Mannose Receptor Deficiency Impacts Bone Marrow and Circulating Immune Cells during High Fat Diet Induced Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Glucose and Insulin Tolerance Tests
2.3. Sample Preparation for Flow Cytometry
2.4. Femurs, Liver, and Adipose Tissues Histology
2.5. Proteomics Analysis
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Statistical Analysis
3. Results
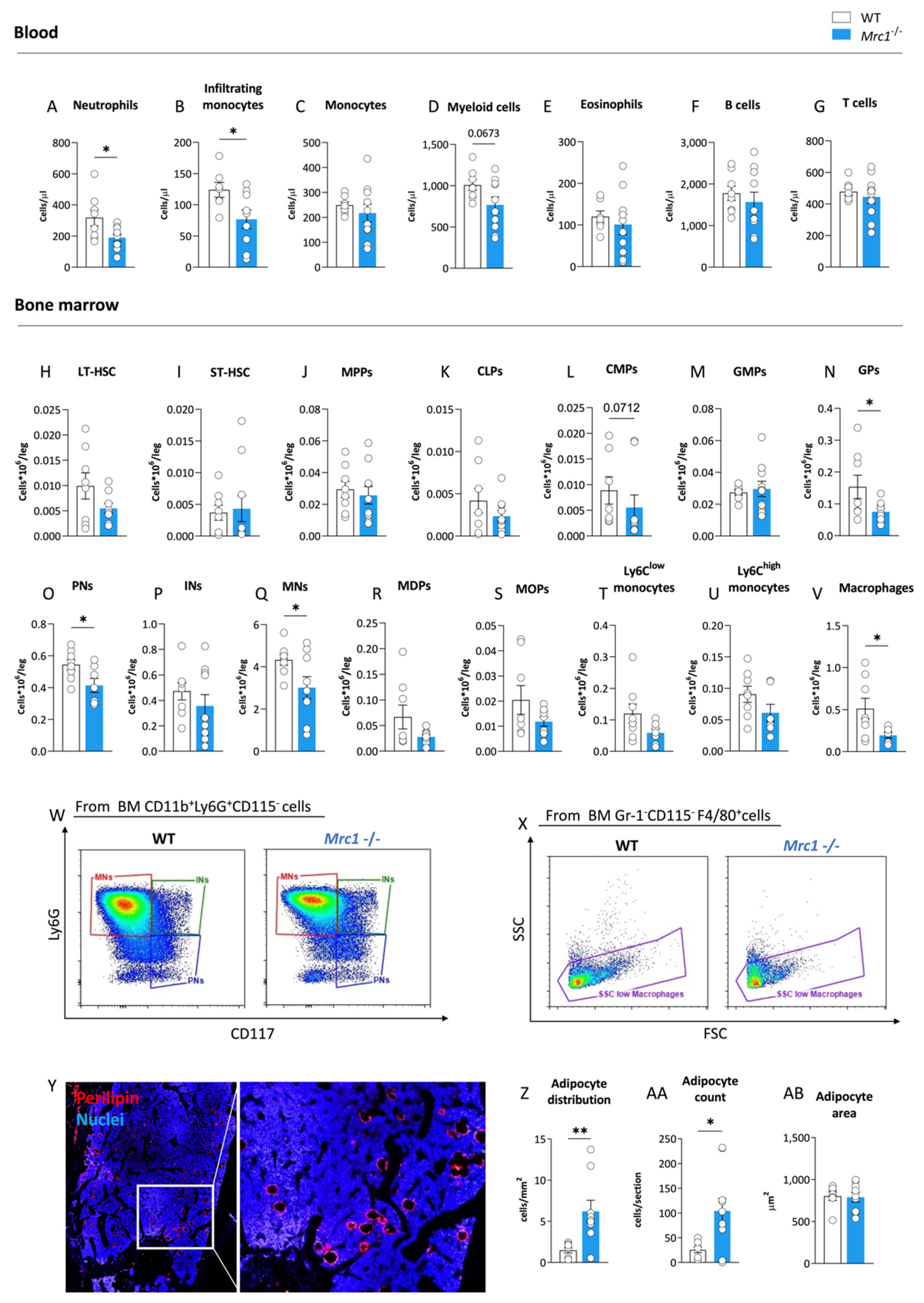
3.1. Mrc1 Deficiency in Mice Fed with a High-Fat Diet Affects Bone Marrow Myelopoiesis and Adiposity, Which Results in Dampened Immune-Inflammatory Response in the Circulation
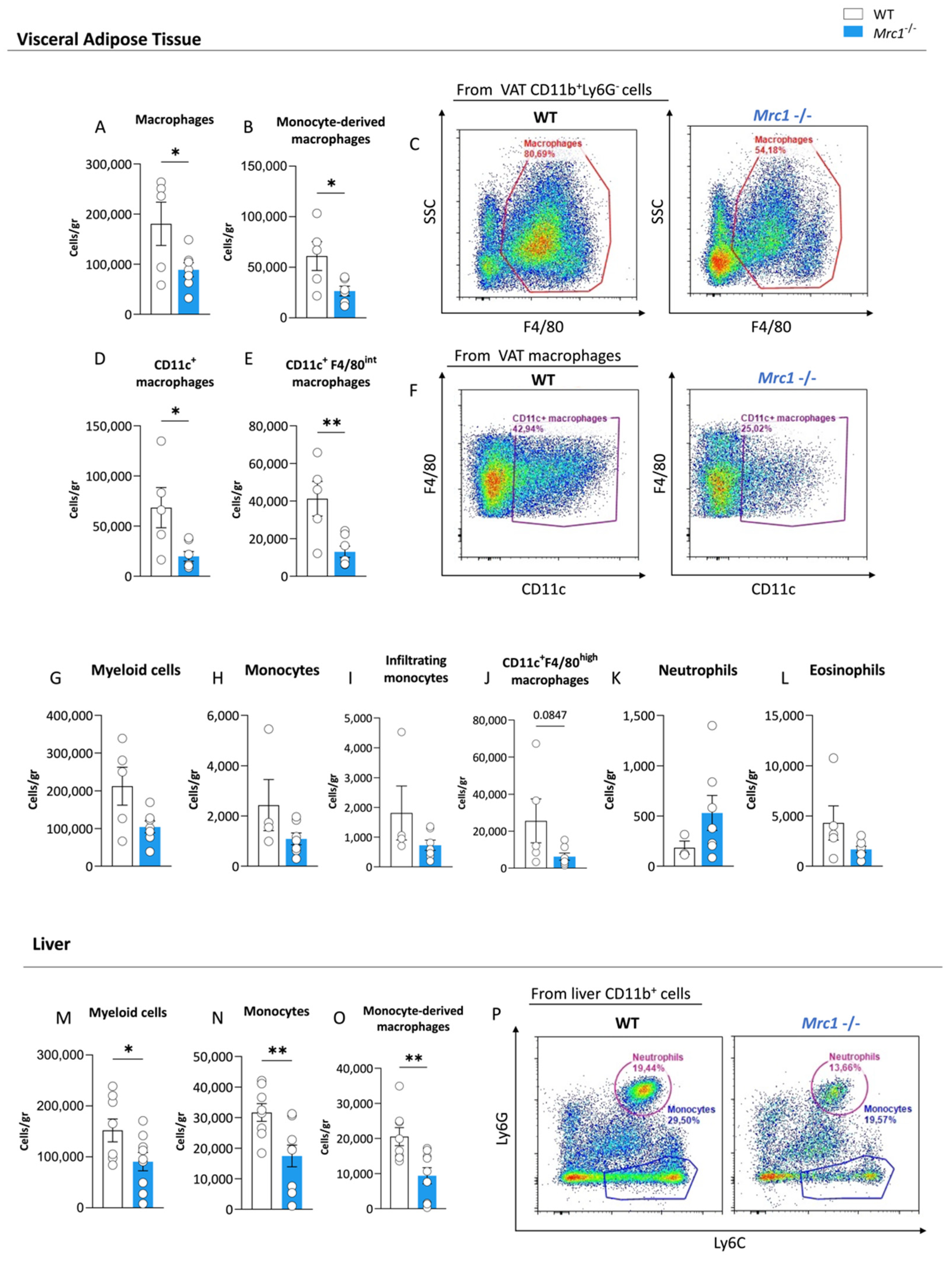
3.2. Mrc1 Deficiency in Mice Fed with a High-Fat Diet Impacts Immune Infiltration of Metabolic Tissues during Obesity
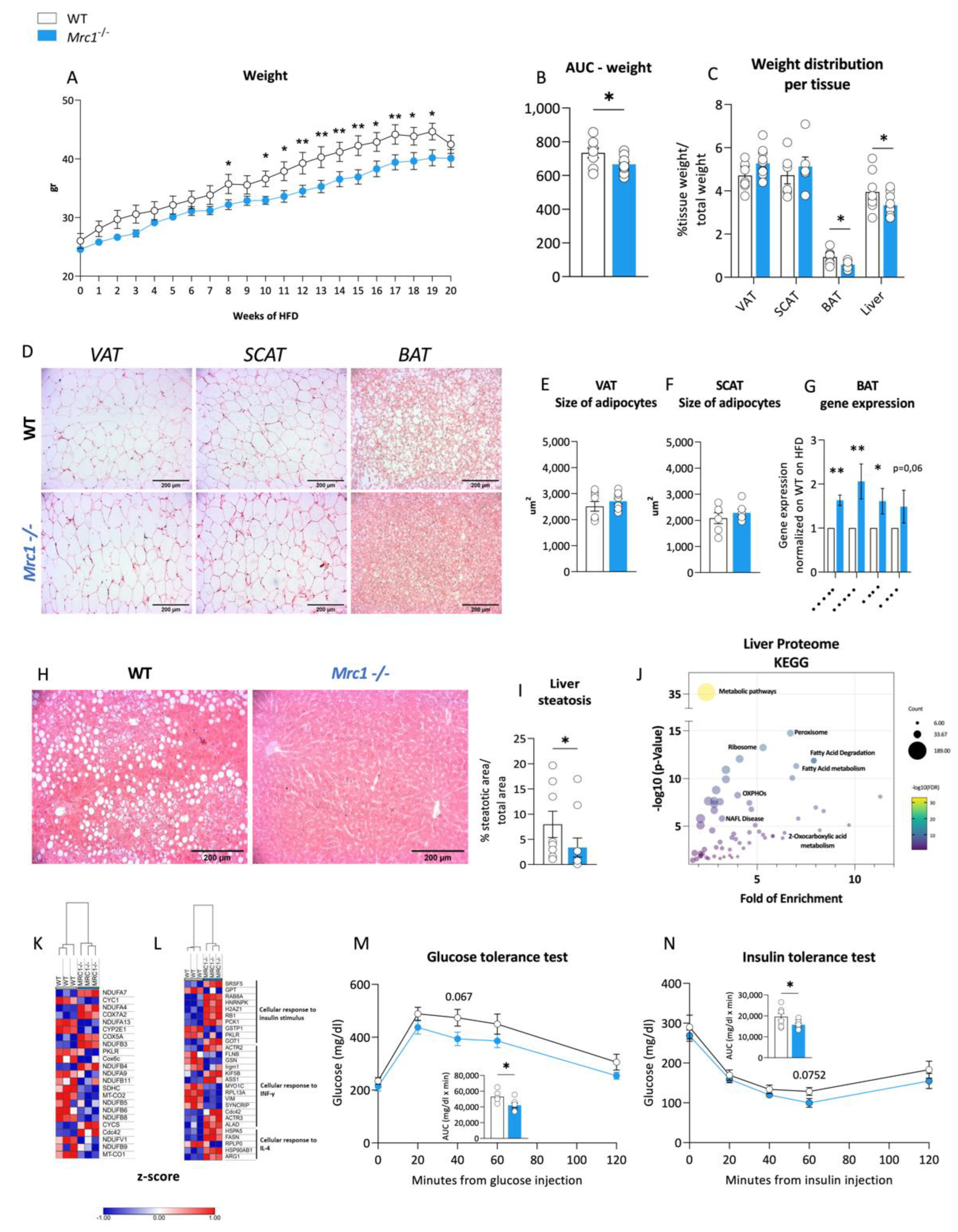
3.3. Immunomodulation in Mrc1−/− Mice Is Associated with Improved Obese Phenotype
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elvevold, K.; Simon-Santamaria, J.; Hasvold, H.; McCourt, P.; Smedsrød, B.; Sørensen, K.K. Liver Sinusoidal Endothelial Cells Depend on Mannose Receptor-Mediated Recruitment of Lysosomal Enzymes for Normal Degradation Capacity. Hepatology 2008, 48, 2007–2015. [Google Scholar] [CrossRef]
- McKenzie, E.J.; Taylor, P.R.; Stillion, R.J.; Lucas, A.D.; Harris, J.; Gordon, S.; Martinez-Pomares, L. Mannose Receptor Expression and Function Define a New Population of Murine Dendritic Cells. J. Immunol. 2007, 178, 4975–4983. [Google Scholar] [CrossRef] [Green Version]
- Rødgaard-Hansen, S.; Rafique, A.; Christensen, P.A.; Maniecki, M.B.; Sandahl, T.D.; Nexø, E.; Møller, H.J. A soluble form of the macrophage-related mannose receptor (MR/CD206) is present in human serum and elevated in critical illness. Clin. Chem. Lab. Med. 2014, 52, 453–461. [Google Scholar] [CrossRef]
