Abstract
Metabolic syndrome (MS), characterized by the presence of risk factors for various metabolic disorders, including impaired glucose tolerance, dyslipidemia, hypertension, and insulin resistance, has a high incidence in the Asian population. Among the various approaches used for improving MS, the combination of exercise and nutrition is of increasing importance. In this randomized controlled trial, we evaluated the effects of combined aqua exercise and burdock extract intake on blood pressure, insulin resistance, arterial stiffness, and vascular regulation factors in older women with MS. A total of 42 participants were randomly assigned into one of four groups (control, exercise, burdock, and exercise + burdock) and underwent a 16-week double-blinded intervention. Blood pressure, insulin resistance, arterial stiffness, and vascular regulation factors were evaluated before and after the intervention. The 16-week intervention of aqua exercise decreased the levels of insulin, glucose, homeostasis model assessment of insulin resistance, and thromboxane A2, but increased the levels of the quantitative insulin sensitivity check index and prostaglandin I2. The combined burdock extract intake and aqua exercise intervention had an additional effect, improving the augmentation index, augmentation index at 75 beats per min, and pulse wave velocity. In conclusion, aqua exercise could improve insulin resistance and vascular regulation factors in older women with MS. Furthermore, combined treatment with burdock extract intake could improve arterial stiffness via a synergistic effect.
1. Introduction
Metabolic syndrome (MS) is characterized by the presence of risk factors for various metabolic disorders, including impaired glucose tolerance, dyslipidemia, hypertension, and insulin resistance [1]. According to the criteria of the National Cholesterol Education Program—Adult Treatment Panel III, MS is defined based on the presence of three or more of the following five risk indicators: abdominal obesity, hyperglycemia, hypertension, reduced levels of high-density lipoprotein (HDL) cholesterol, and increased levels of triglycerides (TG) [2]. Compared with that in Caucasians and African-Americans, the incidence of MS is high among Asians [3].
With insulin resistance and atherosclerosis as key factors, MS accelerates the increase in the risk of cardiovascular disease (CVD) [4]. Moreover, complications often arise in relation to diabetes and reduced vascular function [4]. In this regard, the incidence of CVD is correlated with the incidence of insulin resistance or hyperinsulinemia through sympathetic activation and parasympathetic inhibition [5].
Furthermore, individuals with MS have impaired endothelial function [6]. Indeed, the incidence of atherosclerosis is increased by MS, which was shown to reduce the ability of vascular endothelial cells to regulate the secretion of various compounds that promote healthy blood flow, including vasodilators—nitric oxide (NO) and prostacyclin (prostaglandin I2; PGI2)—and vasoconstrictors—endothelin-1, angiotensin II, and thromboxane A2 (TXA2) [7,8,9,10,11].
According to the obesity, hypertension, and dyslipidemia criteria, MS results from increased arterial stiffness due to carotid artery hypertrophy and increased central arterial pressure. Accordingly, individuals with MS have increased pulse wave velocity (PWV) [12,13]. In addition, CVD-related mortality is three-fold higher among individuals with MS than among healthy individuals [14,15]. Furthermore, CVD and MS risks are higher in women than in men [3,16], and older women are more susceptible to MS due to an increase in adipose tissue and insulin resistance caused by hormonal imbalances after menopause [17]. Thus, it is essential to reduce the risk of CVD in this population through effective interventions that will achieve improvement in insulin resistance and vascular function.
Exercise is a non-invasive method for increasing the resistance to oxidative stress because it increases the activity of oxidation and antioxidation damage repair enzymes through an increase in reactive oxygen species levels [18]. Exercise is well-known as an effective treatment for MS and CVD [18]. Notably, aqua exercise is an ideal form of exercise for overweight and older women, as it is a systemic exercise in which the buoyancy of water decreases the body weight by 90%, thus minimizing the risk of injury [19,20,21]. In previous studies on older women, aqua exercise was shown to effectively improve blood pressure, insulin resistance, and CVD risk factors [22,23]. Nonetheless, few studies have investigated the effectiveness of aqua exercise in older women with MS.
