The Cardiovascular Benefits and Infections Risk of SGLT2i versus Metformin in Type 2 Diabetes: A Systemic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Outcome Indicators
2.5. Data Synthesis and Analysis
3. Results
3.1. Search Results
3.2. Study Characteristics
3.3. Methodological Quality
3.4. Outcomes
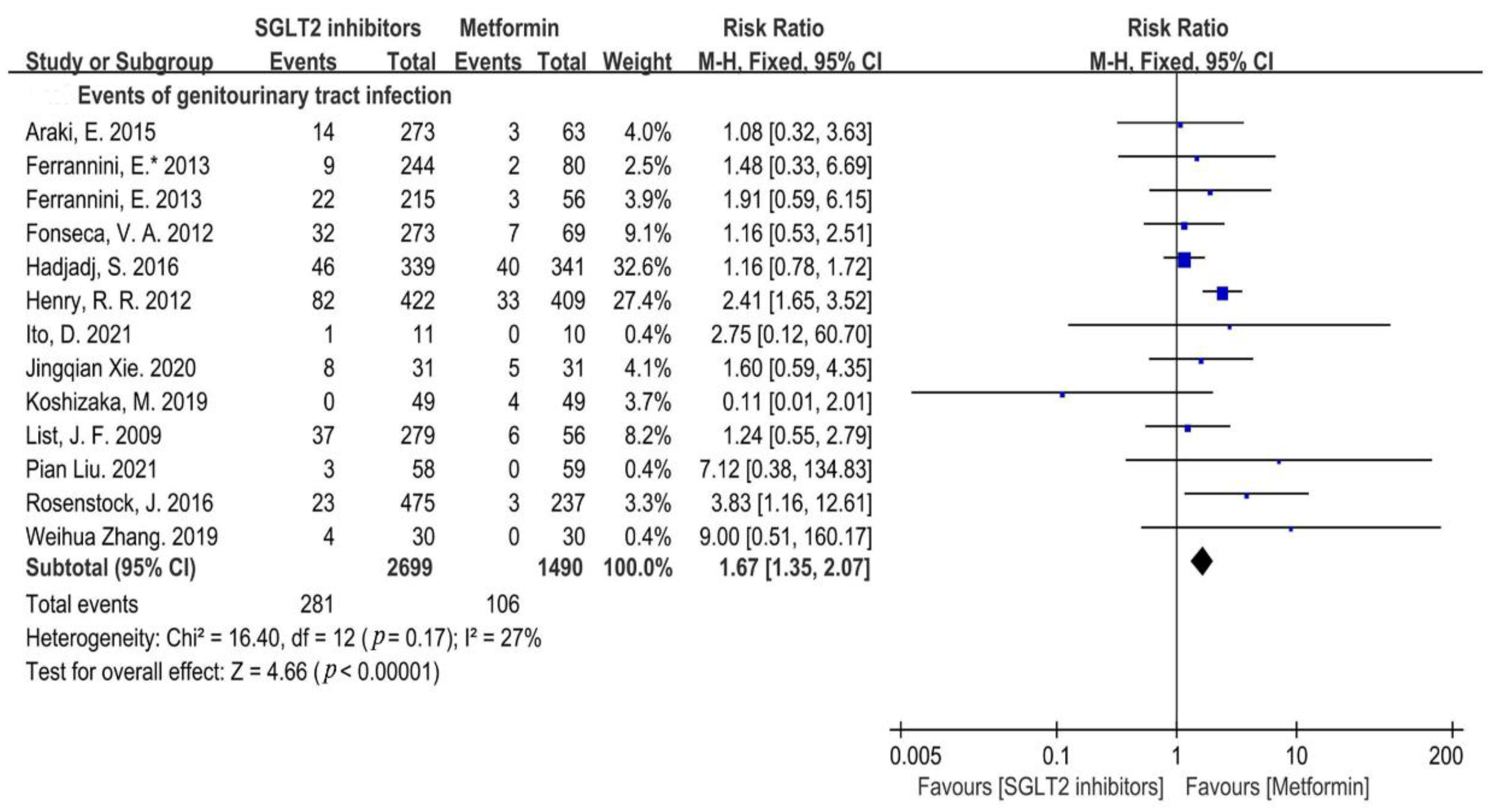
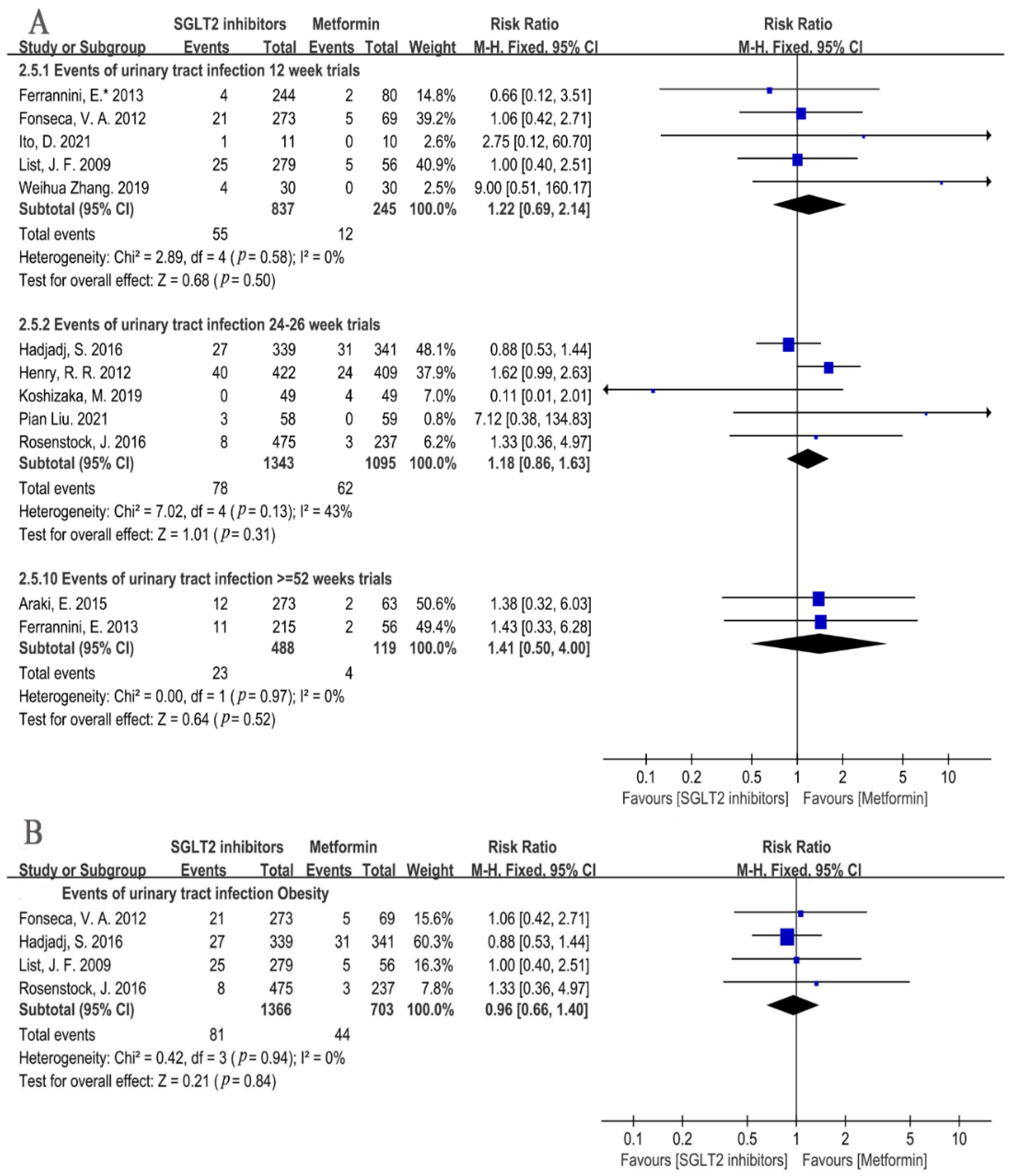
3.4.1. Infection Incidence Risk