- Mullin, N.P.; Hall, K.T.; Taylor, M.E. Characterization of ligand binding to a carbohydrate-recognition domain of the macrophage mannose receptor. J. Biol. Chem. 1994, 269, 28405–28413. [Google Scholar] [CrossRef]
- Martinez-Pomares, L.; Wienke, D.; Stillion, R.; McKenzie, E.J.; Arnold, J.; Harris, J.; McGreal, E.; Sim, R.; Isacke, C.; Gordon, S. Carbohydrate-independent recognition of collagens by the macrophage mannose receptor. Eur. J. Immunol. 2006, 36, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chirino, A.J.; Misulovin, Z.; Leteux, C.; Feizi, T.; Nussenzweig, M.C.; Bjorkman, P.J. Crystal structure of the cysteine-rich domain of mannose receptor complexed with a sulfated carbohydrate ligand. J. Exp. Med. 2000, 191, 1105–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, Y.; Shapiro, S.D.; Baenziger, J.U. Regulation of lutropin circulatory half-life by the mannose/N-acetylgalactosamine-4-SO4 receptor is critical for implantation in vivo. J. Clin. Investig. 2002, 109, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Coonce, M.; Fiete, D.; Steirer, L.; Dveksler, G.; Townsend, R.R.; Baenziger, J.U. Functional consequences of mannose and asialoglycoprotein receptor ablation. J. Biol. Chem. 2016, 291, 18700–18717. [Google Scholar] [CrossRef] [Green Version]
- Salmi, M.; Karikoski, M.; Elima, K.; Rantakari, P.; Jalkanen, S. CD44 Binds to Macrophage Mannose Receptor on Lymphatic Endothelium and Supports Lymphocyte Migration via Afferent Lymphatics. Circ. Res. 2013, 112, 1577–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Pomares, L. The mannose receptor. J. Leukoc. Biol. 2012, 92, 1177–1186. [Google Scholar] [CrossRef]
- Lee, S.J.; Evers, S.; Roeder, D.; Parlow, A.F.; Risteli, J.; Risteli, L.; Lee, Y.C.; Feizi, T.; Langen, H.; Nussenzweig, M.C. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 2002, 295, 1898–1901. [Google Scholar] [CrossRef]
- Gazi, U.; Martinez-Pomares, L. Influence of the mannose receptor in host immune responses. Immunobiology 2009, 214, 554–561. [Google Scholar] [CrossRef]