Recently, as a combined treatment approach involving exercise and nutrition, studies have actively investigated natural products with converged efforts. Burdock is a plant species from the Asteraceae family, which has long been used as food in East Asian regions, including Korea, China, and Japan [20,21,24,25]. Burdock has an 80% water content and is enriched with carbohydrates and dietary fibers. It is considered a healthy food with diverse bioactive compounds and was reported to improve diabetes as well as CVD. Moreover, these compounds can prevent oxidation by suppressing NO overproduction in inflammation and CVD [24,26]. Based on these previous findings, we speculated that combined treatment with aquatic exercise and burdock intake would have a more positive effect than each intervention alone in older women.
Therefore, the aim of this study was to assess the effects of combined aqua exercise and burdock intake on blood pressure, insulin resistance, arterial stiffness, and vascular regulation factors in older women with MS. We hypothesized that a 16-week combined intervention in this population would improve blood pressure and insulin resistance and induce beneficial changes in the level of arterial stiffness through improvement in vascular regulation factors.
2. Materials and Methods
2.1. Participants
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human participants were approved by the Institutional Review Board of Dongguk University (DUIRB-202009-07). This trial was retrospectively registered in the Clinical Research Information Service (CRIS) (Republic of Korea, KCT0007627). After explaining the study’s purpose and contents, written informed consent was obtained from all participants.
The study population included older women aged 70−80 years, who were selected according to the Korea Adult MS Guidelines [27]. The inclusion criteria were as follows: (1) waist circumference ≥ 85 cm; (2) blood pressure ≥ 130/85 mmHg or taking antihypertensive medication; (3) fasting blood glucose level ≥ 100 mg/dL or taking antidiabetic medication; (4) TG level ≥ 150 mg/dL; and (5) HDL cholesterol level < 50 mg/dL. Individuals satisfying three or more of the above five criteria were selected.
2.2. Study Design
The study was designed as a randomized, double-blind, controlled trial. To determine the effects of a 16-week intervention of burdock intake and aqua exercise, the participants were randomly divided into four groups: placebo control (CON), exercise + placebo (EX), burdock (BD), and exercise + burdock (EXBD). The CON and EX groups took placebo beverages in the same manner in which the BD and EXBD groups took the investigational beverage. The color and odor of the burdock extract and placebo beverage were similar and their containers were identical. Unblinded personnel, who were not involved in any study assessments, labeled the investigational beverage. Investigators, other site personnel, and the participants were blinded to the beverage. The total daily required beverage intake was 300 mL; the participants drank 100 mL of burdock extract or placebo beverage after breakfast, lunch, and dinner. They were instructed not to take any other health supplements or drugs. All measurements were taken twice, before and after the intervention. The study design is presented in Figure 1.
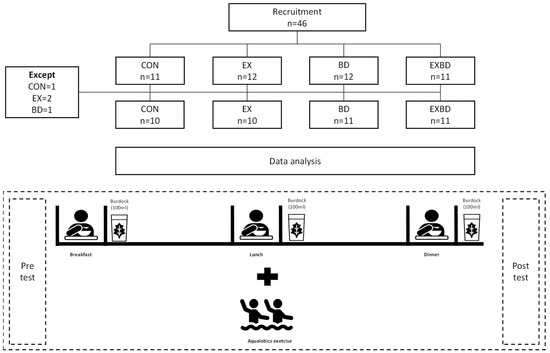
Figure 1.
Study design. CON: placebo control, EX: exercise + placebo, BD: burdock, EXBD: exercise + burdock.