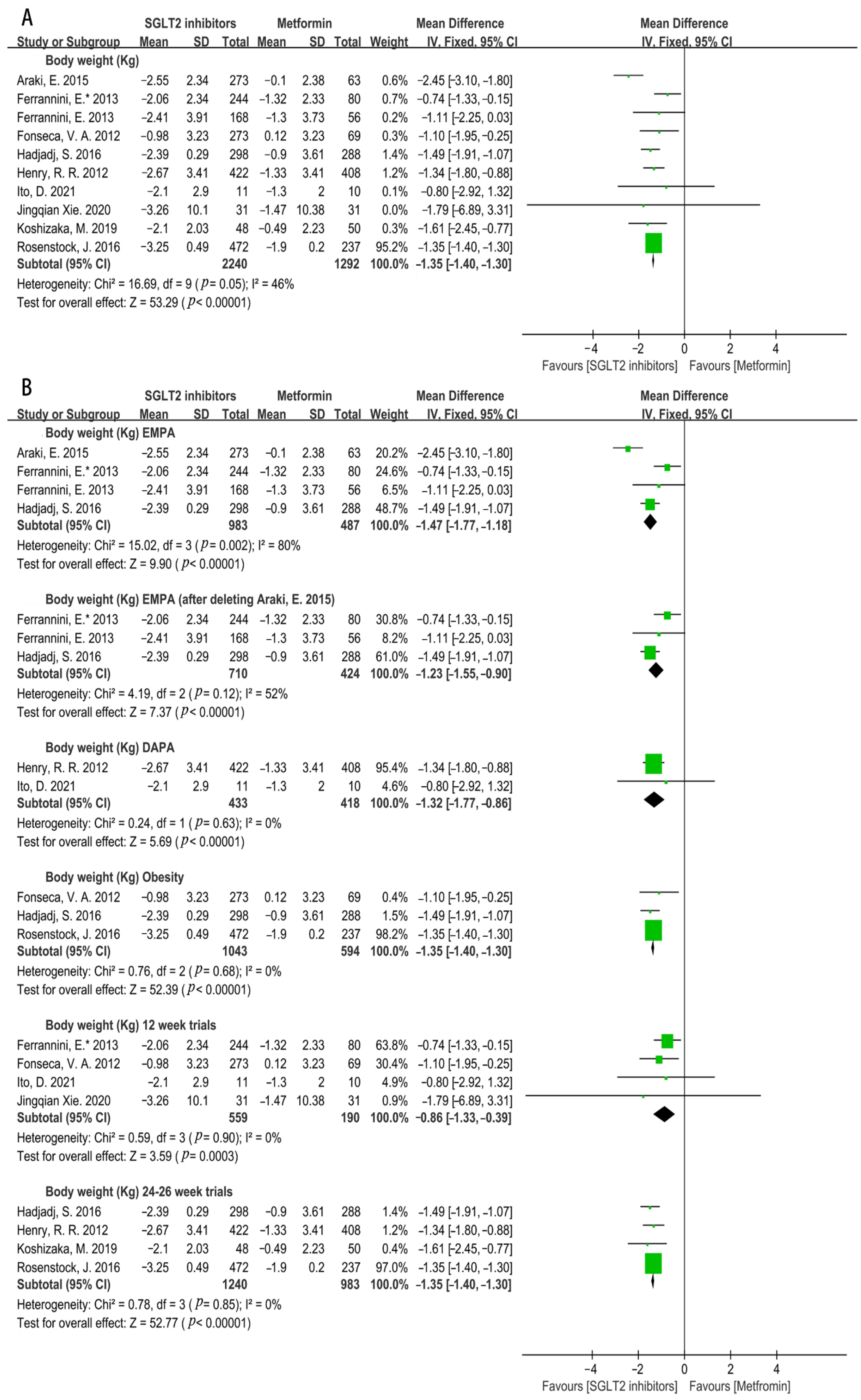
3.4.2. Effects on Cardiovascular Risk Factors
3.4.3. Efficacy on Glycemic Control
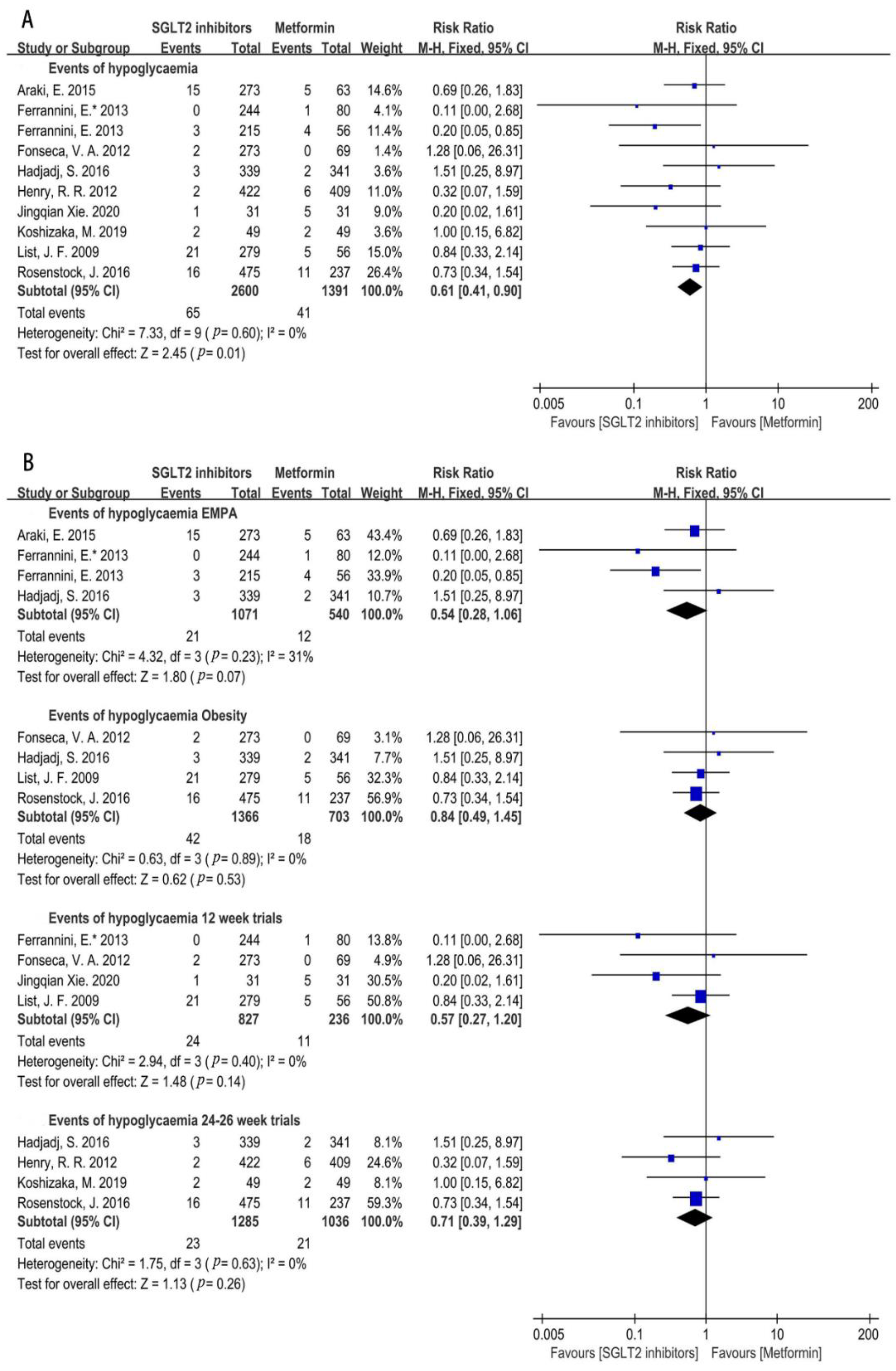
3.4.4. Hypoglycemia Incidence Risk
3.4.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Foley, R.N.; Collins, A.J. End-stage renal disease in the United States: An update from the United States Renal Data System. J. Am. Soc. Nephrol. 2007, 18, 2644–2648. [Google Scholar] [CrossRef]
- Hu, C.; Jia, W. Diabetes in China: Epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes 2018, 67, 3–11. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Guidance for Industry: Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes 2008. Available online: http://www.fda.gov/downloads/Drugs/Guidances/ucm071627.pdf (accessed on 4 February 2022).
