyleShahid, Momina, Kanwal Rehman, Muhammad Sajid Hamid Akash, Shaleem Suhail, Shagufta Kamal, Muhammad Imran, and Mohammed A. Assiri. 2022. "Genetic Polymorphism in Angiotensinogen and Its Association with Cardiometabolic Diseases" Metabolites 12, no. 12: 1291. https://doi.org/10.3390/metabo12121291
APA StyleShahid, M., Rehman, K., Akash, M. S. H., Suhail, S., Kamal, S., Imran, M., & Assiri, M. A. (2022). Genetic Polymorphism in Angiotensinogen and Its Association with Cardiometabolic Diseases. Metabolites, 12(12), 1291. https://doi.org/10.3390/metabo12121291