2.3. Burdock Extract Preparation and Composition
The burdock extract was prepared according to a method previously described by our research group [20,21,25]. In brief, after harvest in the Sangcheong region (Gyeongnam, Korea), the burdock root was washed and dried, subsequently heated at 100 °C for 3 h, and extracted at 0.7 kg/cm2 pressure. The main ingredients of the extract were water (98.02% ± 0.02%), crude ash (0.10% ± 0.00%), crude fat (1.12% ± 0.00%), crude protein (0.20% ± 0.00%), crude fiber (0.03%), calcium (0.004% ± 0.00%), and phosphorus (0.009% ± 0.00%) (Pukyong National University Feed & Foods Nutrition Research Center, Busan, Korea). The burdock extract was placed in small, sealed plastic containers of 100 mL, which were provided to the participants for intake. The composition of the burdock extract is summarized in Table S1.
2.4. Aqua Exercise Program
The aqua exercise program used in this study was developed by revising and complementing the program designed by our research group [20]. The temperature of the swimming pool was maintained at 26–28 °C. The aqua exercise program consisted of 50 min exercise sessions performed three times per week for 16 weeks. Each session included a 5 min warm-up exercise, 40 min of main exercises, and a 5 min cool-down exercise. The exercise intensity was established in the manner conducted in our study group based on the Rating of Perceived Exertion (RPE) scores and the Polar system (RS400sd; model APAC, 90026360; Polar, New York, NY, USA), as follows: W1–5: RPE 9–10 (30–40% heart rate reserve [HRR]), W6–10: RPE 11–12 (40–50% HRR), and W11–16: RPE 13–14 (50–60% HRR) [28]. The details of the aqua exercise program are presented in Table S2.
2.5. Blood Pressure
Blood pressure was measured using a digital blood pressure monitor (Jawon Medical, Daejeon, Korea) after a 10 min rest in the supine position. Measurements were taken twice with a 3 min interval in between. The mean value from the two measurements was used for analysis. When the first and second measurements differed by ≥10 mmHg, an additional measurement was taken to obtain the mean value without significant variation.
2.6. Blood Sampling
All participants were instructed to fast for ≥8 h before sample collection. At 8–10 a.m., 10 mL of blood was collected from the antebrachial vein by a clinical pathologist. The blood was centrifuged at 3000 rpm for 10 min in Combi-514R (Hanil, Seoul, Korea) for further analysis. All blood analyses were performed according to the procedures described by our research group [29].
2.6.1. Glucose
Glucose levels were measured in serum samples. After marking the sample, reference, and blank, 20 μL of plasma and 20 μL of standard reagent were added to the sample and reference, respectively, with the addition of 3.0 mL of coloring agent. The mixture was then incubated in a 37 °C water bath. Absorbance was measured at 505 nm.
2.6.2. Insulin
Insulin levels were measured in serum samples. After centrifugation, 200 μL of the supernatant was transferred to a test tube coated with anti-insulin antibody. After addition of 1.0 mL of insulin (DPC, Los Angeles, CA, USA), the mixture was incubated at 24 °C for 20 h, followed by aspiration and chemiluminescence immunoassay using an automated immunoanalyzer.
2.6.3. Homeostasis Model Assessment of Insulin Resistance and Quantitative Insulin Sensitivity Check Index
The homeostasis model assessment of insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) are widely used as simpler and less invasive methods to evaluate insulin resistance based on fasting serum insulin and glucose levels. In this study, the below formulae reported by Matthews et al. and Katz et al. were used to calculate the HOMA-IR [30] and QUICKI [31], respectively.
HOMA-IR = [fasting insulin (mU/L) × fasting glucose (mg/dL)]/22.5
QUICKI = 1/[log (fasting insulin) + log (fasting glucose)]
QUICKI = 1/[log (fasting insulin) + log (fasting glucose)]
2.6.4. PGI2
Whole blood was collected in an anticoagulant tube and added to a plate coated with a PGI2 reagent (Amersham, IL, USA). Next, 50 μL of detection reagent A was added, and the sample was incubated for 1 h at 37 °C. Subsequently, the plate was washed four times with 350 μL of wash buffer, and 100 μL of detection reagent B was added. After incubation at 37 °C for 30 min, the plate was again washed four times with 350 μL of wash buffer and incubated with 90 μL of substrate solution for 15–22 min at ambient temperature in the dark. When the reaction was completed, 50 μL of stop solution was added to each well, and absorbance was measured at 450 nm using Manifold-24 (Amersham, IL, USA).