- Ferrannini, E.; Solini, A. SGLT2 inhibition in diabetes mellitus: Rationale and clinical prospects. Nat. Rev. Endocrinol. 2012, 8, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Min, K.W.; Chuang, L.M.; Kokubo, S.; Yoshida, S.; Cha, B.S. Efficacy, safety, and tolerability of ipragliflozin in Asian patients with type 2 diabetes mellitus and inadequate glycemic control with metformin: Results of a phase 3 randomized, placebo-controlled, double-blind, multicenter trial. J. Diabetes Investig. 2016, 7, 366–373. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Gibson, A.K.; McGill, J.B.; Maddukuri, G.; Al-Aly, Z. Comparative effectiveness of sodium-glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern. Med. 2021, 181, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Vasilakou, D.; Karagiannis, T.; Athanasiadou, E.; Mainou, M.; Liakos, A.; Bekiari, E.; Sarigianni, M.; Matthews, D.R.; Tsapas, A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ortega, G.; Goring, S.; Bennett, H.A.; Bergenheim, K.; Sternhufvud, C.; Mukherjee, J. Network meta-analysis of treatments for type 2 diabetes mellitus following failure with metformin plus sulfonylurea. Curr. Med. Res. Opin. 2016, 32, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Foote, C.; Blomster, J.; Toyama, T.; Perkovic, V.; Sundström, J.; Neal, B. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: A systematic review and meta-analysis. Lancet. Diabetes Endocrinol. 2016, 4, 411–419. [Google Scholar] [CrossRef]
- Puckrin, R.; Saltiel, M.P.; Reynier, P.; Azoulay, L.; Yu, O.H.Y.; Filion, K.B. SGLT-2 inhibitors and the risk of infections: A systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018, 55, 503–514. [Google Scholar] [CrossRef]
- Li, D.; Wang, T.; Shen, S.; Fang, Z.; Dong, Y.; Tang, H. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2017, 19, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Rådholm, K.; Wu, J.H.; Wong, M.G.; Foote, C.; Fulcher, G.; Mahaffey, K.W.; Perkovic, V.; Neal, B. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular disease, death and safety outcomes in type 2 diabetes—A systematic review. Diabetes Res. Clin. Pract. 2018, 140, 118–128. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhu, Q.Q.; Chen, Y.H.; Li, X.L.; Chen, F.; Huang, J.A.; Xu, B. Cardiovascular safety, long-term noncardiovascular safety, and efficacy of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: A systemic review and meta-analysis with trial sequential analysis. J. Am. Heart Assoc. 2018, 7, e007165. [Google Scholar] [CrossRef]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: A meta-analysis. JAMA Cardiol. 2021, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Al’Aref, S.J.; Khan, M.S.; Al-Hawwas, M.; Vallurupalli, S.; Mehta, J.L.; Mounsey, J.P.; Greene, S.J.; McGuire, D.K.; Lopes, R.D.; et al. Effect of sodium-glucose cotransporter 2 inhibitors on cardiovascular and kidney outcomes-Systematic review and meta-analysis of randomized placebo-controlled trials. Am. Heart J. 2021, 232, 10–22. [Google Scholar] [CrossRef]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef]
- Araki, E.; Tanizawa, Y.; Tanaka, Y.; Taniguchi, A.; Koiwai, K.; Kim, G.; Salsali, A.; Woerle, H.J.; Broedl, U.C. Long-term treatment with empagliflozin as add-on to oral antidiabetes therapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2015, 17, 665–674. [Google Scholar] [CrossRef]
- Ferrannini, E.; Seman, L.; Seewaldt-Becker, E.; Hantel, S.; Pinnetti, S.; Woerle, H.J. A Phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, S.; Rosenstock, J.; Meinicke, T.; Woerle, H.J.; Broedl, U.C. Initial combination of empagliflozin and metformin in patients with type 2 diabetes. Diabetes Care 2016, 39, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Berk, A.; Hantel, S.; Pinnetti, S.; Hach, T.; Woerle, H.J.; Broedl, U.C. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: An active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care 2013, 36, 4015–4021. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.R.; Murray, A.V.; Marmolejo, M.H.; Hennicken, D.; Ptaszynska, A.; List, J.F. Dapagliflozin, metformin XR, or both: Initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int. J. Clin. Pract. 2012, 66, 446–456. [Google Scholar] [CrossRef]
- List, J.F.; Woo, V.; Morales, E.; Tang, W.; Fiedorek, F.T. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009, 32, 650–657. [Google Scholar] [CrossRef]