- Heideveld, E.; van den Akker, E. Digesting the role of bone marrow macrophages on hematopoiesis. Immunobiology 2017, 222, 814–822. [Google Scholar] [CrossRef]
- Helbling, P.M.; Piñeiro-Yanez, E.; Gerosa, R.; Boettcher, S.; Al-Shahrour, F.; Manz, M.G.; Nombela-Arrieta, C. Global Transcriptomic Profiling of the Bone Marrow Stromal Microenvironment during Postnatal Resource Global Transcriptomic Profiling of the Bone Marrow Stromal Microenvironment during Postnatal Development, Aging, and Inflammation. Cell Rep. 2019, 29, 3313–3330. [Google Scholar] [CrossRef] [Green Version]
- Ashok, D.; Polcik, L.; Prosseda, S.D.; Hartmann, T.N. Insights into Bone Marrow Niche Stability: An Adhesion and Metabolism Route. Front. Cell Dev. Biol. 2022, 9, 798604. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Caligiuri, G.; Chavakis, T.; Matarese, G.; Netea, M.G.; Nicoletti, A.; O’Neill, L.A.J.; Marelli-Berg, F.M. The Cellular and Molecular Basis of Translational Immunometabolism. Immunity 2015, 43, 421–434. [Google Scholar] [CrossRef] [Green Version]
- Hoyer, F.F.; Zhang, X.; Coppin, E.; Vasamsetti, S.B.; Modugu, G.; Schloss, M.J.; Rohde, D.; McAlpine, C.S.; Iwamoto, Y.; Libby, P.; et al. Bone marrow endothelial cells regulate myelopoiesis in diabetes mellitus. Circulation 2020, 142, 244–258. [Google Scholar] [CrossRef]
- Fan, Y.; Hanai, J.-I.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017, 25, 661–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonacina, F.; Moregola, A.; Porte, R.; Baragetti, A.; Bonavita, E.; Salatin, A.; Grigore, L.; Pellegatta, F.; Molgora, M.; Sironi, M.; et al. Pentraxin 3 deficiency protects from the metabolic inflammation associated to diet-induced obesity. Cardiovasc. Res. 2019, 115, 1861–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svecla, M.; Garrone, G.; Faré, F.; Aletti, G.; Norata, G.D.; Beretta, G. DDASSQ: An open-source, multiple peptide sequencing strategy for label free quantification based on an OpenMS pipeline in the KNIME analytics platform. Proteomics 2021, 21, e2000319. [Google Scholar] [CrossRef]