2.6.5. TXA2
For TXA2 measurement, 0.9 mL of blood was collected and immediately placed in a polystyrene tube. After adding 0.1 mL of 3.8% trisodium citrate, followed by 1 mL of physiological saline and collagen at 2 μL/mL, the mixture was heated for 15 min in a shaking water bath at 37 °C to stimulate the production of TXA2. After a 5 min centrifugation at 2000 rpm, the supernatant was collected for the quantification of thromboxane B2, an unstable product of TXA2 conversion, using a radioimmunoassay kit (Amersham, TRK780, IL, USA).
2.7. Arterial Stiffness
Arterial stiffness was measured using a non-invasive, tonometry-based PW detector (SphygmoCor; AtCor Medical, Sydney, Australia), according to the guidelines described in the Clinical Application of Arterial Stiffness, Task Force III [32]. PWV was measured based on the PW flow from the carotid to the brachial artery. The automated software of the device was used to record the PW on both ends of the artery, and the interdistance was measured using a tape measure. Next, the PWV formula was used to divide the distance (L) by the time variation (Δt) between the PWs recorded on both sides [33].
PWV = L/Δt
For the augmentation index (AIx), we calculated the pressure difference between the highest level of the central blood pressure and the augmentation point that arises at the PW refraction generated by the traveling wave advancing to the periphery to encounter the reflected wave returning to the periphery and divided it by the PWV [34]. In addition, the heart rate-corrected augmentation index at 75 beats per min (AIx@75) was estimated.
2.8. Sample Size Calculation
The sample size was calculated using G-power version 3.1 for Windows (Kiel University, Kiel, Germany). We estimated the sample size for this study as n = 40 based on the following conditions: effect size of 0.25 (default), significance of 0.05, and power of 0.70. Considering potential dropouts, a total of 46 participants were recruited.
2.9. Statistical Analysis
All data were statistically analyzed using IBM SPSS Statistics 27.0 (IBM Corp., Armonk, NY, USA) and expressed as means with standard deviations. The level of significance was set at p < 0.05. To determine the effects of the 16-week intervention on MS indicators (insulin resistance, vascular regulation factors, and arterial stiffness), two-way repeated measures analysis of variance (ANOVA) was performed with the treatment (CON, EX, BD, and EXBD) and time (pre-test and post-test) as independent variables. The Bonferroni test was used for post-hoc analysis. The post-treatment differences in the response of each variable were analyzed using the pre-test-post-test variation (Δ) by employing one-way ANOVA and Pearson’s correlation analysis. The effect size for the pre-test-post-test variation (Cohen’s d) was expressed as the mean variation [35].
3. Results
3.1. Participants’ Characteristics
Of the 46 enrolled participants, four dropped out of the study owing to personal reasons. Therefore, 42 participants completed the study and were included in the analysis. The characteristics of the participants are presented in Table S3.
3.2. Blood Pressure
The effects of the 16-week intervention of burdock intake and aqua exercise on blood pressure and the relevant variations are shown in Figure 2. A significant time effect was detected for the systolic blood pressure (SBP; p = 0.039), but only the BD group showed a significant decrease from 157.13 ± 24.37 mmHg before the intervention to 146.18 ± 17.53 mmHg after the intervention (p = 0.029). There was also a significant group effect for the diastolic blood pressure (DBP; p = 0.05; Table S4). In addition, one-way ANOVA showed no significant difference in ∆SBP and ∆DBP across all groups (Table S5).
