Protective Effect of Catharanthus roseus Extract on Cadmium-Induced Toxicity in Albino Rats: A Putative Mechanism of Detoxification
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Plant
2.2. Haematological Parameters
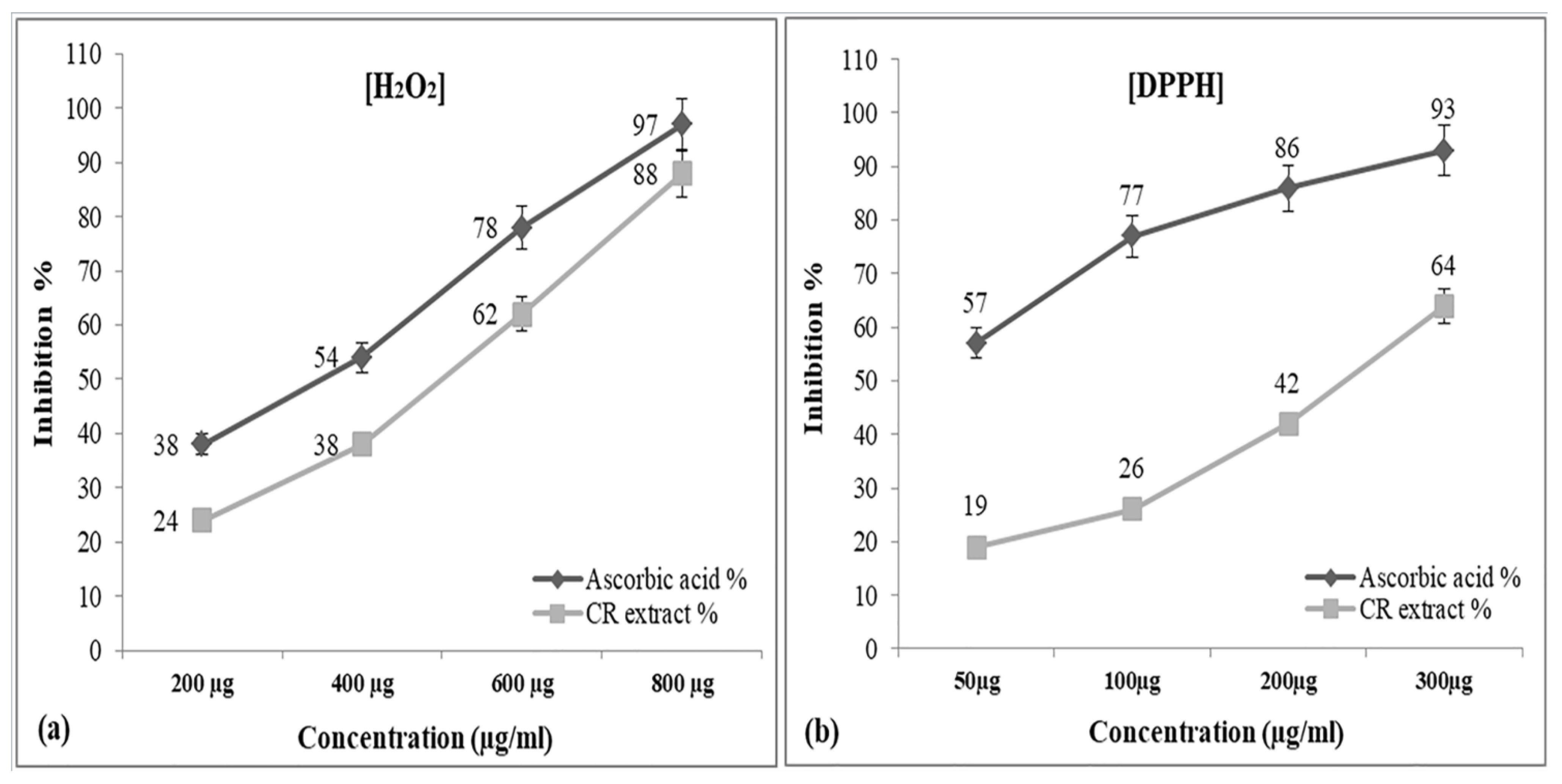
2.3. Hydrogen Peroxide Radical Scavenging Assay
2.4. DPPH (1,1-Diphenyl-2-picrylhydrazyl) Radical Scavenging Assay
