Identification of D-Limonene Metabolites by LC-HRMS: An Exploratory Metabolic Switching Approach in a Mouse Model of Diet-Induced Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Animal Study
2.3. Analysis of D-Limonene in Essential Oil and Feed
2.3.1. Analysis of Essential Oil Rich in D-Limonene GC-MS
2.3.2. Sample Extraction
2.3.3. Quantification of D-Limonene by GC-FID
2.4. Identification of Limonene Metabolites by LC-ESI-qToF-MS/MS
2.4.1. Sample Extraction
2.4.2. Identification of D-Limonene Metabolites by LC-HRMS
2.5. Creatinine Analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of D-Limonene-Supplemented Diet on Body Weight Gain and Feed Intake
3.2. Quantification of D-Limonene Intake
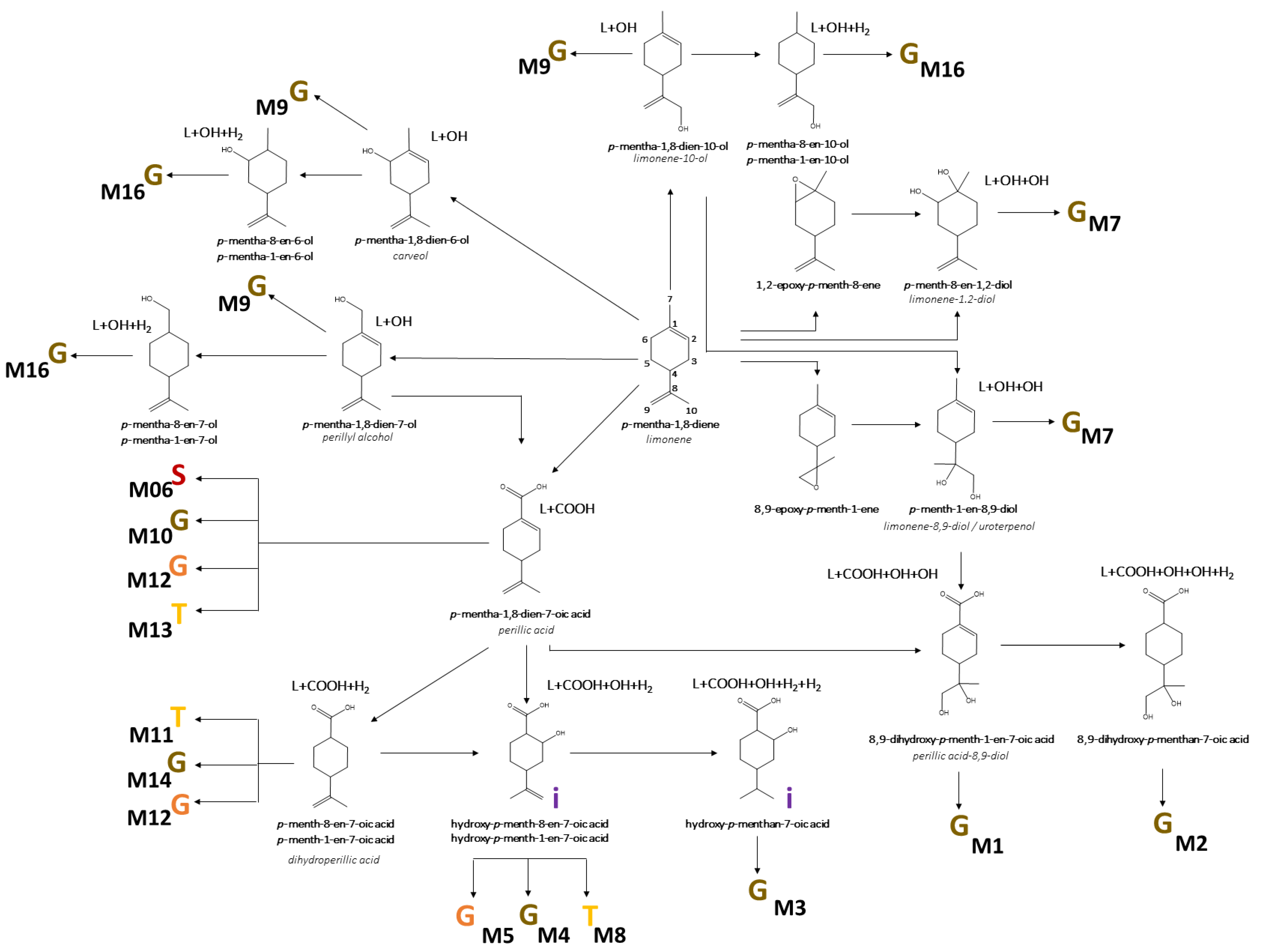
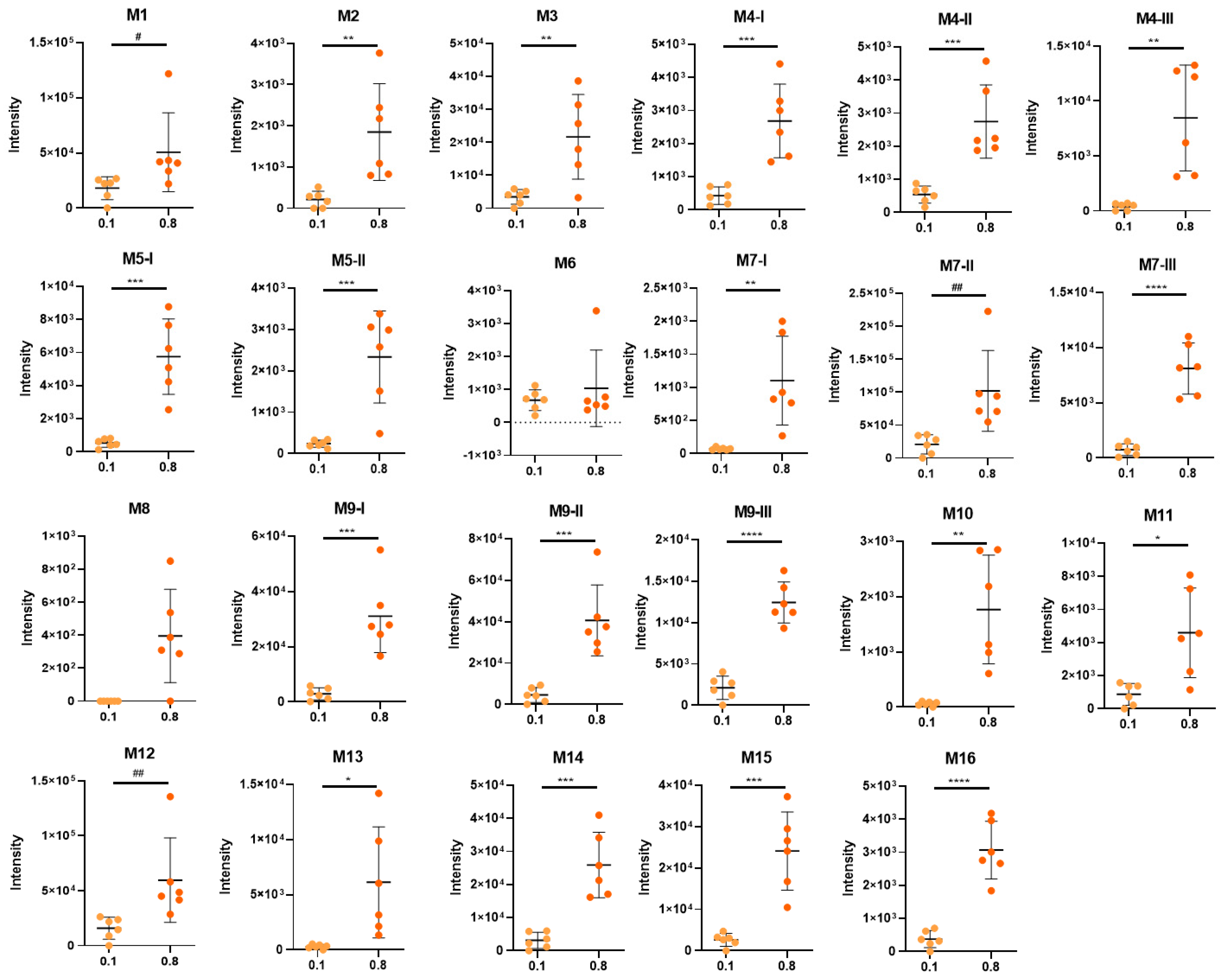
3.3. Identification of D-Limonene Metabolites
3.4. Biotransformation of D-Limonene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goff, S.A.; Klee, H.J. Plant Volatile Compounds: Sensory Cues for Health and Nutritional Value? Science 2006, 311, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Cohen, S.M.; Fukushima, S.; Gooderham, N.J.; Hecht, S.S.; Guengerich, F.P.; Rietjens, I.M.C.M.; Bastaki, M.; Harman, C.L.; McGowen, M.M.; et al. The Safety Evaluation of Food Flavouring Substances: The Role of Metabolic Studies. Toxicol. Res. 2018, 7, 618–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oz, M.; Lozon, Y.; Sultan, A.; Yang, K.H.S.; Galadari, S. Effects of Monoterpenes on Ion Channels of Excitable Cells. Pharmacol. Ther. 2015, 152, 83–97. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Quintans, J.S.S.; Shanmugam, S.; Heimfarth, L.; Araújo, A.A.S.; Almeida, J.R.G.d.S.; Picot, L.; Quintans-Júnior, L.J. Monoterpenes Modulating Cytokines—A Review. Food Chem. Toxicol. 2019, 123, 233–257. [Google Scholar] [CrossRef] [PubMed]
- de Alvarenga, J.F.R.; Genaro, B.; Costa, B.L.; Purgatto, E.; Manach, C.; Fiamoncini, J. Monoterpenes: Current Knowledge on Food Source, Metabolism, and Health Effects. Crit. Rev. Food Sci. Nutr. 2021, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Antidiabetic Potential of Monoterpenes: A Case of Small Molecules Punching above Their Weight. Int. J. Mol. Sci. 2017, 19, 4. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of Innovation in Health and Disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Sun, J. D-Limonene: Safety and Clinical Applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Li, D.; Wu, H.; Dou, H. Weight Loss Effect of Sweet Orange Essential Oil Microcapsules on Obese SD Rats Induced by High-Fat Diet. Biosci. Biotechnol. Biochem. 2019, 83, 923–932. [Google Scholar] [CrossRef]
- Valerii, M.C.; Turroni, S.; Ferreri, C.; Zaro, M.; Sansone, A.; Dalpiaz, A.; Botti, G.; Ferraro, L.; Spigarelli, R.; Bellocchio, I.; et al. Effect of a Fiber D-Limonene-Enriched Food Supplement on Intestinal Microbiota and Metabolic Parameters of Mice on a High-Fat Diet. Pharmaceutics 2021, 13, 1753. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Zhang, Y.; Fan, S.; Gu, M.; Guan, Y.; Lu, X.; Huang, C.; Zhou, Z. Preventive and Ameliorating Effects of Citrus D-Limonene on Dyslipidemia and Hyperglycemia in Mice with High-Fat Diet-Induced Obesity. Eur. J. Pharm. 2013, 715, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.V.A.; Jayachitra, J.; Shenbagam, M.; Nalini, N. Dietary D-Limonene Alleviates Insulin Resistance and Oxidative Stress-Induced Liver Injury in High-Fat Diet and L-NAME-Treated Rats. Eur. J. Nutr. 2012, 51, 57–68. [Google Scholar] [CrossRef]
- Bacanlı, M.; Anlar, H.G.; Aydın, S.; Çal, T.; Arı, N.; Ündeğer Bucurgat, Ü.; Başaran, A.A.; Başaran, N. D-Limonene Ameliorates Diabetes and Its Complications in Streptozotocin-Induced Diabetic Rats. Food Chem. Toxicol. 2017, 110, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Kornbausch, N.; Debong, M.W.; Buettner, A.; Heydel, J.; Loos, H.M. Odorant Metabolism in Humans. Angew. Chem. Int. Ed. 2022, 61, e202202866. [Google Scholar] [CrossRef] [PubMed]
- Rendic, S. Summary of Information on Human CYP Enzymes: Human P450 Metabolism Data. Drug Metab. Rev. 2002, 34, 83–448. [Google Scholar] [CrossRef]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The Role of Reporting Standards for Metabolite Annotation and Identification in Metabolomic Studies. Gigascience 2013, 2, 13. [Google Scholar] [CrossRef]
- FDA Guidance for Industry: Characterization and Qualification of Cell Substrates and Other Biological Starting Materials Used in the Production of Viral Vaccines for the Prevention and Treatment of Infectious Diseases; FDA: Silver Spring, MD, USA, 2005.
