4-PBA Attenuates Fat Accumulation in Cultured Spotted Seabass Fed High-Fat-Diet via Regulating Endoplasmic Reticulum Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish and Experimental Diets
2.2. Experimental Design
2.2.1. Experiment I: High-Fat-Diet Feeding Trial
2.2.2. Experiment II: Effects of 4-PBA on Fat Deposition
2.3. Liver Histology
2.4. Gene Expression
2.5. Western Blotting
2.6. Measures of Biochemical Parameters
2.7. Statistical Analysis
3. Results
3.1. Experiment I: High-Fat-Diet Feeding Trial
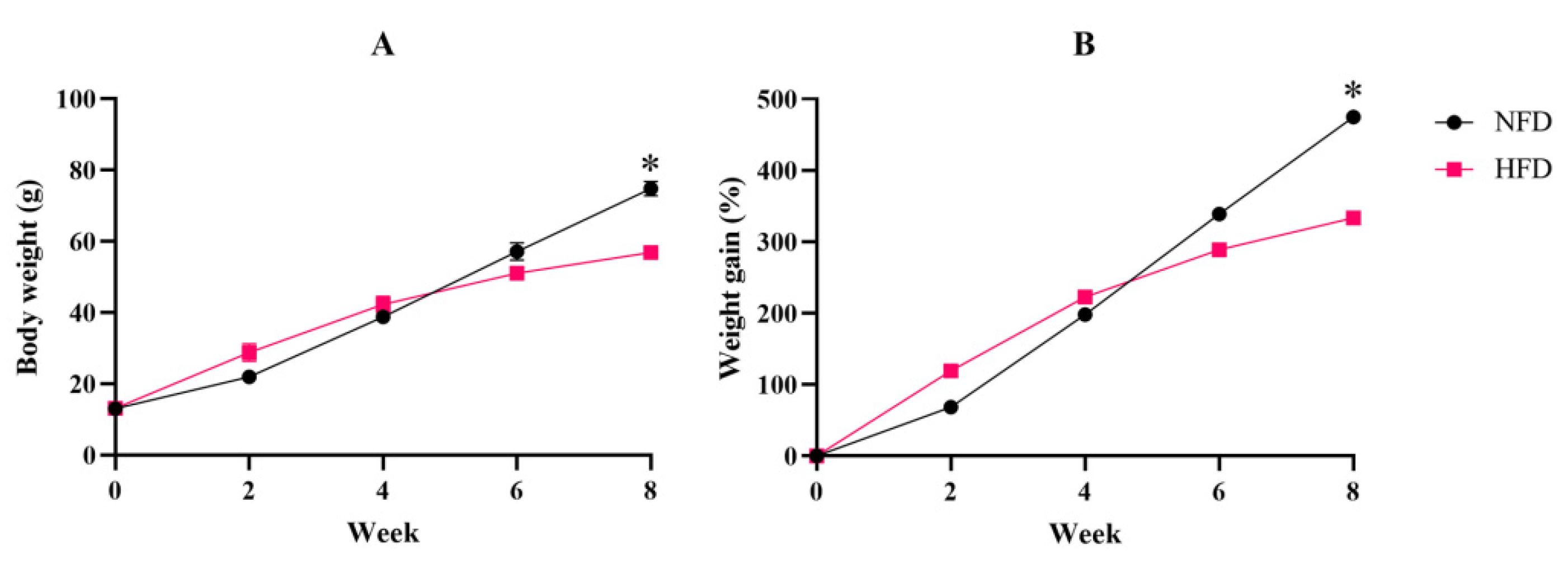
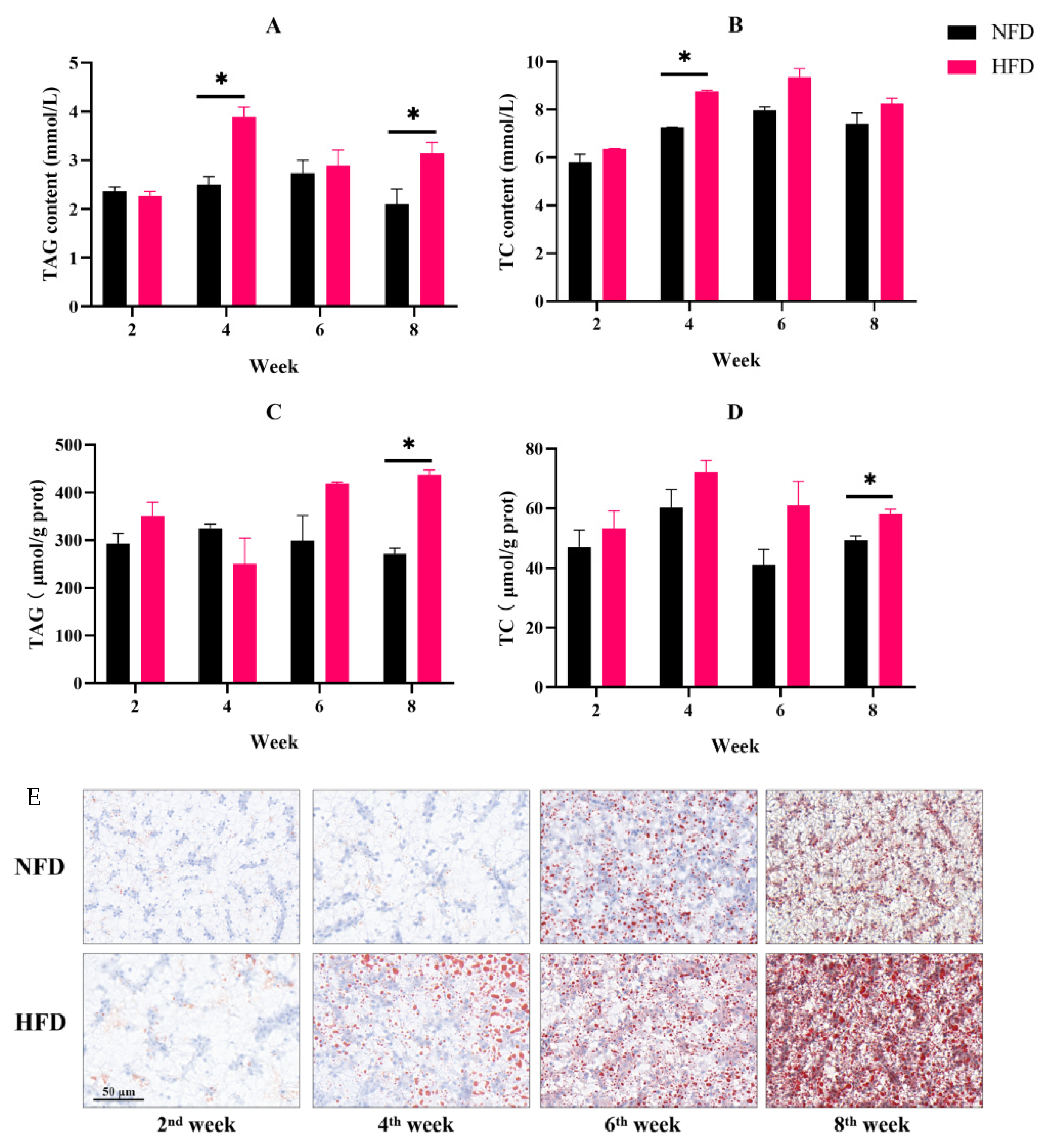
3.1.1. Fish Growth and Fat Accumulation
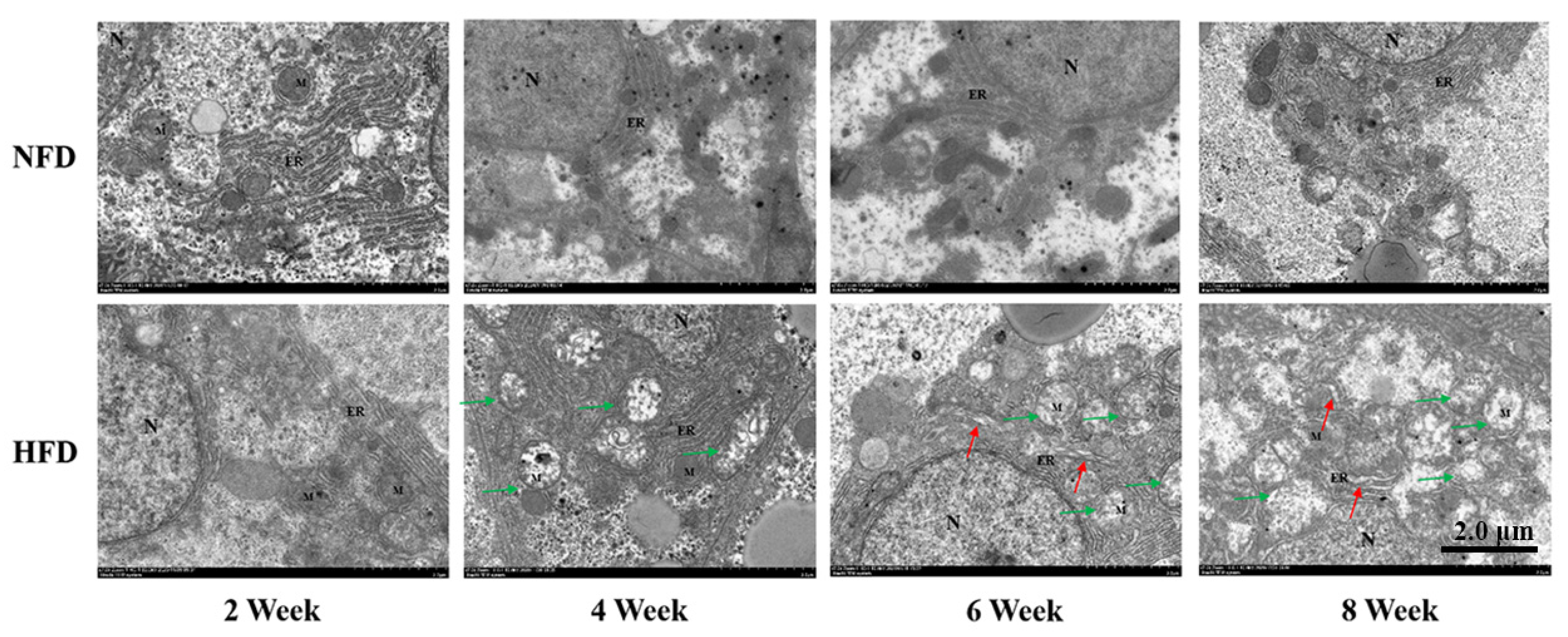
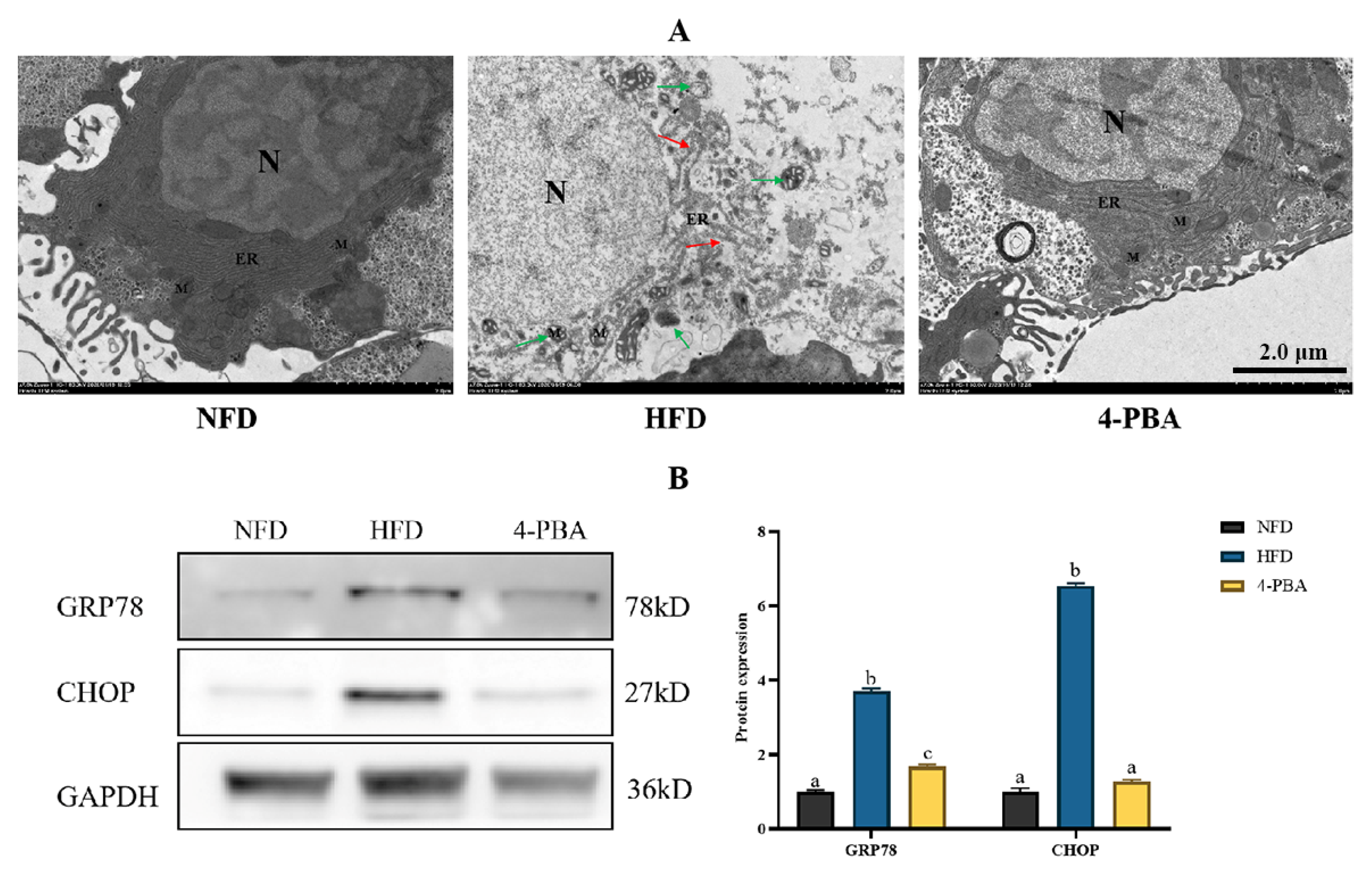
3.1.2. Ultrastructure of Hepatocytes
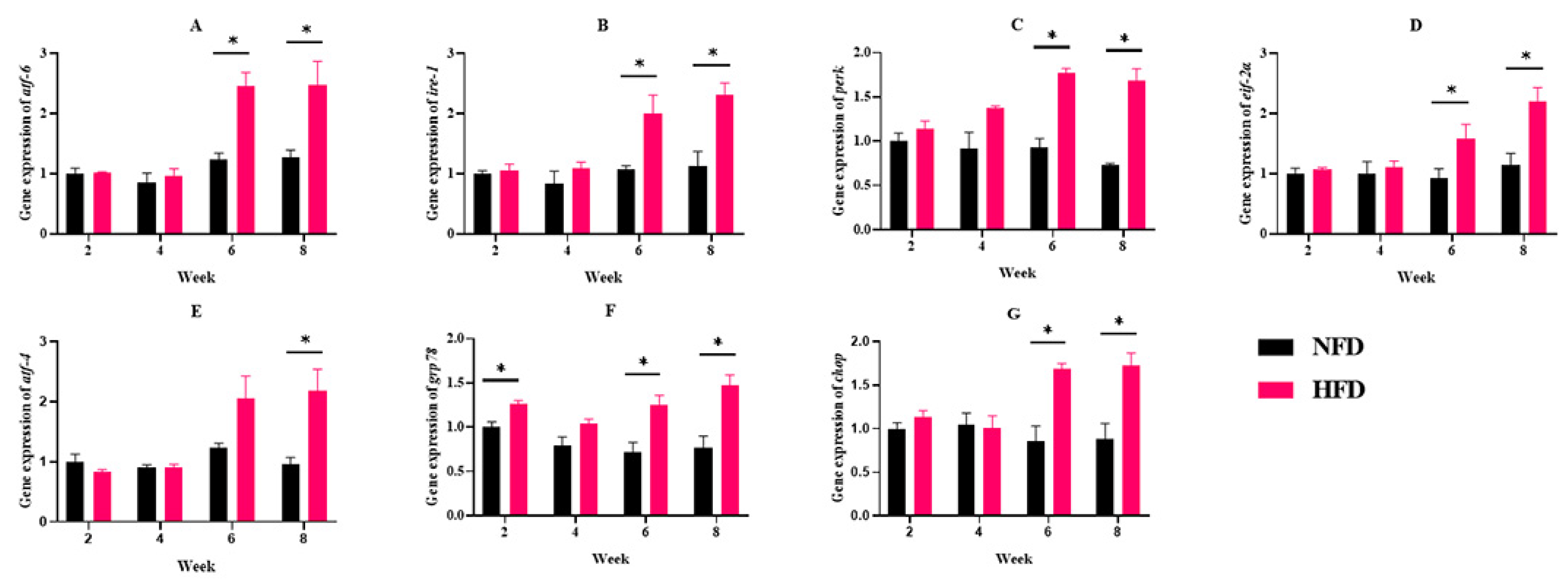
3.1.3. ERs-Related Gene Expressions
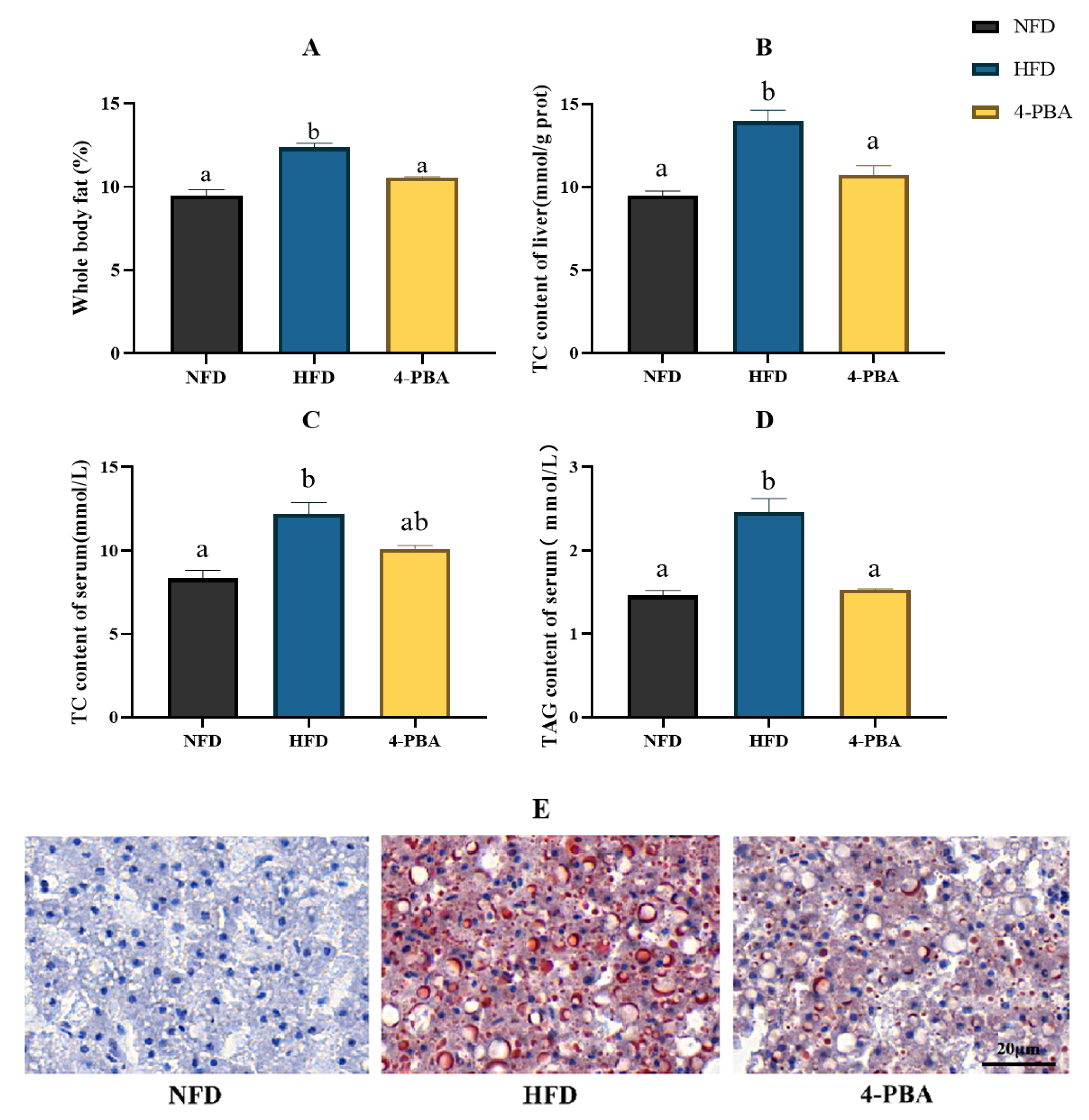
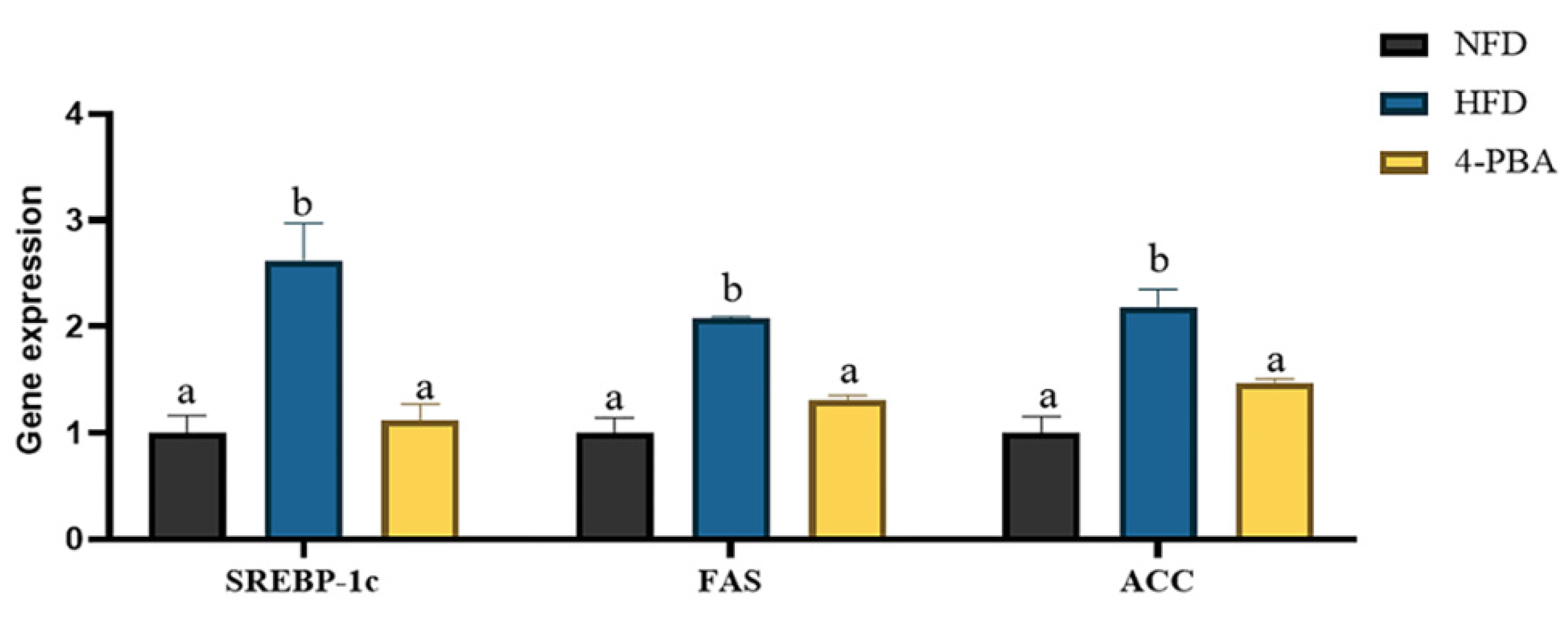
3.2. Experiment II: Effects of 4-PBA on Fat Deposition
Effects of 4-PBA on Fat Accumulation Caused by an HFD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Ding, W.; Li, C.Y.; Liu, Y. HLH-11 modulates lipid metabolism in response to nutrient availability. Nat. Commun. 2020, 11, 5959. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, K.; Kim, K.; Jin Kang, Y.; Lee, S. Influence of lipid level and supplemental lecithin in diet on growth, feed utilization and body composition of juvenile flounder (Paralichthys olivaceus) in suboptimal water temperatures. Aquaculture 2006, 251, 484–490. [Google Scholar] [CrossRef]