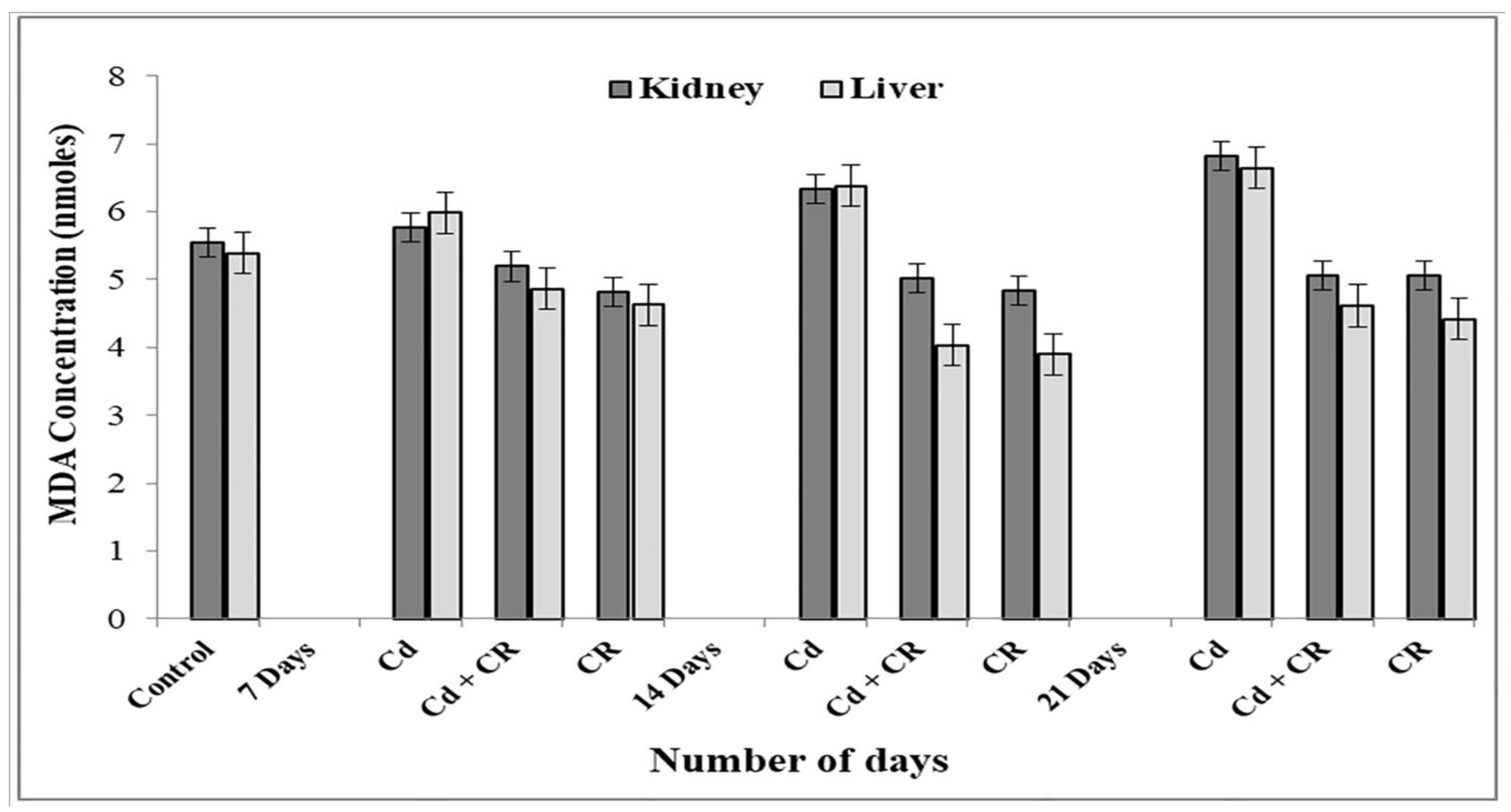
2.5. Lipid Peroxidation Assay
2.6. Detection of DNA Strand Breaks
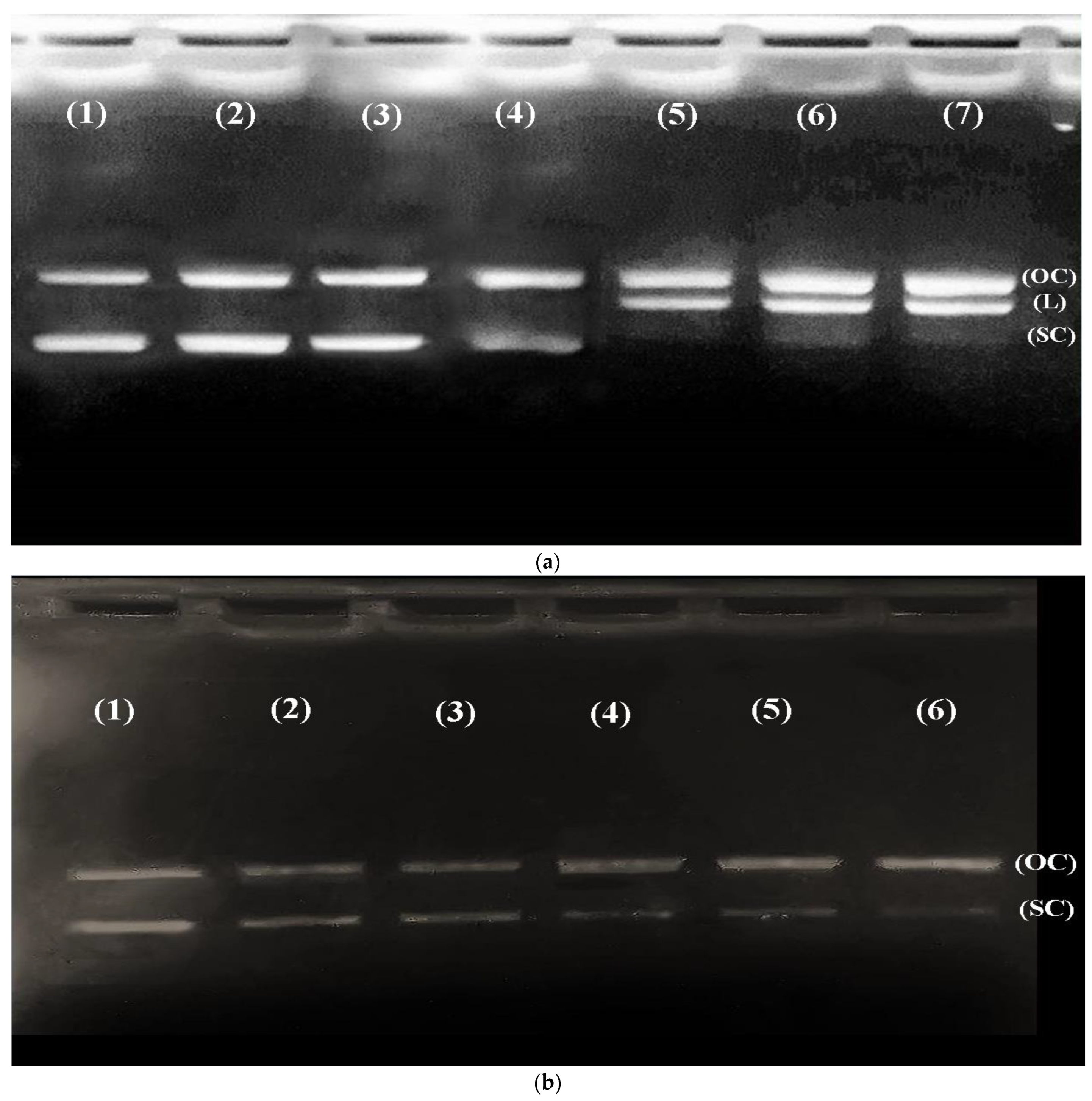
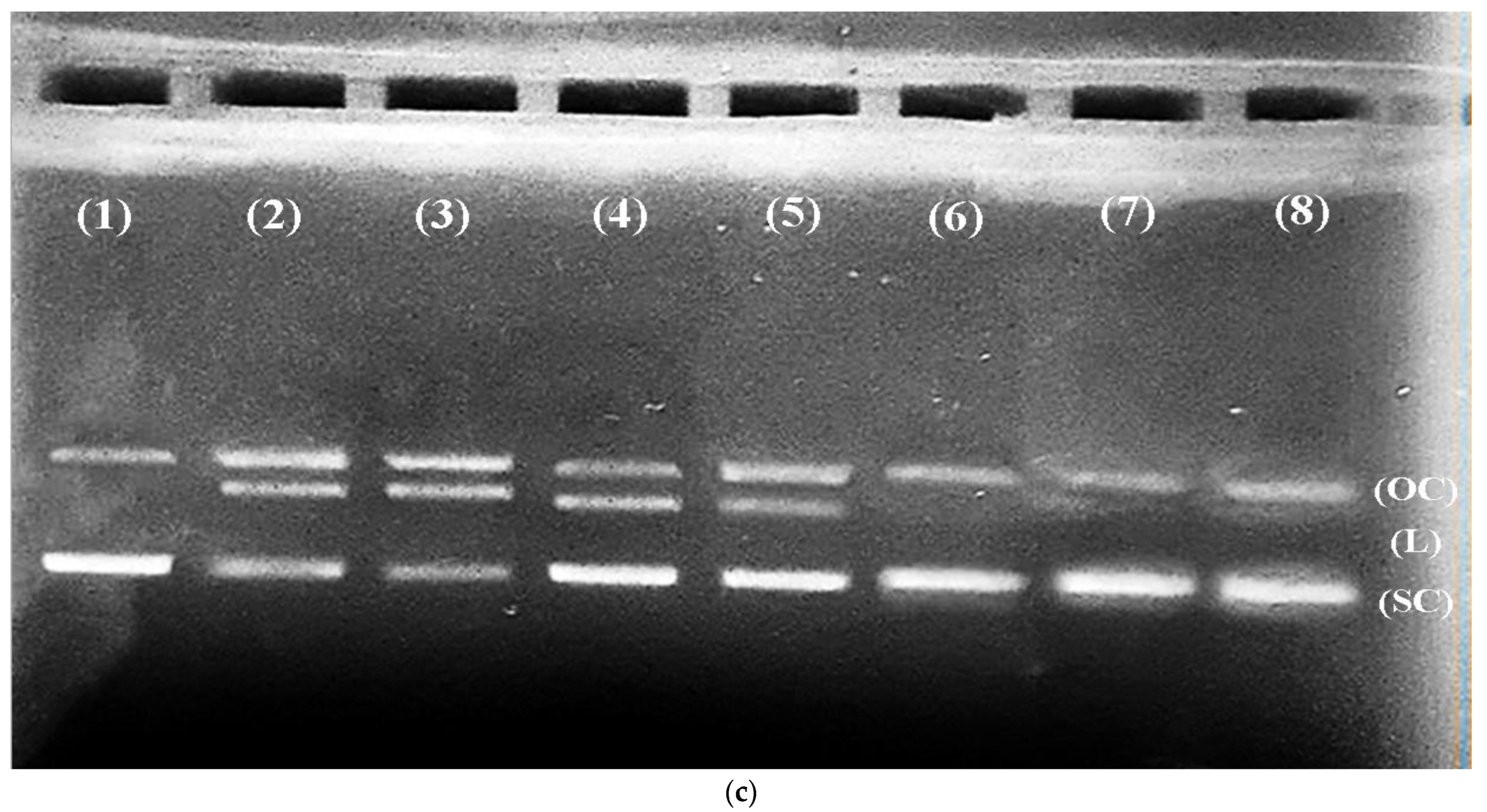
2.7. DNA Protection Assay
2.8. Antioxidant Enzyme Activity Assay
2.9. Comet Assay
3. Results
3.1. CR Extract Reduce Cd Toxicity in Albino Rats (Rattus norvegicus)