- Hakim, I.A.; Hartz, V.; Graver, E.; Whitacre, R.; Alberts, D. Development of a Questionnaire and a Database for Assessing Dietary D-Limonene Intake. Public Health Nutr. 2002, 5, 939–945. [Google Scholar] [CrossRef] [Green Version]
- Djoumbou-Feunang, Y.; Fiamoncini, J.; Gil-de-la-Fuente, A.; Greiner, R.; Manach, C.; Wishart, D.S. BioTransformer: A Comprehensive Computational Tool for Small Molecule Metabolism Prediction and Metabolite Identification. J. Cheminform. 2019, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Kind, T.; Fiehn, O. Advances in Structure Elucidation of Small Molecules Using Mass Spectrometry. Bioanal. Rev. 2010, 2, 23–60. [Google Scholar] [CrossRef] [Green Version]
- Kodama, R.; Yano, T.; Furukawa, K.; Noda, K.; Ide, H. Studies on the Metabolism of d-Limonene (p-Mentha-1, 8-Diene). IV Isolation and Characterization of New Metabolites and Species Differences in Metabolism. Xenobiotica 1976, 6, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Igimi, H.; Nishimura, M.; Kodama, R.; Ide, H. Studies on the Metabolism of D-Limonene (p-Mentha-1,8-Diene): I. The Absorption, Distribution and Excretion of d-Limonene in Rats. Xenobiotica 1974, 4, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.W.; Bjeldanes, L.F. Metabolism of (+)-Limonene in Rats. J. Agric. Food Chem. 1976, 24, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P.L.; Elson, C.E.; Bailey, H.H.; Elegbede, A.; Haag, J.D.; Gould, M.N. Human Metabolism of the Experimental Cancer Therapeutic Agent D-Limonene. Cancer Chemother. Pharm. 1994, 35, 31–37. [Google Scholar] [CrossRef]
- Vigushin, D.M.; Poon, G.K.; Boddy, A.; English, J.; Halbert, G.W.; Pagonis, C.; Jarman, M.; Coombes, R.C. Phase I and Pharmacokinetic Study of D-Limonene in Patients with Advanced Cancer. Cancer Chemother. Pharm. 1998, 42, 111–117. [Google Scholar] [CrossRef]
- Schmidt, L.; Göen, T. R-Limonene Metabolism in Humans and Metabolite Kinetics after Oral Administration. Arch. Toxicol. 2017, 91, 1175–1185. [Google Scholar] [CrossRef]
- Watabe, T.; Hiratsuka, A.; Ozawa, N.; Isobe, M. A Comparative Study on the Metabolism of D-Limonene and 4-Vinylcyclohex-1-Ene by Hepatic Microsomes. Xenobiotica 1981, 11, 333–344. [Google Scholar] [CrossRef]
- Pujos-Guillot, E.; Hubert, J.; Martin, J.F.; Lyan, B.; Quintana, M.; Claude, S.; Chabanas, B.; Rothwell, J.A.; Bennetau-Pelissero, C.; Scalbert, A.; et al. Mass Spectrometry-Based Metabolomics for the Discovery of Biomarkers of Fruit and Vegetable Intake: Citrus Fruit as a Case Study. J. Proteome Res. 2013, 12, 1645–1659. [Google Scholar] [CrossRef]
- Shimada, T.; Shindo, M.; Miyazawa, M. Species Differences in the Metabolism of (+)- and (−)-Limonenes and Their Metabolites, Carveols and Carvones, by Cytochrome P450 Enzymes in Liver Microsomes of Mice, Rats, Guinea Pigs, Rabbits, Dogs, Monkeys, and Humans. Drug Metab. Pharm. 2002, 17, 507–515. [Google Scholar] [CrossRef]
