Proteomic and Metabolomic Evaluation of Insect- and Herbicide-Resistant Maize Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. DNA Extraction and PCR-Based Detection of GM Maize
2.3. Protein Preparation and Trypsin Digestion
2.4. LC–MS/MS Analysis
2.5. Metabolite Preparation
2.6. UPLC Conditions and ESI-Q TRAP-MS/MS
2.7. Data Analysis
2.8. ELISA
3. Results
3.1. Identification of GM Maize Lines
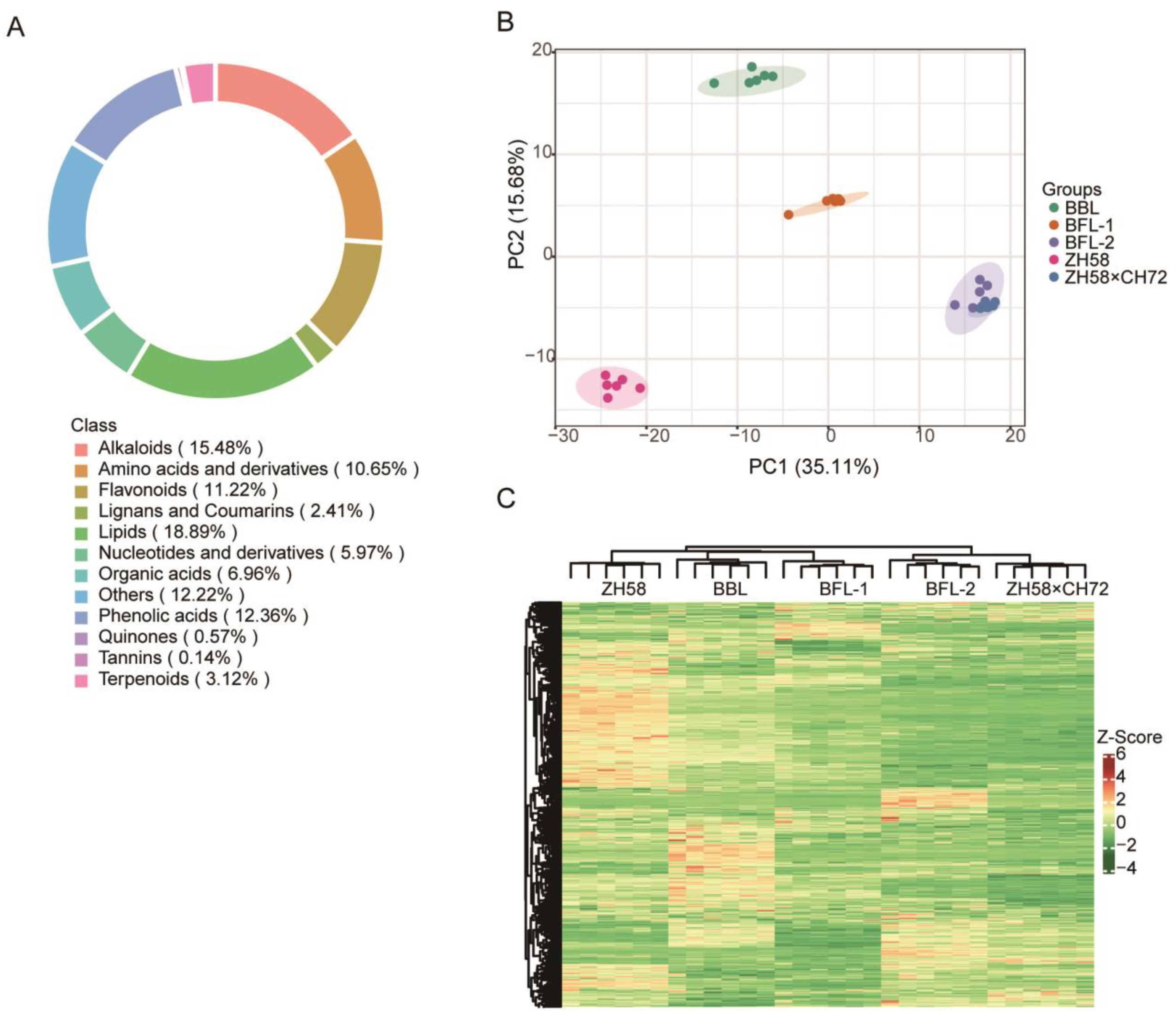
3.2. Protein and Metabolic Profiling of Maize Seeds
3.3. DEPs Detection in Maize Seeds by LFQ Proteomic Analysis
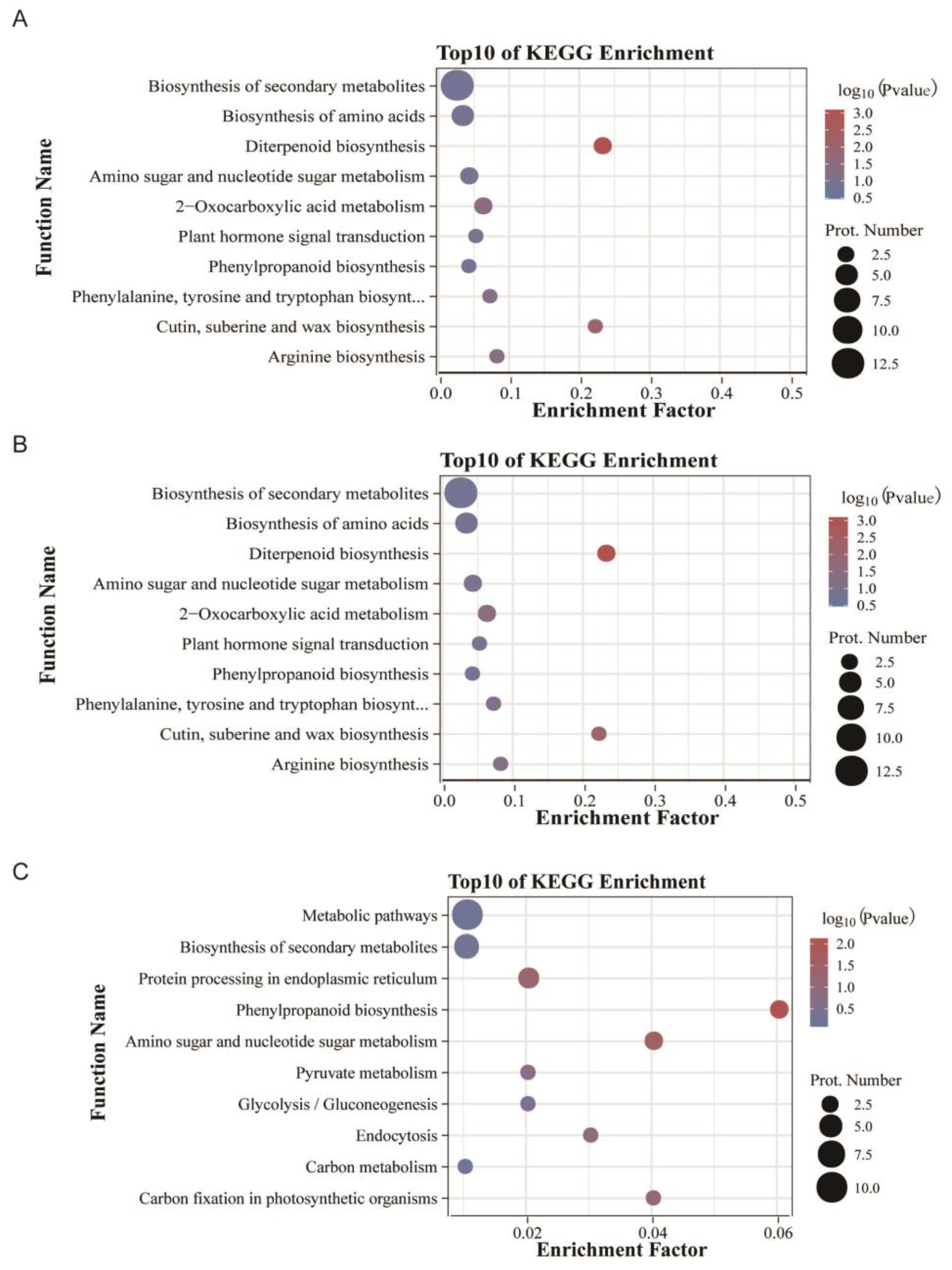
3.4. KEGG Pathway Enrichment Analysis of the Identified DEPs
3.5. DAMs Detection in Maize Seeds by Widely Targeted Metabolomic Analysis
3.6. KEGG Pathway Enrichment Analysis of the Identified DAMs