- Lu, K.L.; Cai, L.S.; Wang, L.; Song, K.; Zhang, C.X.; Rahimnejad, S. Effects of dietary protein/energy ratio and water temperature on growth performance, digestive enzymes activity and non-specific immune response of spotted seabass (Lateolabrax maculatus). Aquacult. Nutr. 2020, 26, 2023–2031. [Google Scholar] [CrossRef]
- Lu, K.L.; Wang, L.N.; Zhang, D.D.; Liu, W.B.; Xu, W.N. Berberine attenuates oxidative stress and hepatocytes apoptosis via protecting mitochondria in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish Physiol. Biochem. 2017, 43, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.J.; Jiang, G.Z.; Yuan, X.Y.; Liu, W.B. High-fat-diet-induced inflammation depresses the appetite of blunt snout bream (Megalobrama amblycephala) through the transcriptional regulation of leptin/mammalian target of rapamycin. Br. J. Nutr. 2018, 120, 1422–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Tian, L.; Zeng, S.; Xie, S.; Yang, H.; Liang, G.; Liu, Y. Dietary lipid requirement on non-specific immune responses in juvenile grass carp (Ctenopharyngodon idella). Fish Shellfish Immun. 2013, 34, 1202–1208. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; Li, Q.; Li, J.; Cui, K.; Zhang, Y.; Kong, A.; Zhang, Y.; Wan, M.; Mai, K.; et al. Effects of High Levels of Dietary Linseed Oil on the Growth Performance, Antioxidant Capacity, Hepatic Lipid Metabolism, and Expression of Inflammatory Genes in Large Yellow Croaker (Larimichthys crocea). Front. Physiol. 2021, 12, 631850. [Google Scholar] [CrossRef]