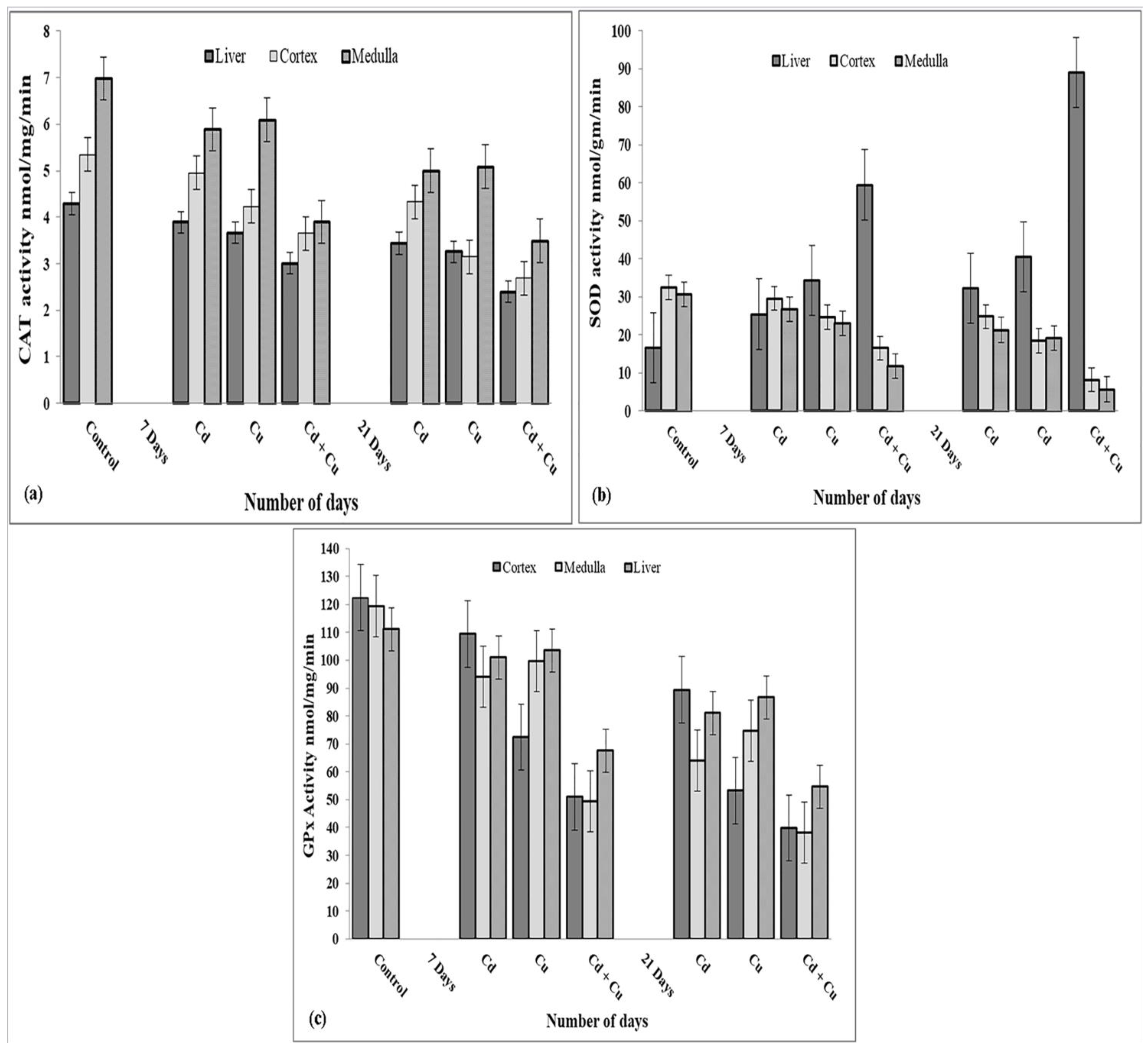
3.2. CR Extract Showed Antioxidant Effects
3.3. CR Extract Protects against Lipid Peroxidation of Rat Tissue
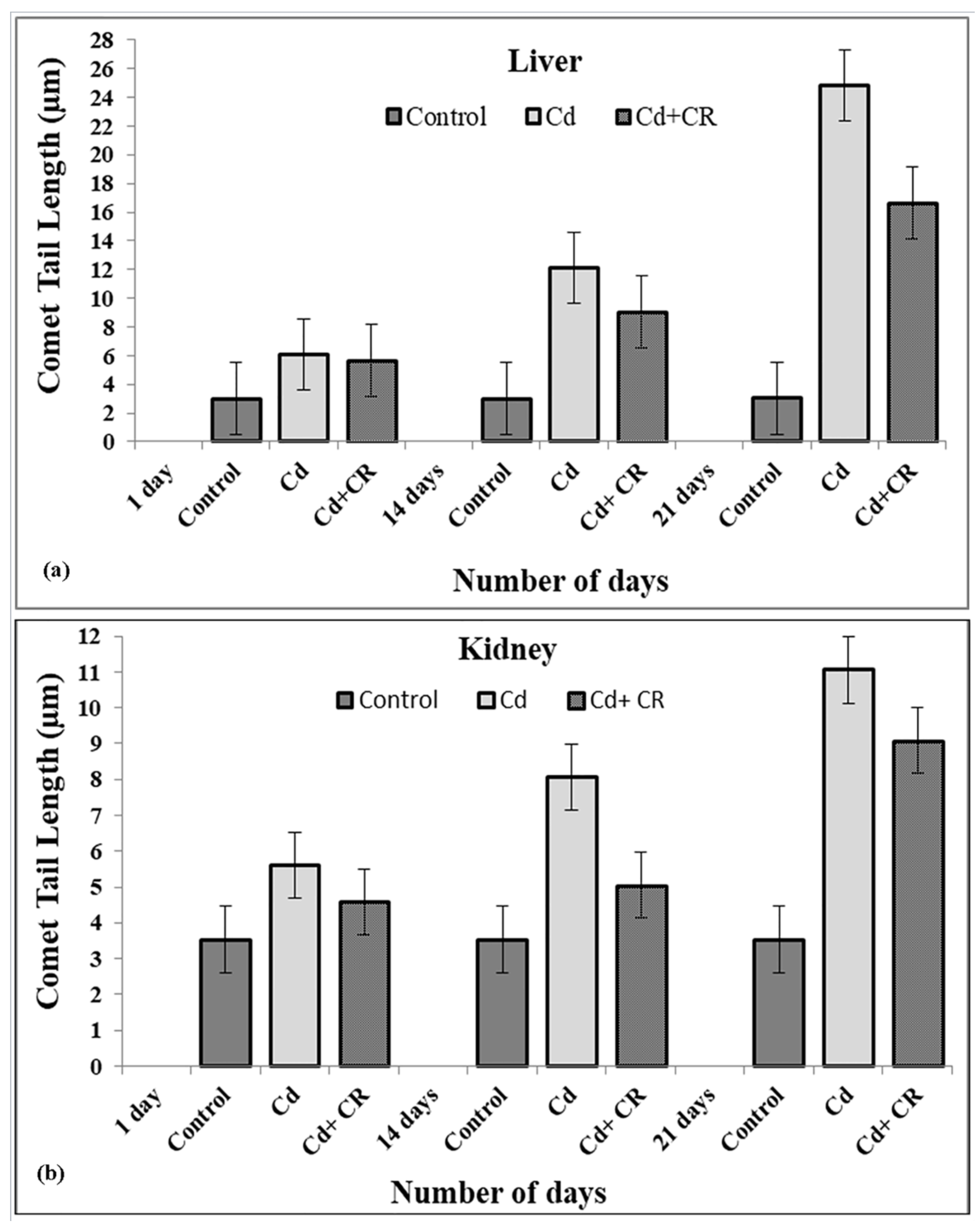
3.4. CR Extract Showed Protection against Cd Induced Oxidative DNA Damage
3.5. Cd Causes Oxidative Stress Independent of Copper
3.6. CR Extract Protects against Cellular DNA Damage Caused by Cd in Rat Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- UNEP; GPA. The State of the Marine Environment: Trends and Processes; Coordination Office; UNEP/GPAArtoos Drukkerijen: Rijswijk, The Netherlands, 2006; Available online: https://wedocs.unep.org/20.500.11822/12469 (accessed on 2 June 2022).