- Ito, D.; Inoue, K.; Saito, D.; Hamaguchi, K.; Kaneko, K.; Sumita, T.; Inukai, K.; Inoue, I.; Shimada, A. Effects of dapagliflozin compared with sitagliptin and metformin in drug-naïve Japanese patients with type 2 diabetes: A 12-week, open-label, randomized, active-controlled trial. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2021, 12, 3201–3215. [Google Scholar] [CrossRef]
- Pian, L.; Xiaocui, Z.; Tao, W. Clinical trial of dapagliflozin tablets in the treatment of patients with type 2 diabetes mellitus complicated with heart failure. Chin. J. Clin. Pharmacol. 2021, 37, 227–230. [Google Scholar] [CrossRef]
- Weihua, Z.; Zhilong, L.; Weilin, Q.; Jiefen, C. Effect of dapagliflozin on blood pressure, blood sugar and cholesterol in patients with type 2 diabetes mellitus compli-cated with metabolic syndrome. Heilongjiang Med. J. 2019, 43, 732–734. [Google Scholar] [CrossRef]
- Rosenstock, J.; Chuck, L.; González-Ortiz, M.; Merton, K.; Craig, J.; Capuano, G.; Qiu, R. Initial combination therapy with canagliflozin plus metformin versus each component as monotherapy for drug-naïve type 2 diabetes. Diabetes Care 2016, 39, 353–362. [Google Scholar] [CrossRef]
- Jingqian, X. The Comparative Study on the Therapeutic Effect and Safety between Canagliflozin and Metformin in Patients with CVD and T2DM. Master’s Thesis, Hebei Medical University, Shijiazhuang, China, 2020. [Google Scholar]
- Fonseca, V.A.; Ferrannini, E.; Wilding, J.P.; Wilpshaar, W.; Dhanjal, P.; Ball, G.; Klasen, S. Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J. Diabetes Complicat. 2013, 27, 268–273. [Google Scholar] [CrossRef]
- Koshizaka, M.; Ishikawa, K.; Ishibashi, R.; Maezawa, Y.; Sakamoto, K.; Uchida, D.; Nakamura, S.; Yamaga, M.; Yokoh, H.; Kobayashi, A.; et al. Comparing the effects of ipragliflozin versus metformin on visceral fat reduction and metabolic dysfunction in Japanese patients with type 2 diabetes treated with sitagliptin: A prospective, multicentre, open-label, blinded-endpoint, randomized controlled study (PRIME-V study). Diabetes Obes. Metab. 2019, 21, 1990–1995. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kompoti, M. Obesity and infection. Lancet Infect. Dis. 2006, 6, 438–446. [Google Scholar] [CrossRef]
- Hecker, K.D.; Kris-Etherton, P.M.; Zhao, G.; Coval, S.; Jeor, S.S. Impact of body weight and weight loss on cardiovascular risk factors. Curr. Atheroscler. Rep. 1999, 1, 236–242. [Google Scholar] [CrossRef]
- Berberich, A.J.; Hegele, R.A. A modern approach to dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef]
- Li, Y.; Teng, D.; Shi, X.; Qin, G.; Qin, Y.; Quan, H.; Shi, B.; Sun, H.; Ba, J.; Chen, B.; et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: National cross sectional study. BMJ 2020, 369, m997. [Google Scholar] [CrossRef]
- Duarte, A.M.; Guarino, M.P.; Barroso, S.; Gil, M.M. Phytopharmacological Strategies in the Management of Type 2 Diabetes Mellitus. Foods 2020, 9, 271. [Google Scholar] [CrossRef]
- Xiang, A.H.; Trigo, E.; Martinez, M.; Katkhouda, N.; Beale, E.; Wang, X.; Wu, J.; Chow, T.; Montgomery, C.; Nayak, K.S.; et al. Impact of gastric banding versus metformin on β-cell function in adults with impaired glucose tolerance or mild type 2 diabetes. Diabetes Care 2018, 41, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Jingfan, Z.; Ling, L.; Cong, L.; Ping, L.; Yu, C. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors in type 2 diabetes mellitus with inadequate glycemic control on metformin: A meta-analysis. Arch. Endocrinol. Metab. 2019, 63, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. A novel approach to control hyperglycemia in type 2 diabetes: Sodium glucose co-transport (SGLT) inhibitors: Systematic review and meta-analysis of randomized trials. Ann. Med. 2012, 44, 375–393. [Google Scholar] [CrossRef]
- Imatoh, T.; Nishi, T.; Yasui, M.; Maeda, T.; Sai, K.; Saito, Y.; Une, H.; Babazono, A. Association between dipeptidyl peptidase-4 inhibitors and urinary tract infection in elderly patients: A retrospective cohort study. Pharmacoepidemiol. Drug Saf. 2018, 27, 931–939. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research FDA Drug Safety Communication. FDA Revises Labels of SGLT2 Inhibitors for Diabetes to Include Warnings about Too Much Acid in the Blood and Serious Urinary Tract Infections 2015. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/ (accessed on 4 February 2022).