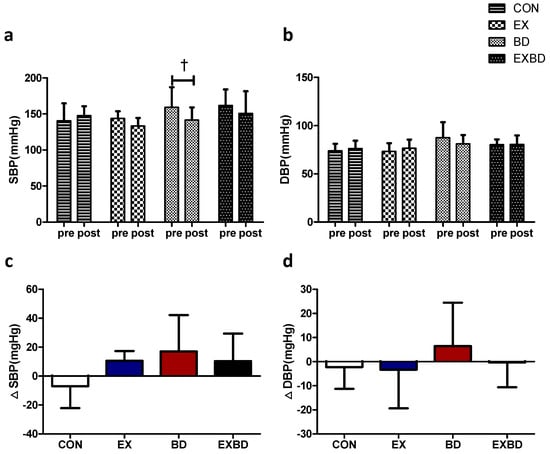
Figure 2.
Effect of 16 weeks of aquatic aqua exercise and burdock intake on the blood pressure in older women with metabolic syndrome. (a) The systolic blood pressure in the BD group decreased after the 16-week intervention compared with the baseline values. (b) No significant changes were detected in the diastolic blood pressure in all groups. Furthermore, no significant differences in systolic (c) or diastolic (d) blood pressure variation were observed among the groups using one-way analysis of variance. Data are presented as the mean ± standard variation. † p < 0.05, significant; before vs. after intervention. SBP, systolic blood pressure; DBP, diastolic blood pressure; CON, placebo control, EX, exercise + placebo, BD, burdock, EXBD, exercise + burdock.
3.3. Insulin Resistance
The effects of the 16-week intervention on insulin resistance and the relevant variation are shown in Figure 3. Insulin levels showed a significant interaction effect (p = 0.003). In the within-group analysis, only the EX group showed a significant decrease from 32.77 ± 20.73 mg/dL to 30.17 ± 22.76 mg/dL (p = 0.002). Glucose levels showed significant differences both for the time (p = 0.029) and interaction effects (p = 0.004). In the within-group analysis, the EX and EXBD groups showed significant decreases from 120.24 ± 23.47 mg/dL to 95.48 ± 13.31 mg/dL (p = 0.001) and from 103.50 ± 24.19 mg/dL to 88.83 ± 10.76 mg/dL (p = 0.05), respectively. The post-hoc test showed significantly smaller (p = 0.034) values for the BD group than for the CON group.
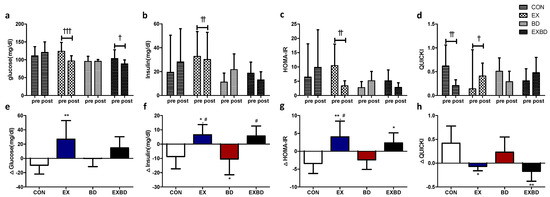
Figure 3.
Effect of 16 weeks of aqua exercise and burdock intake on insulin resistance in older women with metabolic syndrome. After the intervention, compared with the baseline values, (a) glucose levels were decreased in the EX and EXBD groups, (b) insulin levels were decreased in the EX group, (c) the HOMA-IR was decreased in the EX group, and (d) the QUICKI was decreased in the CON group and increased in the EX group. One-way analysis of variance was used to compare the variation in insulin resistance (e–h). Data are presented as the mean ± standard deviation. † p < 0.01, †† p < 0.01, ††† p < 0.001; before vs. after intervention. * p < 0.05, ** p < 0.01 vs. CON, # p < 0.05 vs. BD. HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; CON, placebo control, EX, exercise + placebo, BD, burdock, EXBD, exercise + burdock.
For the HOMA-IR, the interaction effect was significant (p = 0.002) and the within-group analysis showed a significant decrease only in the EX group from 10.45 ± 7.53 to 3.39 ± 1.77 (p = 0.001). For the QUICKI, the interaction effect was also significant (p = 0.002) and the within-group analysis showed a significant decrease in the CON group from 0.62 ± 0.44 to 0.21 ± 0.12 (p = 0.005) and a significant increase in the EX group from 0.14 ± 0.82 to 0.41 ± 0.27 (p = 0.046, Table S4). A one-way ANOVA revealed that ∆insulin showed no significant difference per group (p = 0.002). The post-hoc test indicated higher values for the EX and EXBD groups than for the CON and BD groups (p < 0.05).