- Bradl, H. Heavy Metals in the Environment: Origin, Interaction and Remediation; Academic Press: London, UK, 2002; Volume 6, pp. 1–10. [Google Scholar]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Zhuo, J.Z.; Peng, Y.D.; Wang, Z. Comparative analysis of cadmium-induced toxicity and survival responses in the wolf spider Pirata subpiraticus under low-temperature treatment. Environ. Sci. Pollut. Res. Int. 2022, 29, 32832–32844. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzóska, M.M. Metals in cosmetics: Implications for human health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Preventing Disease through Healthy Environments: Exposure to Cadmium: A Major Public Health Concern. 2019. Available online: https://apps.who.int/iris/handle/10665/329480 (accessed on 2 June 2022).
- Johnston, P.; Santillo, D.; Stringer, R.; Ashton, J.; McKay, B.; Verbeek, M.; Jackson, E.; Landman, J.; van den Broek, J.; Samsom, D.; et al. Report on the World’s Oceans; Greenpeace Research Laboratories Report; Greenpeace: Amsterdam, The Netherlands, 1998; pp. 12–14. [Google Scholar]
- World Health Organization. The Public Health Impact of Chemicals: Knowns and Unknowns: Data Addendum for 2019. 2021. Available online: https://apps.who.int/iris/handle/10665/342273 (accessed on 31 June 2022).
- Ijaz, M.U.; Batool, M.; Batool, A.; Al-Ghanimd, K.A.; Zafar, S.; Ashraf, A.; Al-Misned, F.; Ahmed, Z.; Shahzadi, S.; Samad, A.; et al. Protective effects of vitexin on cadmium-induced renal toxicity in rats. SJBS 2021, 28, 5860–5864. [Google Scholar] [CrossRef]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Chapter 3: Health effects. In Toxicological Profile for Cadmium; ATSDR (US): Atlanta, GA, USA, 2012. [Google Scholar]
- Atef, M.M.A.; Ibrahim, A.A.F.; Abd EL-Latif, A.N.; Aziz, W.S. Antioxidant effects of curcumin against cadmium chloride-induced oxidative stress in the blood of rats. J. Pharmacol. Phytother. 2014, 6, 33–40. [Google Scholar] [CrossRef][Green Version]
- World Health Organization & International Programme on Chemical Safety. Cadmium: Environmental Aspects/Published under the Joint Sponsorship of the United Nations Environment Programme, The International Labour Organisation, and the World Health Organization. 1992. Available online: https://apps.who.int/iris/handle/10665/39366 (accessed on 17 October 2022).
- Takiguchi, M.; Yoshihara, S. New aspects of cadmium as endocrine disruptor. Environ. Sci. 2006, 13, 107–116. [Google Scholar]
- Siu, E.R.; Mruk, D.D.; Porto, C.S.; Cheng, C.Y. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009, 238, 240–249. [Google Scholar] [CrossRef]
- Jin, T.; Lu, J.; Nordberg, M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology 1998, 19, 529–535. [Google Scholar]
- Meplan, C.; Mann, K.; Hainaut, P. Cadmium Induces Conformational Modifications of Wild-type p53 and Suppresses p53 Response to DNA Damage in Cultured Cells. J. Biol. Chem. 1999, 274, 31663–31670. [Google Scholar] [CrossRef]
- Zhong, Z.J.; Troll, W.; Koenig, K.L.; Frenkel, K. Carcinogenic sulfide salts of nickel and cadmium induce H2O2 formation by human polymorphonuclear leukocytes. Cancer Res. 1990, 50, 7564–7570. [Google Scholar]
- Rafati, R.M.; Rafati, R.M.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Caspian J. Intern. Med. 2017, 8, 135–145. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D.; Hassoun, E.; Bagchi, M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 2001, 20, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Badisa, V.L.; Latinwo, L.M.; Odewumi, C.O.; Ikediobi, C.O.; Badisa, R.B.; Ayuk-Takem, L.T.; Nwoga, J.; West, J. Mechanism of DNA damage by cadmium and interplay of antioxidant enzymes and agents. Environ. Toxicol. 2007, 22, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.L.; Phillips, D.H. Oxidative DNA damage mediated by copper (II), iron (II) and nickel (II) Fenton reactions: Evidence for site-specific mechanisms in the formation of double-strand breaks, 8-hydroxydeoxyguanosine and putative intra-strand cross-links. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1999, 424, 23–36. [Google Scholar] [CrossRef]
- Arif, H.; Sohail, A.; Farhan, M.; Rehman, A.A.; Ahmad, A.; Hadi, S.M. Flavonoids-induced redox cycling of copper ions leads to generation of reactive oxygen species: A potential role in cancer chemoprevention. Int. J. Biol. Macromol. 2018, 106, 569–578. [Google Scholar] [CrossRef]