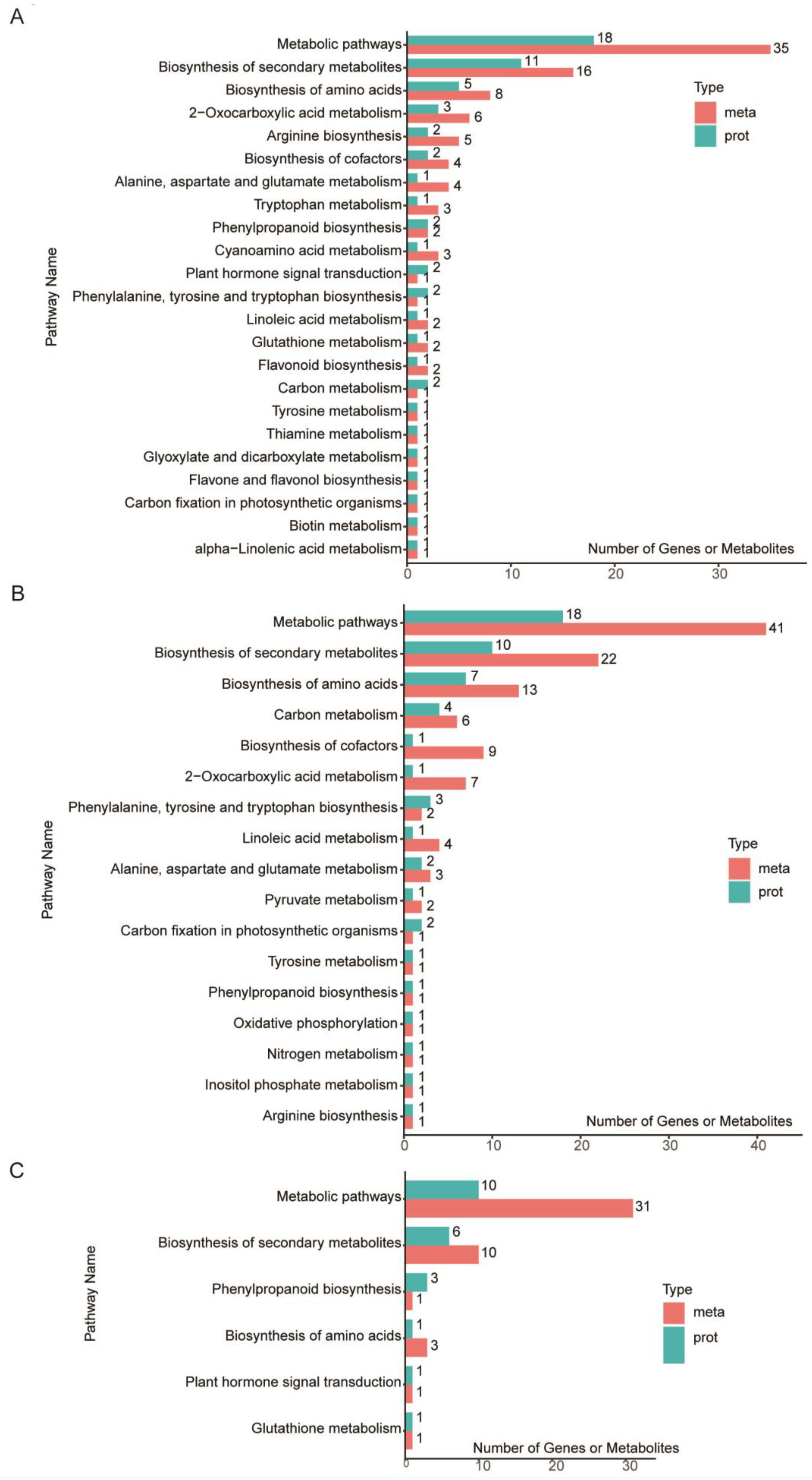
3.7. Identification of co-DEPs and co-DAMs in the Seeds
3.8. Integrated Proteomic and Metabolomic Analyses
3.9. Exogenous Protein Detection by ELISA and LFQ Proteomics of Seeds of GM Maize Varieties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: http://www.isaaa.org/gmapprovaldatabase/default.asp (accessed on 6 May 2022).