- Fu, S.; Watkins, S.M.; Hotamisligil, G.S. The Role of Endoplasmic Reticulum in Hepatic Lipid Homeostasis and Stress Signaling. Cell Metab. 2012, 15, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Jia, R.; Cao, L.; Du, J.; He, Q.; Gu, Z.; Jeney, G.; Xu, P.; Yin, G. Effects of High-Fat Diet on Steatosis, Endoplasmic Reticulum Stress and Autophagy in Liver of Tilapia (Oreochromis niloticus). Front. Mar. Sci. 2020, 7, 363. [Google Scholar] [CrossRef]
- Schr Der, M. Endoplasmic reticulum stress responses. Cell. Mol. Life Sci. 2008, 65, 862–894. [Google Scholar] [CrossRef]
- Maris, M.; Overbergh, L.; Gysemans, C.; Waget, A.; Cardozo, A.K.; Verdrengh, E.; Cunha, J.P.M.; Gotoh, T.; Cnop, M.; Eizirik, D.L.; et al. Deletion of C/EBP homologous protein (Chop) in C57Bl/6 mice dissociates obesity from insulin resistance. Diabetologia 2012, 55, 1167–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Li, L.; Xia, T.; Wang, L.; Xiao, L.; Ding, N.; Wu, Y.; Lu, K. Oxidative Stress Can Be Attenuated by 4-PBA Caused by High-Fat or Ammonia Nitrogen in Cultured Spotted Seabass: The Mechanism Is Related to Endoplasmic Reticulum Stress. Antioxidants 2022, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, L.; Song, K.; Lu, K.; Zhang, C.; Rahimnejad, S. Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture 2020, 516, 734615. [Google Scholar] [CrossRef]