- Chow, A.; Lucas, D.; Hidalgo, A.; Méndez-Ferrer, S.; Hashimoto, D.; Scheiermann, C.; Battista, M.; Leboeuf, M.; Prophete, C.; van Rooijen, N.; et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 2011, 208, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Naveiras, O.; Nardi, V.; Wenzel, P.L.; Hauschka, P.V.; Fahey, F.; Daley, G.Q. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009, 460, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.O.; Yu, H.; Yue, R.; Zhao, Z.; Rios, J.J.; Naveiras, O.; Morrison, S.J. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 2017, 19, 891–903. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S.; Kim, M.-H.; Yi, H.-S.; Kim, S.Y.; Kim, H.-H.; Kim, J.H.; Yeon, J.E.; Byun, K.S.; Byun, J.-S.; Jeong, W.I. CX3CR1 differentiates F4/80low monocytes into pro-inflammatory F4/80high macrophages in the liver. Sci. Rep. 2018, 8, 15076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Embgenbroich, M.; van der Zande, H.J.P.; Hussaarts, L.; Schulte-Schrepping, J.; Pelgrom, L.R.; García-Tardón, N.; Schlautmann, L.; Stoetzel, I.; Händler, K.; Lambooij, J.M.; et al. Soluble mannose receptor induces proinflammatory macrophage activation and metaflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2103304118. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Y.; Takahara, T.; Kawai, K.; Fujino, M.; Sugiyama, T.; Tsuneyama, K.; Tsukada, K.; Nakae, S.; Zhong, L.; Li, X.K. IFN-γ deficiency attenuates hepatic inflammation and fibrosis in a steatohepatitis model induced by a methionine- and choline-deficient high-fat diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Albiero, M.; Poncina, N.; Ciciliot, S.; Cappellari, R.; Menegazzo, L.; Ferraro, F.; Bolego, C.; Cignarella, A.; Avogaro, A.; Fadini, G.P. Bone