- Conner, A.J.; Jacobs, J.M. Genetic engineering of crops as potential source of genetic hazard in the human diet. Mutat. Res. 1999, 443, 223–234. [Google Scholar] [CrossRef]
- Conner, A.J.; Jacobs, J.M. Food risks from transgenic crops in perspective. Nutrition 2000, 16, 709–711. [Google Scholar] [CrossRef]
- Ren, Y.F.; Lv, J.; Wang, H.; Li, L.C.; Peng, Y.F.; Qu, L.J. A comparative proteomics approach to detect unintended effects in transgenic Arabidopsis. J. Genet. Genom. 2009, 36, 629–639. [Google Scholar] [CrossRef]
- Cellini, F.; Chesson, A.; Colquhoun, I.; Constable, A.; Davies, H.V.; Engel, K.H.; Gatehouse, A.M.R.; Karenlampi, S.; Kok, E.J.; Leguay, J.J.; et al. Unintended effects and their detection in genetically modified crops. Food Chem. Toxicol. 2004, 42, 1089–1125. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, X.Y.; Luo, Y.B.; Xiao, G.Y.; Jiang, X.B.; Huang, K.L. Comparative analysis of nutritional composition between herbicide-tolerant rice with bar gene and its non-transgenic counterpart. J. Food Compos. Anal. 2008, 21, 535–539. [Google Scholar] [CrossRef]
- Han, J.H.; Yang, Y.X.; Chen, S.R.; Wang, Z.; Yang, X.L.; Wang, G.D.; Men, J.H. Comparison of nutrient composition of parental rice and rice genetically modified with cowpea trypsin inhibitor in China. J. Food Compos. Anal. 2005, 18, 297–302. [Google Scholar] [CrossRef]
- Bedair, M.; Glenn, K.C. Evaluation of the use of untargeted metabolomics in the safety assessment of genetically modified crops. Metabolomics 2020, 16, 111. [Google Scholar] [CrossRef]
- Ricroch, A.E.; Berge, J.B.; Kuntz, M. Evaluation of Genetically Engineered Crops Using Transcriptomic, Proteomic, and Metabolomic Profiling Techniques. Plant Physiol. 2011, 155, 1752–1761. [Google Scholar] [CrossRef]
- Barros, E.; Lezar, S.; Anttonen, M.J.; van Dijk, J.P.; Rohlig, R.M.; Kok, E.J.; Engel, K.H. Comparison of two GM maize varieties with a near-isogenic non-GM variety using transcriptomics, proteomics and metabolomics. Plant Biotechnol. J. 2010, 8, 436–451. [Google Scholar] [CrossRef]
- Qin, J.; Gu, F.; Liu, D.; Yin, C.; Zhao, S.; Chen, H.; Zhang, J.; Yang, C.; Zhan, X.; Zhang, M. Proteomic analysis of elite soybean Jidou17 and its parents using iTRAQ-based quantitative approaches. Proteome Sci. 2013, 11, 12. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, J.N.; Wang, F.M.; Wang, J.H.; Zheng, Z.; Yin, C.C.; Chen, H.; Shi, A.N.; Zhang, B.; Chen, P.Y.; et al. iTRAQ protein profile analysis of developmental dynamics in soybean [Glycine max (L.) Merr.] leaves. PLoS ONE 2017, 12, e0181910. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.Y.; Sun, Z.D.; Cai, Z.Y.; Chen, H.Z.; Lai, Z.G.; Yang, S.Z.; Tang, X.M. Proteomic analysis by iTRAQ-MRM of soybean resistance to Lamprosema Indicate. BMC Genom. 2017, 18, 444. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Cong, R.; Li, S.; Li, R.; Qin, Z.W.; Li, Y.J.; Zhou, X.L.; Chen, S.X.; Li, J. Comparative Proteomic Analysis of Soybean Leaves and Roots by iTRAQ Provides Insights into Response Mechanisms to Short-Term Salt Stress. Front. Plant Sci. 2016, 7, 573. [Google Scholar] [CrossRef]