- Manach, C.; Milenkovic, D.; van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; et al. Addressing the Inter-Individual Variation in Response to Consumption of Plant Food Bioactives: Towards a Better Understanding of Their Role in Healthy Aging and Cardiometabolic Risk Reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef] [Green Version]
- Lone, J.; Yun, J.W. Monoterpene Limonene Induces Brown Fat-like Phenotype in 3T3-L1 White Adipocytes. Life Sci. 2016, 153, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Soundharrajan, I.; Kim, D.H.; Srisesharam, S.; Kuppusamy, P.; Choi, K.C. R -Limonene Enhances Differentiation and 2-Deoxy-D-Glucose Uptake in 3T3-L1 Preadipocytes by Activating the Akt Signaling Pathway. Evid. Based Complement. Altern. Med. 2018, 2018, 4573254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.C.; Chua, K.H.; Ravishankar Ram, M.; Kuppusamy, U.R. Monoterpenes: Novel Insights into Their Biological Effects and Roles on Glucose Uptake and Lipid Metabolism in 3T3-L1 Adipocytes. Food Chem. 2016, 196, 242–250. [Google Scholar] [CrossRef] [PubMed]


| Compound | rt | Relative Peak Area (%) | Fragments Ions a |
|---|---|---|---|
| α-pinene pin-2,3-ene | 9.12 | 0.57 | 136 (8), 121 (13), 105 (11), 93 (100), 92 (38), 91 (41), 79 (24), 77 (29), 67 (9), 53 (7) |
| β-phellandrene p-menthane-2,7-diene | 10.43 | 0.75 | 136 (13), 121 (5), 105 (3), 93 (100), 92 (10), 91 (40), 79 (27), 77 (35), 69 (9), 53 (7) |
| β-pinene pin-2,10-ene | 11.07 | 1.52 | 136 (4), 121 (5), 107 (3), 93 (100), 92 (12), 91 (22), 79 (18), 77 (16), 69 (73), 53 (12) |
| octanal | 11.42 | 0.23 | 136 (4), 100 (16), 93 (22), 84 (72), 69 (51), 68 (38), 67 (29), 57 (100), 56 (89), 55 (78) |
| Δ3-carene car-3,4-ene | 11.56 | 0.14 | 136 (18), 121 (19), 105 (14), 93 (100), 92 (24), 91 (42), 80 (24), 79 (34), 77 (38), 67 (11) |
| m-cymene m-menthane-1,3,5-triene | 12.03 | 0.01 | 134 (22), 119 (100), 117 (16), 115 (8), 103 (2), 91 (24), 77 (11), 65 (8), 51 (6), 56 (6) |
| limonene p-mentha-1,8-diene | 12.26 | 96.45 | 136 (21), 121 (24), 107 (23), 93 (73), 92 (24), 91 (22), 79 (36), 67 (76), 68 (100), 53 (23) |
| γ-terpinene p-menthane-1,4-diene | 13.04 | 0.07 | 136 (36), 121 (30), 119 (14), 105 (11), 93 (100), 92 (26), 91 (55), 79 (27), 77 (36), 53 (9) |
| linalool 3,7-dimethylocta1,6-dien-3-ol | 14.21 | 0.27 | 154 (6), 136 (8), 121 (22), 105 (7), 93 (78), 83 (17), 80 (32), 71 (100), 69 (44), 55 (58) |
| ID | Metabolite | rt | [M-H]− | MS/MS | Error | [MF]-H | HL0.1% | HL0.8% | T |
|---|---|---|---|---|---|---|---|---|---|
| M1 | limonene+COOH+OH+OH glucuronide dihydroxy-p-mentha-1,8-dien-7-oic acid glucuronide perillic acid-8,9-diol glucuronide | 1.89 | 375.1304 | 375.1333 (20); 357.1104 (5); 183.1088 (5); 172.9903 (100); 160.0421 (15); 113.0224 (40); 93.0353 (85); 85.0291 (45); 75.0086 (65); 59.0141 (75) | −1.8 | C16H23O10 | 23.00 | 12.13 | U |