marrow macrophages contribute to diabetic stem cell mobilopathy by producing oncostatin M. Diabetes 2015, 64, 2957–2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baragetti, A.; Bonacina, F.; Catapano, A.L.; Norata, G.D. Effect of Lipids and Lipoproteins on Hematopoietic Cell Metabolism and Commitment in Atherosclerosis. Immunometabolism 2021, 3, e210014. [Google Scholar] [CrossRef] [PubMed]
- Bowers, E.; Singer, K. Obesity-induced inflammation: The impact of the hematopoietic stem cell niche. JCI Insight 2021, 6, e145295. [Google Scholar] [CrossRef]
- Xu, Y.; Murphy, A.J.; Fleetwood, A.J. Hematopoietic Progenitors and the Bone Marrow Niche Shape the Inflammatory Response and Contribute to Chronic Disease. Int. J. Mol. Sci. 2022, 23, 2234. [Google Scholar] [CrossRef]
- Pirillo, A.; Svecla, M.; Catapano, A.L.; Holleboom, A.G.; Norata, G.D. Impact of protein glycosylation on lipoprotein metabolism and atherosclerosis. Cardiovasc. Res. 2021, 117, 1033–1045. [Google Scholar]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Bugianesi, E.; Lenzi, M.; McCullough, A.J.; Natale, S.; Forlani, G.; Melchionda, N. Non-alcoholic fatty liver disease. Crit. Rev. Clin. Lab. Sci. 2011, 48, 97–113. [Google Scholar]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage polarization: Different gene signatures in M1(Lps+) vs. Classically and M2(LPS-) vs. Alternatively activated macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Aminuddin, A.; Kado, T.; Takikawa, A.; Yamamoto, S.; Tsuneyama, K.; Igarashi, Y.; Ikutani, M.; Nishida, Y.; Nagai, Y.; et al. CD206+ M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat. Commun. 