- Illán-Cabeza, N.A.; Vilaplana, R.A.; Alvarez, Y.; Akdi, K.; Kamah, S.; Hueso-Ureña, F.; Quirós, M.; González-Vílchez, F.; Moreno-Carretero, M.N. Synthesis, structure and biological activity of a new and efficient Cd (II)-uracil derivative complex system for cleavage of DNA. J. Biol. Inorg. Chem. 2005, 10, 924–934. [Google Scholar] [CrossRef]
- Natarajan, A.; Ahmed, K.S.Z.; Sundaresan, S.; Sivaraj, A.; Devi, K.; Kumar, B.S. Effect of aqueous flower extract of catharanthus roseus on alloxan induced diabetes in male albino rats. Int. J. Pharm. Sci. Drug Res. 2012, 4, 150–153. [Google Scholar]
- Saboon, C.S.K.; Arshad, S.; Amjad, M.S.; Akhtar, M.S. Natural Compounds Extracted from Medicinal Plants and Their Applications. In Natural Bio-Active Compounds; Akhtar, M., Swamy, M., Sinniah, U., Eds.; Springer: Singapore, 2019; pp. 193–207. [Google Scholar] [CrossRef]
- Van-Der-Heijden, R.; Jacobs, D.I.; Snoeijer, W.; Hallard, D.; Verpoorte, R. The Catharanthus alkaloids: Pharmacognosy and biotechnology. Curr. Med. Chem. 2004, 11, 607–628. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, X.; Guo, X.; Guo, Q.; Li, D. Metabolomics characterization of two Apocynaceae plants, Catharanthus roseus and Vinca minor, using GC-MS and LC-MS methods in combination. Molecules 2017, 22, 997. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Goyal, S.; Mukim, M.; Meghani, M.; Patwekar, F.; Patwekar, M.; Khan, S.K.; Sharma, G.N. A Comprehensive Review on Catharanthus roseus L. (G.) Don: Clinical Pharmacology, Ethnopharmacology and Phytochemistry. J. Pharmacol. Res. Dev. 2022, 4, 17–36. [Google Scholar] [CrossRef]
- Mustafa, N.R.; Verpoorte, R. Phenolic compounds in Catharanthus roseus. Phytochem Rev. 2007, 6, 243–258. [Google Scholar] [CrossRef]
- Levêque, D.; Jehl, F. Molecular pharmacokinetics of catharanthus (vinca) alkaloids. J. Clin. Pharmacol. 2007, 47, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Arif, H.; Rehmani, N.; Farhan, M.; Ahmad, A.; Hadi, S.M. Mobilization of Copper ions by Flavonoids in Human Peripheral Lymphocytes Leads to Oxidative DNA Breakage: A Structure Activity Study. Int. J. Mol. Sci. 2015, 16, 26754–26769. [Google Scholar] [CrossRef] [PubMed]
- Panchal, H.; Sachdeva, S.N.; Bhardwaj, J.K. Ultrastructural analysis of cadmium-induced toxicity and its alleviation by antioxidant quercetin in caprine testicular germ cells in vitro. Ultrastruct. Pathol. 2022, 46, 259–267. [Google Scholar] [CrossRef]
- Hashim, M.; Tabassum, B.; Abd Allah, E.F.; Hashem, A.; Bajaj, P. Bioremediation of cadmium induced renal toxicity in Rattus norvegicus by medicinal plant Catharanthus roseus. Saudi J. Biol. Sci. 2018, 25, 1739–1742. [Google Scholar] [CrossRef]
- Golla, U.R.; Gajam, P.K.; Mohammad, A.R.; Kumar, K.A.; Raj, B.S.S. Assessment of bioactivity of Desmostachya bipinnata (L.) Stapf using brine shrimp (artemia salina) lethality assay. Pharmacologyonline 2011, 3, 982–990. [Google Scholar]
- Brunning, R.D. Hematology: Basic Principles and Practice. JAMA J. Am. Med. Assoc. 1995, 274, 1722–1736. [Google Scholar] [CrossRef]
- Gülçin, I.; Alici, H.A.; Cesur, M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem. Pharm. Bull. 2005, 53, 281–285. [Google Scholar] [CrossRef]
- Abe, N.; Murata, T.; Hirota, A. Novel DPPH Radical Scavengers, Bisorbicillinol and Demethyltrichodimerol, from a Fungus. Biosci. Biotechnol Biochem. 1998, 62, 661–666. [Google Scholar] [CrossRef]
- Tappel, A.L.; Zalkin, H. Inhibition of lipid peroxidation in mitochondria by vitamin E. Arch. Biochem. Biophys. 1959, 80, 333–336. [Google Scholar] [CrossRef]
- Muiras, M.L.; Giacomoni, P.U.; Tachon, P. Modulation of DNA breakage induced via the Fenton reaction. Mutat. Res. 1993, 295, 47–54. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, H.R.; Kim, J.; Jang, Y.S. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J. Agric. Food Chem. 2002, 50, 6490–6496. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Miller, M.J.; Sadowska-Krowicka, H.; Chotinaruemol, S.; Kakkis, J.L.; Clark, D.A. Amelioration of chronic ileitis by nitric oxide synthase inhibition. J. Pharmacol. Exp. Ther. 1993, 264, 11–16. [Google Scholar]