- de Almeida, S.F.; Picarote, G.; Fleming, J.V.; Carmo-Fonseca, M.; Azevedo, J.E.; de Sousa, M. Chemical chaperones reduce endoplasmic reticulum stress and prevent mutant HFE aggregate formation. J. Biol. Chem. 2007, 282, 27905–27912. [Google Scholar] [CrossRef] [Green Version]
- Basseri, S.; Lhotak, S.; Sharma, A.M.; Austin, R.C. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J. Lipid Res. 2009, 50, 2486–2501. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, C.; Yang, P. c-Jun NH2-terminal kinase 1/2 and endoplasmic reticulum stress as interdependent and reciprocal causation in diabetic embryopathy. Diabetes 2013, 62, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Xu, W.; Li, J.; Li, X.; Huang, G.; Liu, W. Alterations of liver histology and blood biochemistry in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish. Sci. 2013, 79, 661–671. [Google Scholar] [CrossRef]
- Dong, Y.; Xia, T.; Lin, J.; Wang, L.; Song, K.; Zhang, C. Quercetin Attenuates High-Fat Diet-Induced Excessive Fat Deposition of Spotted Seabass (Lateolabrax maculatus) Through the Regulatory for Mitochondria and Endoplasmic Reticulum. Front. Mar. Sci. 2021, 8, 746811. [Google Scholar] [CrossRef]
- Zhou, W.; Rahimnejad, S.; Tocher, D.R.; Lu, K.; Zhang, C.; Sun, Y. Metformin attenuates lipid accumulation in hepatocytes of blunt snout bream (Megalobrama amblycephala) via activation of AMP-activated protein kinase. Aquaculture 2019, 499, 90–100. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Li, L.; Espe, M.; Lu, K.L.; Rahimnejad, S. Hydroxytyrosol Attenuates Hepatic Fat Accumulation via Activating Mitochondrial Biogenesis and Autophagy through the AMPK Pathway. J. Agric. Food Chem. 2020, 68, 9377–9386. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, M.; Wu, Y.; Xia, T.; Wang, L.; Song, K.; Zhang, C.; Lu, K.; Rahimnejad, S. Hydroxytyrosol Promotes the Mitochondrial Function through Activating Mitophagy. Antioxidants 2022, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- Jobling, M.; Koskela, J.; Savolainen, R. Influence of dietary fat level and increased adiposity on growth and fat deposition in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Res. 1998, 29, 601–607. [Google Scholar] [CrossRef]
- Zhou, W.; Rahimnejad, S.; Lu, K.; Wang, L.; Liu, W. Effects of berberine on growth, liver histology, and expression of lipid-related genes in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Fish Physiol. Biochem. 2018, 45, 83–91. [Google Scholar] [CrossRef]
- Li, W.; Wu, K.; Liu, Y.; Yang, Y.; Tang, H. Molecular cloning of SLC35D3 and analysis of its role during porcine intramuscular preadipocyte differentiation. BMC Genet. 2020, 21, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Wang, J.X.; Ren, J.; Wang, J.; Qiao, F.; Zhang, M.L.; Du, Z.Y. Activation of peroxisome proliferator-activated receptor-α stimulated lipid catabolism in liver of largemouth bass, Micropterus salmoides. Aquacult. Nutr. 2021, 27, 2749–2758. [Google Scholar] [CrossRef]
- Zhou, C.; Li, P.; Han, M.; Gao, X. Daidzein stimulates fatty acid-induced fat deposition in C2C12 myoblast cells via the G protein-coupled receptor 30 pathway. Anim. Biotechnol. 2020, 33, 851–863. [Google Scholar] [CrossRef]