- Lim, S.; Borza, T.; Peters, R.D.; Coffin, R.H.; Al-Mughrabi, K.I.; Pinto, D.M.; Wang-Pruski, G.F. Proteomics analysis suggests broad functional changes in potato leaves triggered by phosphites and a complex indirect mode of action against Phytophthora infestans. J. Proteom. 2013, 93, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Tian, L.; Qu, L. Proteomic analysis of endoplasmic reticulum stress responses in rice seeds. Sci. Rep. 2015, 5, 14255. [Google Scholar] [CrossRef]
- Schauer, N.; Semel, Y.; Roessner, U.; Gur, A.; Balbo, I.; Carrari, F.; Pleban, T.; Perez-Melis, A.; Bruedigam, C.; Kopka, J.; et al. Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nat. Biotechnol. 2006, 24, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Schauer, N.; Semel, Y.; Balbo, I.; Steinfath, M.; Repsilber, D.; Selbig, J.; Pleban, T.; Zamir, D.; Fernie, A.R. Mode of inheritance of primary metabolic traits in tomato. Plant Cell 2008, 20, 509–523. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Li, L.; Yang, Y.; Ding, Y.; Guan, H.; Wang, X.; Zhang, A.; Wen, H. Negligible transcriptome and metabolome alterations in RNAi insecticidal maize against Monolepta hieroglyphica. Plant Cell Rep. 2020, 39, 1539–1547. [Google Scholar] [CrossRef]
- Liu, Q.S.; Yang, X.W.; Tzin, V.; Peng, Y.F.; Romeis, J.; Li, Y.H. Plant breeding involving genetic engineering does not result in unacceptable unintended effects in rice relative to conventional cross-breeding. Plant J. 2020, 103, 2236–2249. [Google Scholar] [CrossRef]
- Fu, W.; Zhu, P.; Qu, M.; Zhi, W.; Zhang, Y.; Li, F.; Zhu, S. Evaluation on reprogramed biological processes in transgenic maize varieties using transcriptomics and metabolomics. Sci. Rep. 2021, 11, 2050. [Google Scholar] [CrossRef] [PubMed]
- Chakroun, M.; Banyuls, N.; Bel, Y.; Escriche, B.; Ferre, J. Bacterial Vegetative Insecticidal Proteins (Vip) from Entomopathogenic Bacteria. Microbiol. Mol. Biol. Rev. MMBR 2016, 80, 329–350. [Google Scholar] [CrossRef]
- Arruda, S.C.; Barbosa, H.S.; Azevedo, R.A.; Arruda, M.A. Comparative studies focusing on transgenic through cp4EPSPS gene and non-transgenic soybean plants: An analysis of protein species and enzymes. J. Proteom. 2013, 93, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.J.; Palma-Bautista, C.; Rojano-Delgado, A.M.; Bracamonte, E.; Portugal, J.; Alcantara-de la Cruz, R.; De Prado, R. The Triple Amino Acid Substitution TAP-IVS in the EPSPS Gene Confers High Glyphosate Resistance to the Superweed Amaranthus hybridus. Int. J. Mol. Sci. 2019, 20, 2396. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yang, Z.Y.; Nie, Y.F.; Wang, J.; Xu, P. A new type of class I bacterial 5-enopyruvylshikimate-3-phosphate synthase mutants with enhanced tolerance to glyphosate. Biochim. Et Biophys. Acta 2001, 1568, 1–6. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Baldrianova, J.; Cerny, M.; Novak, J.; Jedelsky, P.L.; Diviskova, E.; Brzobohaty, B. Arabidopsis proteome responses to the smoke-derived growth regulator karrikin. J. Proteom. 2015, 120, 7–20. [Google Scholar] [CrossRef]
- Wang, L.M.; Wang, X.C.; Jin, X.; Jia, R.Z.; Huang, Q.X.; Tan, Y.H.; Guo, A.P. Comparative proteomics of Bt-transgenic and non-transgenic cotton leaves. Proteome Sci. 2015, 13, 15. [Google Scholar] [CrossRef]
- Liu, Y.B.; Zhang, Y.X.; Song, S.Q.; Li, J.S.; Stewart, C.N.; Wei, W.; Zhao, Y.J.; Wang, W.Q. A proteomic analysis of seeds from Bt-transgenic Brassica napus and hybrids with wild B. juncea. Sci. Rep. 2015, 5, 15480. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, H.; Miao, C.; Jin, W. Integrated proteomics and metabolomics analysis of transgenic and gene-stacked maize line seeds. GM Crops Food 2021, 12, 361–375. [Google Scholar] [CrossRef]
- Yuan, H.; Zeng, X.; Shi, J.; Xu, Q.; Wang, Y.; Jabu, D.; Sang, Z.; Nyima, T. Time-Course Comparative Metabolite Profiling under Osmotic Stress in Tolerant and Sensitive Tibetan Hulless Barley. Biomed. Res. Int. 2018, 2018, 9415409. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Plischke, A.; Choi, Y.H.; Brakefield, P.M.; Klinkhamer, P.G.L.; Bruinsma, M. Metabolomic Plasticity in GM and Non-GM Potato Leaves in Response to Aphid Herbivory and Virus Infection. J. Agr. Food Chem. 2012, 60, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, L.; Chang, Y.W.; Lu, X.; Zhu, Z.; Xu, G.W. Alteration of Leaf Metabolism in Bt-Transgenic Rice (Oryza sativa L.) and Its Wild Type under Insecticide Stress. J. Proteome Res. 2012, 11, 4351–4360. [Google Scholar] [CrossRef]
- Christ, B.; Hochstrasser, R.; Guyer, L.; Francisco, R.; Aubry, S.; Hortensteiner, S.; Weng, J.K. Non-specific activities of the major herbicide-resistance gene BAR. Nat. Plants 2017, 3, 937–945. [Google Scholar] [CrossRef]
- Hao, W.Y.; Li, F.W.; Yan, W.; Li, C.C.; Hao, D.Y. Comparative metabolic profiling of four transgenic maize lines and two non-transgenic maize lines using high-performance liquid chromatography mass spectrometry. Acta Physiol. Plant 2017, 39, 167. [Google Scholar] [CrossRef]
- Chang, Y.W.; Zhao, C.X.; Zhu, Z.; Wu, Z.M.; Zhou, J.; Zhao, Y.N.; Lu, X.; Xu, G.W. Metabolic profiling based on LC/MS to evaluate unintended effects of transgenic rice with cry1Ac and sck genes. Plant Mol. Biol. 2012, 78, 477–487. [Google Scholar] [CrossRef]