| M2 | limonene+COOH+OH+OH+H2 glucuronide dihydroxy-p-mentha-1-en-7-oic acid/dihydroxy-p-mentha-8-en-7-oic acid glucuronide | 2.01 | 377.1460 | 377.1469 (100); 204.0368 (10); 201.1180 (5); 178.0497 (20); 175.0236 (5); 172.9923 (60); 160.0399 (33); 113.0251 (30); 93.0344 (55); 85.0293 (35); 75.0087 (60); 71.0145 (70); 59.0147 (70) | −1.9 | C16H25O10 | 0.27 | 0.44 | U |
| M3 | limonene+COOH+OH+H2+H2 glucuronide dihydroxy-p-menthan-7-oic acid glucuronide | 2.83 | 361.1504 | 361.1500 (85); 301.1299 (5); 185.1181 (10); 175.0256 (3) 157.0128 (5); 113.0239 (60); 85.0294 (65); 71.0138 (90); 59.0141 (100) | −0.1 | C16H25O9 | 4.37 | 5.18 | U |
| M4-I | limonene+COOH+OH+H2 glucuronide I hydroxy-p-menth-8-en-7-oic acid glucuronide | 2.95 | 359.1344 | 359.1347 (35); 297.1331 (5); 241.1095 (5); 183.1047 (15); 175.0242 (5); 113.0241 (45); 99.0084 (15); 85.0290 (60); 75.0088 (100); 71.0131 (80); 59.0143 (95); 44.9989 (20) | 1.0 | C16H23O9 | 0.54 | 0.64 | U |
| M4-II | limonene+COOH+OH+H2 glucuronide II hydroxy-p-menth-8-en-7-oic acid glucuronide | 3.18 | 359.1345 | 359.1332 (10);315.1646 (5); 183.1020 (65); 175.0240 (5); 155.1070 (25); 137.0971 (10); 113.0249 (30); 99.0097 (10); 85.0294 (40); 71.0137 (45); 59.0137 (100); 44.9985 (20) | 0.8 | C16H23O9 | 0.68 | 0.66 | U |
| M5-I | limonene+COOH+OH+H2 glycine I hydroxy-p-menth-8-en-7-oic acid glycine | 3.41 | 240.1224 | 240.1228 (5); 178.1213 (1); 111.0801 (5); 74.0242 (100); 72.0453 (20); 56.0152 (2) | 7.2 | C12H18NO4 | 0.68 | 1.38 | U |
| M4-III | limonene+COOH+OH+H2 glucuronide III hydroxy-p-menth-8-en-7-oic acid glucuronide | 3.57 | 359.1326 | 183.1015 (100); 175.0243 (5); 137.0965 (30); 113.0233 (45); 99.0080 (15); 85.0292 (35); 71.0137 (30); 59.0138 (80); 44.9983 (30) | 6.0 | C16H23O9 | 0.50 | 2.02 | U |
| M6 | limonene+COOH sulfate p-mentha-1,8-dien-7-oic acid sulfate perillic acid sulfate | 3.62 | 245.0490 | 245.0517 (20); 165.0919 (10); 79.9569 (20); 74.0241 (20); 59.0151 (45) | −0.3 | C10H13O5S | 0.85 | 0.25 | U |
| M5-II | limonene+COOH+OH+H2 glycine II hydroxy-p-menth-8-en-7-oic acid glycine | 3.62 | 240.1229 | 240.1229 (5); 178.1244 (1); 111.0793 (5); 74.0244 (100); 72.0446 (20); 56.0148 (2) | 5.3 | C12H18NO4 | 0.30 | 0.56 | U |
| M7-I | limonene+OH+OH glucuronide I p-menth-8-en-1,2-diol/p-menth-1-en-8,9-diol glucuronide limonene-1,2-diol/limonene-8,9-diol (uroterpenol) glucuronide | 3.71 | 345.1550 | 345.1546 (50); 327.1414 (5); 285.1317 (5); 269.1388 (5); 193.0382 (5); 175.0227 (5); 169.1228 (5); 157.0139 (10); 113.0236 (60); 99.0082 (10); 85.0291 (70); 75.0084 (100); 59.0138 (60) | 1.4 | C16H25O8 | 0.09 | 0.26 | U |