- Hetz, C.; Chevet, E.; Oakes, S.A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015, 17, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef]
- Lerner, A.G.; Upton, J.; Praveen, P.V.K.; Ghosh, R.; Nakagawa, Y.; Igbaria, A.; Shen, S.; Nguyen, V.; Backes, B.J.; Heiman, M.; et al. IRE1α Induces Thioredoxin-Interacting Protein to Activate the NLRP3 Inflammasome and Promote Programmed Cell Death under Irremediable ER Stress. Cell Metab. 2012, 16, 250–264. [Google Scholar] [CrossRef] [Green Version]
- Faitova, J.; Krekac, D.; Hrstka, R.; Vojtesek, B. Endoplasmic reticulum stress and apoptosis. Cell. Mol. Biol. Lett. 2006, 11, 488–505. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Q.; Lu, M.; Chen, W.; Song, Y.; Jing, F.; Guan, Y.; Wang, L.; Lin, Y.; Bo, T.; et al. Thyrotropin increases hepatic triglyceride content through upregulation of SREBP-1c activity. J. Hepatol. 2014, 61, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.P.; Kim, D.; Lee, Y.; Lee, Y.; Truong, X.T.; Lee, J.; Song, D.; Kwon, T.K.; Park, S.; Jung, C.H.; et al. SREBP-1c impairs ULK1 sulfhydration-mediated autophagic flux to promote hepatic steatosis in high-fat-diet-fed mice. Mol. Cell. 2021, 81, 3820–3832. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Dai, Y.; Liu, M.; Yuan, X.; Wang, C.; Huang, Y.; Liu, W.; Jiang, G. High-fat diet induces aberrant hepatic lipid secretion in blunt snout bream by activating endoplasmic reticulum stress-associated IRE1/XBP1 pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Chen, B.; Wang, X. 4-PBA prevents pressure overload-induced myocardial hypertrophy and interstitial fibrosis by attenuating endoplasmic reticulum stress. Chem. Biol. Interact. 2015, 242, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, M.N.; Homma, K.; Osada, H.; Toda, E.; Ban, N.; Nagai, N.; Negishi, K.; Tsubota, K.; Ozawa, Y. Neuroprotective Effect of 4-Phenylbutyric Acid against Photo-Stress in the Retina. Antioxidants 2021, 10, 1147. [Google Scholar] [CrossRef] [PubMed]
- Souza-Neto, F.V.; Jiménez-González, S.; Delgado-Valero, B.; Jurado-López, R.; Genty, M.; Romero-Miranda, A.; Rodríguez, C.; Nieto, M.L.; Martínez-Martínez, E.; Cachofeiro, V. The Interplay of Mitochondrial Oxidative Stress and Endoplasmic Reticulum Stress in Cardiovascular Fibrosis in Obese Rats. Antioxidants 2021, 10, 1274. [Google Scholar] [CrossRef]
- Pereira, S.; Moore, J.; Li, J.X.; Yu, W.Q.; Ghanim, H.; Vlavcheski, F.; Joseph, Y.D.; Dandona, P.; Volchuk, A.; Cummins, C.L.; et al. 4-Phenylbutyric acid improves free fatty acid-induced hepatic insulin resistance in vivo. Endocr. Connect. 2021, 10, 861–872. [Google Scholar] [CrossRef]
- Randhawa, R.; Bhardwaj, R.; Kaur, T. Amelioration of hyperoxaluria-induced kidney dysfunction by chemical chaperone 4-phenylbutyric acid. Urolithiasis 2018, 47, 171–179. [Google Scholar] [CrossRef]