- Donnan, J.R.; Grandy, C.A.; Chibrikov, E.; Marra, C.A.; Aubrey-Bassler, K.; Johnston, K.; Swab, M.; Hache, J.; Curnew, D.; Nguyen, H.; et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: A systematic review and meta-analysis. BMJ Open 2019, 9, e022577. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Ni, T.; Wang, Y.; Wang, X.; Wu, Y.; Zhu, Z.; Li, Q. Comparison of new oral hypoglycemic agents on risk of urinary tract and genital infections in type 2 diabetes: A network meta-analysis. Adv. Ther. 2021, 38, 2840–2853. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Li, S.; Jia, P.; Deng, K.; Chen, W.; Sun, X. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 2824. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, N.; Chen, R.; Zhao, J.G.; Yu, P. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: A meta-analysis of randomized controlled trials for 1 to 2 years. J. Diabetes Complicat. 2015, 29, 1295–1303. [Google Scholar] [CrossRef]
- Tahara, A.; Takasu, T.; Yokono, M.; Imamura, M.; Kurosaki, E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors in pharmacokinetics, pharmacodynamics, and pharmacologic effects. J. Pharmacol. Sci. 2016, 130, 159–169. [Google Scholar] [CrossRef]
- Johnsson, K.M.; Ptaszynska, A.; Schmitz, B.; Sugg, J.; Parikh, S.J.; List, J.F. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J. Diabetes Complicat. 2013, 27, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Kohler, S.; Salsali, A.; Hantel, S.; Kaspers, S.; Woerle, H.J.; Kim, G.; Broedl, U.C. Safety and tolerability of empagliflozin in patients with type 2 diabetes. Clin. Ther. 2016, 38, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Ding, L.L.; Zhang, M.; Zhou, H.R. Safety of four SGLT2 inhibitors in three chronic diseases: A meta-analysis of large randomized trials of SGLT2 inhibitors. Diabetes Vasc. Dis. Res. 2021, 18, 14791641211011016. [Google Scholar] [CrossRef]
- Bailey, C.J.; Morales Villegas, E.C.; Woo, V.; Tang, W.; Ptaszynska, A.; List, J.F. Efficacy and safety of dapagliflozin monotherapy in people with Type 2 diabetes: A randomized double-blind placebo-controlled 102-week trial. Diabet. Med. 2015, 32, 531–541. [Google Scholar] [CrossRef]
- Leiter, L.A.; Yoon, K.H.; Arias, P.; Langslet, G.; Xie, J.; Balis, D.A.; Millington, D.; Vercruysse, F.; Canovatchel, W.; Meininger, G. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: A randomized, double-blind, phase 3 study. Diabetes Care 2015, 38, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Forst, T.; Guthrie, R.; Goldenberg, R.; Yee, J.; Vijapurkar, U.; Meininger, G.; Stein, P. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes. Metab. 2014, 16, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and type 2 diabetes: Two diseases with a need for combined treatment strategies-EASO can lead the way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef]
- Kohlgruber, A.; Lynch, L. Adipose tissue inflammation in the pathogenesis of type 2 diabetes. Curr. Diabetes Rep. 2015, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Oldridge, N.B.; Stump, T.E.; Nothwehr, F.K.; Clark, D.O. Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle- and older-age adults. J. Clin. Epidemiol. 2001, 54, 928–934. [Google Scholar] [CrossRef]
- Genoni, G.; Prodam, F.; Marolda, A.; Giglione, E.; Demarchi, I.; Bellone, S.; Bona, G. Obesity and infection: Two sides of one coin. Eur. J. Pediatr. 2014, 173, 25–32. [Google Scholar] [CrossRef]
- Vasapollo, P.; Cione, E.; Luciani, F.; Gallelli, L. Generalized intense pruritus during canagliflozin treatment: Is it an adverse drug reaction? Curr. Drug Saf. 2018, 13, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Silverii, G.A.; Dicembrini, I.; Monami, M.; Mannucci, E. Fournier’s gangrene and sodium-glucose co-transporter-2 inhibitors: A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2020, 22, 272–275. [Google Scholar] [CrossRef]