- Sanchez-Moreno, C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, P.K. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Guleria, S.; Tiku, A.K.; Rana, S. Antioxidant and free radical scavenging activity of acetone extract fractions of Terminalia belleria Roxb fruit. Indian J. Biochem. Biophys. 2011, 47, 110–116. [Google Scholar]
- Heinz-Peter, S. The chemical basis of radiation biology: By C. von Sonntag; published by Taylor and Francis, London. pp. 515. J. Photochem. Photobiol B Biol. 1989, 3, 465. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Linder, M.C. Introduction and Overview of Copper as an Element Essential for Life. In Biochemistry of Copper. Biochemistry of the Elements; Springer: Boston, MA, USA, 1991; Volume 10. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, H.M.; Lee, Y.H.; Jeong, C.B.; Park, J.C.; Lee, J.S. Adverse effects of a synthetic pyrethroid insecticide cypermethrin on life parameters and antioxidant responses in the marine copepods Paracyclopina nana and Tigriopus japonicus. Chemosphere 2019, 217, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Arif, A.; Salam, S.; Mahmood, R. Bioallethrin-induced generation of reactive species and oxidative damage in isolated human erythrocytes. Toxicol. In Vitro 2020, 65, 104810. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Punia, S.; Kaur, J.; Kumar, R.; Kumar, K. Catharanthus roseus: A medicinal plant with potent anti-tumor properties. Int. J. Res. Ayurveda Pharm. 2014, 5, 652–656. [Google Scholar] [CrossRef]
- Halsted, C.H.; Villanueva, J.A.; Devlin, A.M.; Niemela, O.; Parkkila, S.; Garrow, T.A.; Wallock, L.M.; Shigenaga, M.K.; Melnyk, S.; James, S.J. Folate deficiency disturbs hepatic methionine metabolism and promotes liver injury in the ethanol-fed micropig. Proc. Natl. Acad. Sci. USA 2002, 99, 10072–10077. [Google Scholar] [CrossRef]
- Pravenec, M.; Kozich, V.; Krijt, J.; Sokolová, J.; Zídek, V.; Landa, V.; Simáková, M.; Mlejnek, P.; Silhavy, J.; Oliyarnyk, O.; et al. Folate deficiency is associated with oxidative stress, increased blood pressure, and insulin resistance in spontaneously hypertensive rats. Am. J. Hypertens. 2013, 26, 135–140. [Google Scholar] [CrossRef]
- Trotta, R.J.; Sullivan, S.G.; Stern, A. Lipid peroxidation and haemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide. The relative roles of haem- and glutathione-dependent decomposition of t-butyl hydroperoxide and membrane lipid hydroperoxides in lipid peroxidation and haemolysis. Biochem. J. 1983, 212, 759–772. [Google Scholar] [CrossRef]
- Tesoriere, L.; D’Arpa, D.; Butera, D.; Pintaudi, A.M.; Allegra, M.; Livrea, M.A. Exposure to malondialdehyde induces an early redox unbalance preceding membrane toxicity in human erythrocytes. Free Radical Res. 2002, 36, 89–97. [Google Scholar] [CrossRef]
- Puppo. A.; Hallwell. B. Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Biochem. J. 1988, 249, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Tsou, T.C.; Yang, J.L. Formation of reactive oxygen species and DNA strand breakage during interaction of chromium (III) and hydrogen peroxide in vitro: Evidence for a chromium (III)-mediated Fenton-like reaction. Chem. Biol. Interact. 1996, 102, 133–153. [Google Scholar] [CrossRef]
- Chater, S.; Douki, T.; Garrel, C.; Favier, A.; Sakly, M.; Abdelmelek, H. Cadmium-induced oxidative stress and DNA damage in kidney of pregnant female rats. Comptes Rendus Biol. 2008, 331, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 6175804. [Google Scholar] [CrossRef]
| 7 days | Parameters | Control | Cadmium | Cadmium + CR | CR |
| RBC | 8.42 ± 3.19 | 5.87 ± 2.23 * | 6.98 ± 2.64 ns | 8.61 ± 3.26 ns | |
| WBC | 12.26 ± 0.001 | 14.98 ± 0.153 * | 12.98 ± 0.053 ns | 12.07 ± 0.022 ns | |
| PLT | 625.25 ± 15.58 | 961.05 ± 156.49 * | 612.04 ± 14.89 * | 621.78± 13.45 ns | |
| Hb | 158.01 ± 7.58 | 115.98 ± 6.08 * | 148.54 ± 6.98 * | 155.06 ± 7.35 ns | |
| PCV | 42.05 ± 0.81 | 45.05 ± 0.02 * | 41.91 ± 0.66 ns | 42.01 ± 0.54 ns | |
| MCV | 59.29 ± 1.78 | 62.01 ± 2.45 * | 58.97 ± 1.49 * | 58.79 ± 1.66 ns | |
| MCH | 18.79 ± 0.56 | 19.17 ± 1.45 * | 18.69 ± 0.43 ns | 16.28 ± 0.73 ns | |
| 21 days | RBC | 8.73 ± 3.30 | 5.67 ± 2.14 * | 7.25 ± 2.74 ns | 8.58 ± 3.25 ns |
| WBC | 11.77 ± 0.009 | 13.78 ± 1.29 * | 12.59 ± 0.034 ns | 11.98 ± 0.01 ns | |
| PLT | 624.29 ± 14.88 | 923.50 ± 186.66 * | 619.14 ± 13.99 ns | 621.68 ± 12.45 ns | |
| Hb | 156.98 ± 7.56 | 118.02 ± 6.57 * | 157.08 ± 7.54 ns | 142.58 ± 6.57 ns | |
| PCV | 43.03 ± 089 | 44.58 ± 0.03 * | 42.88 ± 0.65 ns | 42.98 ± 0.52 ns | |