| M7-II | limonene+OH+OH glucuronide II p-menth-8-en-1,2-diol/p-menth-1-en-8,9-diol glucuronide limonene-1,2-diol/limonene-8,9-diol (uroterpenol) glucuronide | 3.80 | 345.1548 | 345.1537 (50); 327.1414 (5); 285.1324 (5); 269.1388 (5); 193.0382 (5); 175.0227 (5); 169.1207 (5); 157.0133 (10); 113.0237 (60); 99.0082 (10); 85.0291 (70); 75.0084 (100); 59.0141 (60) | 2.1 | C16H25O8 | 26.15 | 24.36 | U, L |
| M8 | limonene+COOH+OH+H2 taurine I hydroxy-p-menth-8-en-7-oic acid taurine | 3.92 | 290.1054 | 290.2051 (45); 152.0006 (10); 124.0063 (30); 106.9814 (10); 79.9570 (100); 74.0242 (85) | 4.6 | C12H20NO5S | 0.00 | 0.09 | U |
| M7-III | limonene+OH+OH glucuronide III p-menth-8-en-1,2-diol/p-menth-1-en-8,9-diol glucuronide limonene-1,2-diol/limonene-8,9-diol (uroterpenol) glucuronide | 4.13 | 345.1538 | n.d. | 5.0 | C16H25O8 | 0.95 | 1.94 | U |
| M9-I | limonene+OH glucuronide I p-mentha-1,8-dien-1-ol/p-mentha-1,8-dien-6-ol/p-mentha-1,8-dien-7-ol/p-mentha-1,8-dien-10-ol glucuronide p-mentha-1,8-dien-1-ol/carveol/perillyl alcohol/limonene-10-ol glucuronide | 4.36 | 327.1437 | 327.1439 (20); 309.1323 (5); 209.1191 (5); 175.0247 (3); 157.0141 (5); 151.1132 (2); 113.0233 (30); 85.0290 (60); 75.0085 (100); 59.0139 (80) | 4.1 | C16H23O7 | 3.71 | 7.44 | U |
| M10 | limonene+COOH glucuronide p-mentha-1,8-dien-7-oic acid glucuronide perillic acid glucuronide | 4.49 | 341.1243 | 261.0763 (10); 175.0230 (3); 165.0923 (45); 113.0241 (35); 99.0081 (20); 85.0295 (55); 71.0141 (40); 59.0142 (100); 44.9993 (25) | −0.3 | C16H21O8 | 0.08 | 0.42 | U |
| M9-II | limonene+OH glucuronide II p-mentha-1,8-dien-1-ol/p-mentha-1,8-dien-6-ol/p-mentha-1,8-dien-7-ol/p-mentha-1,8-dien-10-ol glucuronide p-mentha-1,8-dien-1-ol/carveol/perillyl alcohol/limonene-10-ol glucuronide | 4.51 | 327.1435 | 327.1424 (15); 209.1177 (5); 175.0237 (1); 157.0123 (5); 151.1120 (1); 113.0235 (30); 85.0287 (10); 75.0083 (100); 57.0348 (45); 44.9985 (20) | 4.1 | C16H23O7 | 5.84 | 9.73 | U, L |
| M11 | limonene+COOH+H2 taurine p-menth-8-en-7-oic acid/p-menth-1-en-7-oic acid taurine dihydroperillic acid taurine | 4.55 | 274.1101 | 274.1092 (60); 206.0483 (5); 124.0062 (5); 106.9791 (10); 79.9570 (100); 72.0445 (5) | 6.2 | C12H20NO4S | 1.10 | 1.10 | U |
| M12 | limonene+COOH glycine p-mentha-1,8-dien-7-oic acid glycine perillic acid glycine | 4.63 | 222.1134 | 222.1120 (25); 178.1218 (75); 176.1078 (80); 121.1019 (25); 119.0852 (45); 110.0612 (35); 108.0453 (20); 74.0246 (100); 56.0143 (15) | 0.8 | C12H16NO3 | 20.04 | 14.26 | U |
| M13 | limonene+COOH taurine p-mentha-1,8-dien-7-oic acid taurine perillic acid taurine | 4.66 | 272.0949 | 272.0949 (35); 204.0322 (25); 164.1039 (3); 124.0070 (5); 106.9803 (10); 79.9567 (100) | 5 | C12H18NO4S | 0.38 | 1.47 | U |