- Brunetti, V.C.; Reynier, P.; Azoulay, L.; Yu, O.H.Y.; Ernst, P.; Platt, R.W.; Filion, K.B. SGLT-2 inhibitors and the risk of hospitalization for community-acquired pneumonia: A population-based cohort study. Pharmacoepidemiol. Drug Saf. 2021, 30, 740–748. [Google Scholar] [CrossRef]
- Mudaliar, S.; Polidori, D.; Zambrowicz, B.; Henry, R.R. Sodium-glucose cotransporter inhibitors: Effects on renal and intestinal glucose transport: From bench to bedside. Diabetes Care 2015, 38, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Bailey, C.J.; Gross, J.L.; Pieters, A.; Bastien, A.; List, J.F. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 375, 2223–2233. [Google Scholar] [CrossRef]
- Ferrannini, E.; Ramos, S.J.; Salsali, A.; Tang, W.; List, J.F. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: A randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010, 33, 2217–2224. [Google Scholar] [CrossRef]
- Jojima, T.; Sakurai, S.; Wakamatsu, S.; Iijima, T.; Saito, M.; Tomaru, T.; Kogai, T.; Usui, I.; Aso, Y. Empagliflozin increases plasma levels of campesterol, a marker of cholesterol absorption, in patients with type 2 diabetes: Association with a slight increase in high-density lipoprotein cholesterol. Int. J. Cardiol. 2021, 331, 243–248. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Nardini, C.; Mannucci, E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: A meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 2014, 16, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Muto, S.; Fukuda, K.; Watanabe, M.; Ohara, K.; Koepsell, H.; Vallon, V.; Nagata, D. Osmotic diuresis by SGLT2 inhibition stimulates vasopressin-induced water reabsorption to maintain body fluid volume. Physiol. Rep. 2020, 8, e14360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, M.; Lv, Q.; Tong, N. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes and moderate renal function impairment: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2018, 140, 295–303. [Google Scholar] [CrossRef]













| Study | Interventions | Study Duration (Weeks) | Number of Participants | Male (N) | Age (Years) | HbA1c (%) | Body Weight (Kg) | BMI (Kg/M) |
|---|---|---|---|---|---|---|---|---|
| Araki, E. 2015, [20] (NCT01368081) | EMPA 10 mg | 52 | 136 | 99 | 61.3 ± 9.9 | 8.0 ± 0.7 | 65.8 ± 12.2 | 24.6 ± 3.8 |
| EMPA 25 mg | 137 | 96 | 61.8 ± 9.6 | 8.1 ± 0.8 | 67.0 ± 13.7 | 25.2 ± 4.2 | ||
| MET 1111 mg § | 63 | 47 | 60.0 ± 10.2 | 7.9 ± 0.8 | 68.2 ± 12.2 | 25.2 ± 3.6 | ||
| Ferrannini, E.* 2013, [21] (NCT00789035) | EMPA 5 mg | 12 | 81 | 46 | 59.0 (37–78) * | 7.9 ± 0.8 | 82.8 (51.9–116.0) * | 28.5 (20.5–38.8) * |
| EMPA 10 mg | 81 | 40 | 58.0 (30–76) * | 8.0 ± 0.8 | 76.8 (45.5–118.0) * | 28.1 (21.5–39.3) * | ||
| EMPA 25 mg | 82 | 41 | 57.0 (30–79) * | 7.8 ± 0.8 | 81.2 (49.1–130.0) * | 28.3 (20.1–38.8) * | ||
| MET † | 80 | 39 | 58.0 (34–73) * | 8.1 ± 0.9 | 81.1 (42.0–126.0) * | 28.6 (18.7–40.6) * | ||
| Hadjadj, S. 2016, [22] (NCT01719003) | EMPA 10 mg | 24 | 169 | 97 | 53.1 ± 10.7 | 8.6 ± 1.2 | 83.8 ± 19.8 | 30.3 ± 5.2 |
| EMPA 25 mg | 164 | 83 | 53.3 ± 10.7 | 8.9 ± 1.3 | 83.1 ± 20.3 | 30.6 ± 5.9 | ||
| MET 500 mg | 168 | 86 | 53.4 ± 10.9 | 8.7 ± 1.0 | 82.7 ± 21.2 | 30.3 ± 5.8 | ||
| MET 1000 mg | 164 | 92 | 51.6 ± 10.8 | 8.6 ± 1.1 | 83.7 ± 20.1 | 30.5 ± 5.9 | ||
| Ferrannini, E. 2013, [23] (NCT00881530) | EMPA 10 mg | 78 | 106 | 49 | 59 (30–76) * | 7.9 ± 0.9 | 82.9 ± 16.4 | 28.9 (20.3–39.2) * |
| EMPA 25 mg | 109 | 57 | 59 (35–79) * | 8.0 ± 0.9 | 84.6 ± 18.1 | 28.1 (19.3–40.0) * | ||