| MCV | 60.01 ± 1.97 | 63.47 ± 0.71 * | 60.96 ± 1.73 ns | 60.00 ± 1.49 ns | |
| MCH | 18.49 ± 1.24 | 22.03 ± 0.66 * | 19.46 ± 1.78 * | 18.20 ± 1.59 ns |
| Liver | Groups | Control | Cadmium Treated | Cadmium + CR | CR | |
| Sets | ||||||
| Set I: Acute (1d) | 5.23 ± 0.003 | 3.40 ± 0.006 * | 5.347 ± 0.003 * | 4.18 ± 0.008 ns | ||
| Set II: Sub-acute (7ds) | 5.31 ± 0.004 | 3.03 ± 0.001 * | 5.38 ± 0.007 * | 5.22 ± 0.008 ns | ||
| Set III: Sub-acute (14ds) | 5.29 ± 0.005 | 3.02 ± 0.003 * | 5.35 ± 0.004 * | 4.98 ± 0.007 ns | ||
| Set IV: Sub-acute (21ds) | 5.38 ± 0.003 | 2.66 ± 0.006 * | 5.37 ± 0.004 ns | 5.42 ± 0.006 ns | ||
| Kidney | Set I: Acute (1d) | 6.42 ± 0.003 | 4.58 ± 0.006 * | 6.47 ± 0.003 * | 5.88 ± 0.008 ns | |
| Set II: Sub-acute (7ds) | 6.53 ± 0.004 | 4.08 ± 0.001 * | 6.68 ± 0.007 * | 6.49 ± 0.008 ns | ||
| Set III: Sub-acute (14ds) | 6.41 ± 0.005 | 4.09 ± 0.003 * | 6.40 ± 0.004 ns | 6.27± 0.007 ns | ||
| Set IV: Sub-acute (21ds) | 6.57 ± 0.003 | 3.71 ± 0.006 * | 6.56 ± 0.004 ns | 6.25 ± 0.006 ns | ||
| Liver | Groups | Control | Cadmium Treated | Cadmium + CR | CR | |
| Sets | ||||||
| Set I: Acute (1d) | 9.04 ± 0.004 | 4.82 ± 0.024 * | 10.01 ± 0.003 * | 7.85 ± 0.004 ns | ||
| Set II: Sub-acute (7ds) | 9.13 ± 0.005 | 4.24 ± 0.010 * | 9.80 ± 0.078 * | 7.06 ± 0.012 ns | ||
| Set III: Sub-acute (14ds) | 9.11 ± 0.004 | 4.05 ± 0.003 * | 10.13 ± 0.093 * | 8.98 ± 0.007 ns | ||
| Set IV: Sub-acute (21ds) | 9.25 ± 0.009 | 3.96 ± 0.006 * | 9.94 ± 0.066 * | 9.03 ± 0.006 ns | ||
| Kidney | Set I: Acute (1d) | 16.01 ± 0.004 | 10.79 ± 0.004 * | 18.29 ± 0.003 * | 9.35 ± 0.004 ns | |
| Set II: Sub-acute (7ds) | 16.13 ± 0.005 | 11.09 ± 0.003 * | 14.59 ± 0.018 * | 10.46 ± 0.002 ns | ||
| Set III: Sub-acute (14ds) | 16.51 ± 0.004 | 9.09 ± 0.003 * | 12.13 ± 0.023 * | 11.98 ± 0.007 ns | ||
| Set IV: Sub-acute (21ds) | 16.51 ± 0.009 | 8.86 ± 0.006 * | 13.94± 0.046 * | 15.93 ± 0.003 ns | ||
| Liver | Groups | Control | Cadmium Treated | Cadmium + CR | CR | |
| Sets | ||||||
| Set I: Acute (1d) | 42.14 ± 0.004 | 24.82 ± 0.024 * | 43.01 ± 0.003 * | 37.26 ± 0.004 ns | ||
| Set II: Sub-acute (7ds) | 42.17 ± 0.005 | 24.24 ± 0.010 * | 41.60 ± 0.078 * | 37.36 ± 0.012 ns | ||
| Set III: Sub-acute (14ds) | 42.15 ± 0.004 | 24.05 ± 0.003 * | 40.13 ± 0.093 * | 38.98 ± 0.004 ns | ||
| Set IV: Sub-acute (21ds) | 43.55 ± 0.009 | 23.96 ± 0.002 * | 39.94 ± 0.006 * | 39.23 ± 0.003 ns | ||
| Kidney | Set I: Acute (1d) | 52.43 ± 0.010 | 34.31 ± 0.002 * | 53.24 ± 0.103 * | 39.56 ± 0.005 ns | |
| Set II: Sub-acute (7ds) | 52 ± 0.004 | 31.78 ± 0.002 * | 52.68 ± 0.003 * | 41.98 ± 0.002 ns | ||
| Set III: Sub-acute (14ds) | 51.88 ± 0.004 | 29.66 ± 0.003 * | 52.28 ± 0.007 * | 49.36 ± 0.003 ns | ||
| Set IV: Sub-acute (21ds) | 52.30 ± 0.042 | 27.03 ± 0.014 * | 53.17 ± 0.005 * | 51.96 ± 0.014 ns | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashim, M.; Arif, H.; Tabassum, B.; Arif, A.; Rehman, A.A.; Rehman, S.; Khanam, R.; Khan, B.; Hussain, A.; Barnawi, J.; et al. Protective Effect of Catharanthus roseus Extract on Cadmium-Induced Toxicity in Albino Rats: A Putative Mechanism of Detoxification. Metabolites 2022, 12, 1059. https://doi.org/10.3390/metabo12111059
Hashim M, Arif H, Tabassum B, Arif A, Rehman AA, Rehman S, Khanam R, Khan B, Hussain A, Barnawi J, et al. Protective Effect of Catharanthus roseus Extract on Cadmium-Induced Toxicity in Albino Rats: A Putative Mechanism of Detoxification. Metabolites. 2022; 12(11):1059. https://doi.org/10.3390/metabo12111059
Chicago/Turabian StyleHashim, Mohammad, Hussain Arif, Baby Tabassum, Amin Arif, Ahmed A. Rehman, Shahnawaz Rehman, Rehnuma Khanam, Bushra Khan, Arif Hussain, Jameel Barnawi, and et al. 2022. "Protective Effect of Catharanthus roseus Extract on Cadmium-Induced Toxicity in Albino Rats: A Putative Mechanism of Detoxification" Metabolites 12, no. 11: 1059. https://doi.org/10.3390/metabo12111059
APA StyleHashim, M., Arif, H., Tabassum, B., Arif, A., Rehman, A. A., Rehman, S., Khanam, R., Khan, B., Hussain, A., Barnawi, J., Tayeb, F. J., Algehainy, N., Altemani, F. H., Mir, R., Almutairi, F. M., Ullah, M. F., Elfaki, I., & Ajmal, M. R. (2022). Protective Effect of Catharanthus roseus Extract on Cadmium-Induced Toxicity in Albino Rats: A Putative Mechanism of Detoxification. Metabolites, 12(11), 1059. https://doi.org/10.3390/metabo12111059