| M9-III | limonene+OH glucuronide III p-mentha-1,8-dien-1-ol/p-mentha-1,8-dien-6-ol/p-mentha-1,8-dien-7-ol/p-mentha-1,8-dien-10-ol glucuronide p-mentha-1,8-dien-1-ol/carveol/perillyl alcohol/limonene-10-ol glucuronide | 4.71 | 327.1433 | 327.1429 (15); 309.1361 (3); 209.1166 (5); 151.1120 (15); 113.0235 (10); 85.0289 (50); 83.0498 (65); 75.0083 (100); 57.0348 (35); 44.9984 (10) | 4.1 | C16H23O7 | 2.69 | 2.98 | U |
| M14 | limonene+COOH+H2 glucuronide p-menth-8-en-7-oic acid/p-menth-1-en-7-oic acid glucuronide dihydroperillic acid glucuronide | 4.71 | 343.1389 | 175.0235 (3); 167.1069 (75); 113.0238 (40); 99.0081 (20); 85.0288 (50); 71.0133 (35); 59.0139 (100) | 1.3 | C16H23O8 | 3.97 | 6.20 | U, L |
| M15 | limonene+COOH+H2 glycine p-menth-8-en-7-oic acid/p-menth-1-en-7-oic acid glycine dihydroperillic acid glycine | 4.71 | 224.1277 | 224.1287 (5); 180.1399 (5); 178.1203 (10); 54.0479 (5); 74.0247 (100); 56.0151 (5) | 6.7 | C12H18NO3 | 3.30 | 5.77 | U |
| M16 | limonene+OH+H2 glucuronide p-mentha-1-en-1-ol/p-mentha-1-en-6-ol/p-mentha-1-en-7-ol/p-mentha-1-en-10-ol/p-mentha-8-en-1-ol/p-mentha-8-en-6-ol/p-mentha-8-en-7-ol/p-mentha-8-en-10-ol glucuronide | 4.90 | 329.1585 | 329.1575 (15); 269.1356 (5); 211.1321 (5); 153.1355 (3); 113.0245 (20); 85.0264 (65); 75.0080 (100); 55.0186 (35) | 5.6 | C16H25O7 | 0.47 | 0.73 | U |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi de Alvarenga, J.F.; Lei Preti, C.; Santos Martins, L.; Noronha Hernandez, G.; Genaro, B.; Lamesa Costa, B.; Gieseler Dias, C.; Purgatto, E.; Fiamoncini, J. Identification of D-Limonene Metabolites by LC-HRMS: An Exploratory Metabolic Switching Approach in a Mouse Model of Diet-Induced Obesity. Metabolites 2022, 12, 1246. https://doi.org/10.3390/metabo12121246
Rinaldi de Alvarenga JF, Lei Preti C, Santos Martins L, Noronha Hernandez G, Genaro B, Lamesa Costa B, Gieseler Dias C, Purgatto E, Fiamoncini J. Identification of D-Limonene Metabolites by LC-HRMS: An Exploratory Metabolic Switching Approach in a Mouse Model of Diet-Induced Obesity. Metabolites. 2022; 12(12):1246. https://doi.org/10.3390/metabo12121246
Chicago/Turabian StyleRinaldi de Alvarenga, José Fernando, Caroline Lei Preti, Lara Santos Martins, Guilherme Noronha Hernandez, Brunna Genaro, Bruna Lamesa Costa, Caroline Gieseler Dias, Eduardo Purgatto, and Jarlei Fiamoncini. 2022. "Identification of D-Limonene Metabolites by LC-HRMS: An Exploratory Metabolic Switching Approach in a Mouse Model of Diet-Induced Obesity" Metabolites 12, no. 12: 1246. https://doi.org/10.3390/metabo12121246
APA StyleRinaldi de Alvarenga, J. F., Lei Preti, C., Santos Martins, L., Noronha Hernandez, G., Genaro, B., Lamesa Costa, B., Gieseler Dias, C., Purgatto, E., & Fiamoncini, J. (2022). Identification of D-Limonene Metabolites by LC-HRMS: An Exploratory Metabolic Switching Approach in a Mouse Model of Diet-Induced Obesity. Metabolites, 12(12), 1246. https://doi.org/10.3390/metabo12121246