| MET † | 56 | 28 | 58 (35–73) * | 8.2 ± 1.0 | 85.8 ± 15.6 | 28.6 (22.4–39.3) * | ||
| Henry, R.R. 2012, [24] (NCT00643851 NCT00859898) | DAPA 5 mg | 24 | 203 | 92 | 52.3 ±10.2 | 9.1 ± 1.4 | 86.2 ± 21.1 | NO |
| DAPA 10 mg | 219 | 105 | 51.1 ± 11.5 | 9.1 ± 1.3 | 88.5 ± 19.3 | |||
| MET 2000 mg | 409 | 192 | 52.3 ± 10.1 | 9.1 ± 1.3 | 86.4 ± 19.7 | |||
| List, J.F. 2009, [25] (NCT00263276) | DAPA 2.5 mg | 12 | 59 | 29 | 55.0 ± 11.0 | 7.6 ± 0.7 | 90.0 ± 20.0 | 32.0 ± 5.0 |
| DAPA 5 mg | 58 | 28 | 55.0 ± 12.0 | 8.0 ± 0.9 | 89.0 ± 17.0 | 32.0 ± 5.0 | ||
| DAPA 10 mg | 47 | 25 | 54.0 ± 9.0 | 8.0 ± 0.8 | 86.0 ± 17.0 | 31.0 ± 5.0 | ||
| DAPA 20 mg | 59 | 32 | 55.0 ± 10.0 | 7.7 ± 0.9 | 88.0 ± 18.0 | 31.0 ± 5.0 | ||
| DAPA 50 mg | 56 | 25 | 53.0 ± 10.0 | 7.8 ± 1.0 | 92.0 ± 19.0 | 32.0 ± 4.0 | ||
| MET 1500 mg | 56 | 27 | 54.0 ± 9.0 | 7.6 ± 0.8 | 88.0 ± 20.0 | 32.0 ± 5.0 | ||
| Ito, D. 2021, [26] | DAPA 5 mg | 12 | 11 | 8 | 55.9 ± 7.5 | 7.9 ± 0.9 | 77.5 ± 18.1 | 27.7 ± 4.9 |
| MET 1000 mg | 10 | 9 | 57.5 ± 9.6 | 7.9 ± 0.9 | 74.8 ± 8.7 | 26.7 ± 3.4 | ||
| Pian Liu. 2021, [27] | DAPA 10 mg | 26 | 58 | 31 | 66.6 ± 8.4 | 8.1 ± 1.2 | 70.1 ± 7.8 | 24.7 ± 1.8 |
| MET 1000 mg | 59 | 32 | 66.3 ± 9.3 | 8.5 ± 1.1 | 68.6 ± 7.7 | 24.1 ± 2.3 | ||
| Weihua Zhang. 2019, [28] | DAPA 10 mg | 12 | 30 | 19 | 44.9 ± 10.2 | 8.5 ± 1.7 | 76.3 ± 13.6 | 27.9 ± 4.3 |
| MET 1500 mg | 30 | 20 | 44.1 ± 10.8 | 8.3 ± 1.4 | 75.4 ± 14.3 | 27.5 ± 4.5 | ||
| Rosenstock, J. 2016, [29] (NCT01809327) | CANA 100 mg | 26 | 237 | 105 | 54.1 ± 10.7 | 8.8 ± 1.2 | 90.2 ± 18.6 | 32.4 ± 5.4 |
| CANA 300 mg | 238 | 125 | 55.9 ± 9.6 | 8.8 ± 1.2 | 93.0 ± 19.9 | 32.6 ± 5.8 | ||
| MET 2000 mg | 237 | 116 | 55.3 ± 9.8 | 8.8 ± 1.2 | 92.1 ± 20.1 | 33.0 ± 6.0 | ||
| Jingqian Xie. 2020, [30] | CANA 100 mg | 12 | 31 | 11 | 63.8 ± 8.6 | 9.1 ± 1.7 | 73.3 ± 10.3 | NO |
| MET (1000–1500 mg) | 31 | 13 | 63.0 ± 9.7 | 8.3 ± 1.5 | 72.5 ± 10.2 | |||
| Fonseca, V.A. 2012, [31] (NCT01071850) | IPRA 12.5 mg | 12 | 70 | 39 | 53.9 ± 9.6 | 8.0 ± 0.8 | 86.0 ± 22.3 | 31.0 ± 5.9 |
| IPRA 50 mg | 67 | 34 | 52.6 ± 10.7 | 8.1 ± 0.8 | 90.7 ± 20.8 | 32.2 ± 5.9 | ||
| IPRA 150 mg | 68 | 29 | 54.2 ± 10.3 | 7.8 ± 0.7 | 83.3 ± 21.6 | 30.9 ± 6.3 | ||
| IPRA 300 mg | 68 | 37 | 54.2 ± 10.7 | 7.9 ± 0.7 | 86.7 ± 19.6 | 30.7 ± 5.0 | ||
| MET 1500 mg | 69 | 40 | 53.1 ± 11.7 | 8.0 ± 0.9 | 84.1 ± 21.8 | 29.8 ± 5.5 | ||
| Koshizaka, M. 2019, [32] | IPRA 50 mg | 12 | 48 | 31 | 56.6 ± 11.9 | 8.0 ± 0.7 | 73.1 ± 14.2 | 27.6 ± 4.2 |
| MET 1124 mg§ | 50 | 28 | 55.7 ± 12.2 | 8.1 ± 0.9 | 78.3 ± 18.4 | 28.8 ± 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; He, L.; Zhang, J.; Xu, L.; Dong, J.; Liao, L. The Cardiovascular Benefits and Infections Risk of SGLT2i versus Metformin in Type 2 Diabetes: A Systemic Review and Meta-Analysis. Metabolites 2022, 12, 979. https://doi.org/10.3390/metabo12100979
Xu C, He L, Zhang J, Xu L, Dong J, Liao L. The Cardiovascular Benefits and Infections Risk of SGLT2i versus Metformin in Type 2 Diabetes: A Systemic Review and Meta-Analysis. Metabolites. 2022; 12(10):979. https://doi.org/10.3390/metabo12100979
Chicago/Turabian StyleXu, Chunmei, Liping He, Jing Zhang, Lusi Xu, Jianjun Dong, and Lin Liao. 2022. "The Cardiovascular Benefits and Infections Risk of SGLT2i versus Metformin in Type 2 Diabetes: A Systemic Review and Meta-Analysis" Metabolites 12, no. 10: 979. https://doi.org/10.3390/metabo12100979
APA StyleXu, C., He, L., Zhang, J., Xu, L., Dong, J., & Liao, L. (2022). The Cardiovascular Benefits and Infections Risk of SGLT2i versus Metformin in Type 2 Diabetes: A Systemic Review and Meta-Analysis. Metabolites, 12(10), 979. https://doi.org/10.3390/metabo12100979