- Frank, T.; Rohlig, R.M.; Davies, H.V.; Barros, E.; Engel, K.H. Metabolite Profiling of Maize Kernels-Genetic Modification versus Environmental Influence. J. Agric. Food Chem. 2012, 60, 3005–3012. [Google Scholar] [CrossRef]
- Chen, M.J.; Rao, R.S.P.; Zhang, Y.M.; Zhong, C.; Thelen, J.J. Metabolite variation in hybrid corn grain from a large-scale multisite study. Crop J. 2016, 4, 177–187. [Google Scholar] [CrossRef]
- Tang, W.J.; Hazebroek, J.; Zhong, C.; Harp, T.; Vlahakis, C.; Baumhover, B.; Asiago, V. Effect of Genetics, Environment, and Phenotype on the Metabolome of Maize Hybrids Using GC/MS and LC/MS. J. Agric. Food Chem. 2017, 65, 5215–5225. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.Y.; Li, Q.; Yu, H.T.; Wang, Z.Z.; Wang, T. Proteomics Insight into the Biological Safety of Transgenic Modification of Rice As Compared with Conventional Genetic Breeding and Spontaneous Genotypic Variation. J. Proteome Res. 2012, 11, 3019–3029. [Google Scholar] [CrossRef]
- Zabalza, A.; Orcaray, L.; Fernandez-Escalada, M.; Zulet-Gonzalez, A.; Royuela, M. The pattern of shikimate pathway and phenylpropanoids after inhibition by glyphosate or quinate feeding in pea roots. Pestic. Biochem. Phys. 2017, 141, 96–102. [Google Scholar] [CrossRef]
- Herrmann, K.M. The Shikimate Pathway—Early Steps in the Biosynthesis of Aromatic-Compounds. Plant Cell 1995, 7, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.; Jallu, S.; Singh, T.P. The shikimate pathway: Review of amino acid sequence, function and three-dimensional structures of the enzymes. Crit. Rev. Microbiol. 2015, 41, 172–189. [Google Scholar] [CrossRef] [PubMed]
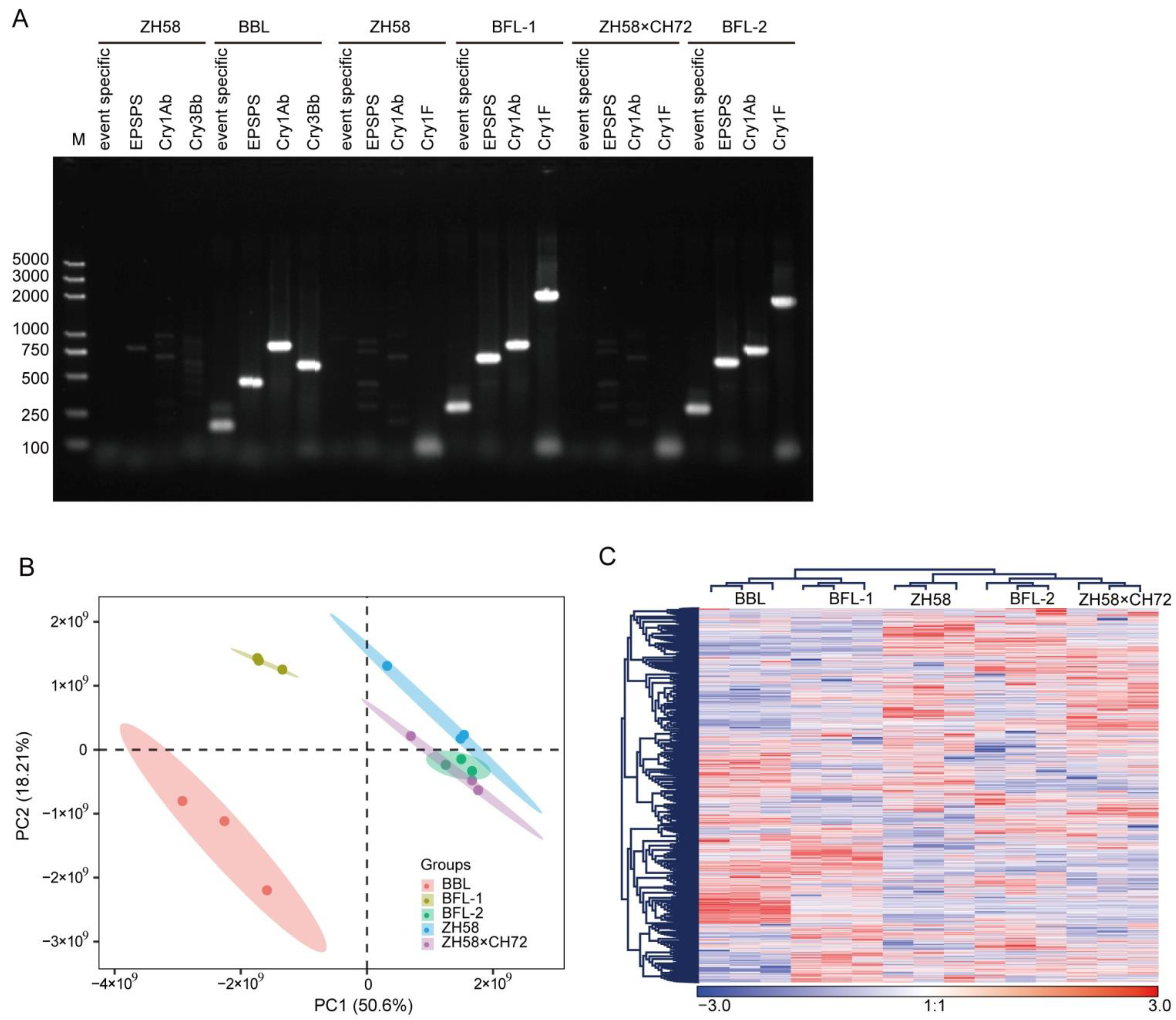


| Natural Genotypic Maize Lines | GM Maize Lines | Foreign Proteins |
|---|---|---|
| ZH58 | BBL | EPSPS |
| Cry1Ab | ||
| Cry3Bb | ||
| BFL-1 | EPSPS | |
| Cry1Ab | ||
| Cry1F | ||
| ZH58×CH72 | BFL-2 | EPSPS |
| Cry1Ab | ||
| Cry1F |
| Comparison Groups | No. of Upregulated Proteins | No. of Downregulated Proteins | No. of DEPs |
|---|---|---|---|
| BBL/ZH58 | 47 | 29 | 76 |
| BFL-1/ZH58 | 20 | 20 | 40 |
| BFL-2/ZH58×CH72 | 19 | 6 | 25 |
| BFL-1/BFL-2 | 42 | ||
| ZH58/ZH58×CH72 | 30 |
| Comparison Groups | No. of Upregulated Metabolites | No. of Downregulated Metabolites | No. of DAMs |
|---|---|---|---|
| BBL/ZH58 | 66 | 79 | 145 |
| BFL-1/ZH58 | 18 | 160 | 178 |
| BFL-2/ZH58×CH72 | 72 | 16 | 88 |
| BFL-2/BFL-1 | 189 | ||
| ZH58×CH72/ZH58 | 286 |
| Accession | Name | State | KEGG Enrichment Pathway |
|---|---|---|---|
| EPSPS | Up | Phenylalanine, tyrosine, and tryptophan biosynthesis (ko00400); Metabolic pathways (ko01100); Biosynthesis of secondary metabolites (ko01110); Biosynthesis of amino acids (ko01230) | |
| B4FR99 | Acidic endochitinase | Up | Amino sugar and nucleotide sugar metabolism (ko00520); Metabolic pathways (ko01100) |