2017, 8, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoedel, K.B.; Morcos, M.N.F.; Zerjatke, T.; Roeder, I.; Grinenko, T.; Voehringer, D.; Göthert, J.R.; Waskow, C.; Roers, A.; Gerbaulet, A. The bulk of the hematopoietic stem cell population is dispensable for murine steady-state and stress hematopoiesis. Blood 2016, 128, 2285–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nour, J.; Moregola, A.; Svecla, M.; Da Dalt, L.; Bellini, R.; Neyrolles, O.; Fadini, G.P.; Rombouts, Y.; Albiero, M.; Bonacina, F.; et al. Mannose Receptor Deficiency Impacts Bone Marrow and Circulating Immune Cells during High Fat Diet Induced Obesity FigShare Dataset. Figshare Dataset 2022. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nour, J.; Moregola, A.; Svecla, M.; Da Dalt, L.; Bellini, R.; Neyrolles, O.; Fadini, G.P.; Rombouts, Y.; Albiero, M.; Bonacina, F.; et al. Mannose Receptor Deficiency Impacts Bone Marrow and Circulating Immune Cells during High Fat Diet Induced Obesity. Metabolites 2022, 12, 1205. https://doi.org/10.3390/metabo12121205
Nour J, Moregola A, Svecla M, Da Dalt L, Bellini R, Neyrolles O, Fadini GP, Rombouts Y, Albiero M, Bonacina F, et al. Mannose Receptor Deficiency Impacts Bone Marrow and Circulating Immune Cells during High Fat Diet Induced Obesity. Metabolites. 2022; 12(12):1205. https://doi.org/10.3390/metabo12121205
Chicago/Turabian StyleNour, Jasmine, Annalisa Moregola, Monika Svecla, Lorenzo Da Dalt, Rossella Bellini, Olivier Neyrolles, Gian Paolo Fadini, Yoann Rombouts, Mattia Albiero, Fabrizia Bonacina, and et al. 2022. "Mannose Receptor Deficiency Impacts Bone Marrow and Circulating Immune Cells during High Fat Diet Induced Obesity" Metabolites 12, no. 12: 1205. https://doi.org/10.3390/metabo12121205
APA StyleNour, J., Moregola, A., Svecla, M., Da Dalt, L., Bellini, R., Neyrolles, O., Fadini, G. P., Rombouts, Y., Albiero, M., Bonacina, F., & Norata, G. D. (2022). Mannose Receptor Deficiency Impacts Bone Marrow and Circulating Immune Cells during High Fat Diet Induced Obesity. Metabolites, 12(12), 1205. https://doi.org/10.3390/metabo12121205