- Kolb, P.S.; Ayaub, E.A.; Zhou, W.; Yum, V.; Dickhout, J.G.; Ask, K. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int. J. Biochem. Cell Biol. 2015, 61, 45–52. [Google Scholar] [CrossRef]
- Wang, T.; Wei, X.; Sun, Y.; Hu, Y.; Li, J.; Zhang, X.; Yin, S.; Shi, Y.; Zhu, Y. Copper nanoparticles induce the formation of fatty liver in Takifugu fasciatus triggered by the PERK-EIF2α-SREBP-1c pathway. NanoImpact 2021, 21, 100280. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, T.; Liao, Y.-Q.; Li, L.; Sun, L.-Y.; Ding, N.-S.; Wu, Y.-L.; Lu, K.-L. 4-PBA Attenuates Fat Accumulation in Cultured Spotted Seabass Fed High-Fat-Diet via Regulating Endoplasmic Reticulum Stress. Metabolites 2022, 12, 1197. https://doi.org/10.3390/metabo12121197
Xia T, Liao Y-Q, Li L, Sun L-Y, Ding N-S, Wu Y-L, Lu K-L. 4-PBA Attenuates Fat Accumulation in Cultured Spotted Seabass Fed High-Fat-Diet via Regulating Endoplasmic Reticulum Stress. Metabolites. 2022; 12(12):1197. https://doi.org/10.3390/metabo12121197
Chicago/Turabian StyleXia, Tian, Yan-Qin Liao, Lei Li, Lu-Yu Sun, Neng-Shui Ding, You-Lin Wu, and Kang-Le Lu. 2022. "4-PBA Attenuates Fat Accumulation in Cultured Spotted Seabass Fed High-Fat-Diet via Regulating Endoplasmic Reticulum Stress" Metabolites 12, no. 12: 1197. https://doi.org/10.3390/metabo12121197
APA StyleXia, T., Liao, Y.-Q., Li, L., Sun, L.-Y., Ding, N.-S., Wu, Y.-L., & Lu, K.-L. (2022). 4-PBA Attenuates Fat Accumulation in Cultured Spotted Seabass Fed High-Fat-Diet via Regulating Endoplasmic Reticulum Stress. Metabolites, 12(12), 1197. https://doi.org/10.3390/metabo12121197