| A0A804MXV9 | Uncharacterized protein | Up | - |
| Accession | Name | BBL | BFL-1 | BFL-2 | KEGG Enrichment Pathway |
|---|---|---|---|---|---|
| mws0520 | N-Acetyl-L-tyrosine | Up | Up | Up | - |
| Smpn009074 | 2α,3α,19α,23-tetrahydroxy-12-ursen-28-oic acid | Up | Up | Up | - |
| Lmsn009824 | 2α,3β,19α,23-Tetrahydroxyolean-12-en-28-oic acid | Up | Up | Up | - |
| pmn001319 | 1-O-Feruloyl-3-O-p-Coumaroylglycerol | Down | Down | Down | - |
| pme1173 | Allopurinol | Down | Down | Up | - |
| Lmlp003161 | N-Feruloylputrescine | Down | Down | Up | Arginine and proline metabolism (ko00330); Metabolic pathways (ko01100) |
| pme2693 | N-Acetylputrescine | Down | Down | Up | Arginine and proline metabolism (ko00330); Metabolic pathways (ko01100) |
| mws0005 | Tryptamine | Down | Down | Up | Tryptophan metabolism (ko00380); Indole alkaloid biosynthesis (ko00901); Metabolic pathways (ko01100); Biosynthesis of secondary metabolites (ko01110) |
| mws0133 | Nicotinamide | Down | Down | Up | Nicotinate and nicotinamide metabolism (ko00760); Metabolic pathways (ko01100); Biosynthesis of cofactors (ko01240) |
| Lmmp002013 | Dihydroferuloylputrescine | Down | Down | Up | - |
| pme2914 | 3-Hydroxy-3-methylpentane-1,5-dioic acid | Up | Down | Up | - |
| Comparison Group | Exogenous Proteins | Fold Change | p-Value | Exogenous Protein Content (μg/g) inGM Maize Seeds |
|---|---|---|---|---|
| BBL/ZH58 | Cry1Ab | 3.199 | 0.000658169 | 3.35 ± 0.24 |
| Cry3Bb | - | - | 2.26 ± 0.01 | |
| CP4-EPSPS | 172.77 | 1.46348 × 10−6 | 20.86 ± 3.41 | |
| BFL-1/ZH58 | Cry1Ab | - | - | 1.95 ± 0.01 |
| Cry1F | - | - | 39.46 ± 0.22 | |
| CP4-EPSPS | 23.718 | 0.000121842 | 3.79 ± 0.08 | |
| BFL-2/ZH58×CH72 | Cry1Ab | - | - | 1.81 ± 0.07 |
| Cry1F | 26.586 | 0.004223503 | 33.72 ± 0.71 | |
| CP4-EPSPS | 16.396 | 1.3585 × 10−7 | 3.49 ± 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Meng, L.; Zhao, W.; Wang, Z.; Miao, C.; Wan, Y.; Jin, W. Proteomic and Metabolomic Evaluation of Insect- and Herbicide-Resistant Maize Seeds. Metabolites 2022, 12, 1078. https://doi.org/10.3390/metabo12111078
Liu W, Meng L, Zhao W, Wang Z, Miao C, Wan Y, Jin W. Proteomic and Metabolomic Evaluation of Insect- and Herbicide-Resistant Maize Seeds. Metabolites. 2022; 12(11):1078. https://doi.org/10.3390/metabo12111078
Chicago/Turabian StyleLiu, Weixiao, Lixia Meng, Weiling Zhao, Zhanchao Wang, Chaohua Miao, Yusong Wan, and Wujun Jin. 2022. "Proteomic and Metabolomic Evaluation of Insect- and Herbicide-Resistant Maize Seeds" Metabolites 12, no. 11: 1078. https://doi.org/10.3390/metabo12111078
APA StyleLiu, W., Meng, L., Zhao, W., Wang, Z., Miao, C., Wan, Y., & Jin, W. (2022). Proteomic and Metabolomic Evaluation of Insect- and Herbicide-Resistant Maize Seeds. Metabolites, 12(11), 1078. https://doi.org/10.3390/metabo12111078