Moreover, ∆glucose showed a significant between-group difference (p < 0.007) and the post-hoc test indicated significantly higher values for the EX group than for the CON group (p < 0.01). The ∆HOMA-IR also showed a significant between-group difference (p = 0.001), with higher values for the EX and EXBD groups than for the CON group (p < 0.05 and p < 0.01, respectively) and for the EX group than for the BD group (p < 0.05) in the post-hoc test. Similarly, the ∆QUICKI showed a significant difference among groups (p = 0.003), with higher values for the EX group than for the CON group (p < 0.05) and for the CON group than for the EXBD group (p < 0.01) in the post-hoc test (Table S5).
3.4. Vascular Regulation Factors
The effects of the 16-week intervention on vascular regulation factors and the relevant variations are shown in Figure 4. For PGI2 levels, only the EX group showed a significant increase from 15.98 ± 2.95 pg/mL to 19.32 ± 6.43 pg/mL in the within-group analysis (p = 0.050). TXA2 levels showed a significant interaction effect (p = 0.002), and the within-group analysis showed a significant increase in the CON group from 22.43 ± 6.32 pg/mL to 24.73 ± 6.69 pg/mL (p = 0.003). Conversely, the EX and EXBD groups showed a significant decrease from 18.39 ± 5.11 pg/mL to 16.88 ± 4.48 pg/mL (p = 0.049) and from 22.42 ± 6.66 pg/mL to 20.56 ± 5.88 pg/mL (p = 0.021), respectively (Table S6).
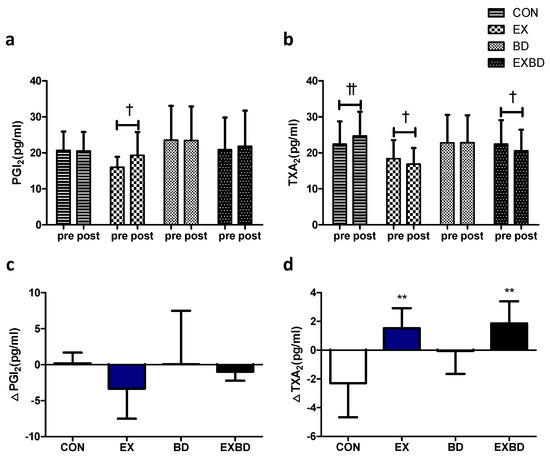
Figure 4.
Effect of 16 weeks of aqua exercise and burdock intake on vascular regulation factors in older women with metabolic syndrome. Compared with the baseline values, (a) PGI2 levels were increased in the EX group, and (b) TXA2 levels were decreased in the CON, EX, and EXBD groups after the 16-week intervention. One-way analysis of variance was used to compare the variation in the levels of vascular regulation factors (c,d). Data are presented as the mean ± standard deviation. † p < 0.01, †† p < 0.01; before vs. after intervention. ** p < 0.01 vs. CON. PGI2, prostacyclin, i.e., prostaglandin I2; TXA2, thromboxane A2; CON, placebo control, EX, exercise + placebo, BD, burdock, EXBD, exercise + burdock.
The one-way ANOVA showed that no significant between-group difference was found for ∆PGI2, whereas ∆TXA2 showed a significant difference among groups (p = 0.002), with significantly higher values for the CON group than for the EX and EXBD groups (p < 0.01; Table S7).
3.5. Arterial Stiffness
The effects of the 16-week intervention of burdock intake and aqua exercise on arterial stiffness and the relevant variations are shown in Figure 5. The AIx showed a significant interaction effect (p = 0.026), and the within-group analysis showed a significant increase in the CON group from 33.14 ± 8.09% to 42.00 ± 11.15% (p = 0.042) and a significant decrease in the EXBD group from 42.00 ± 11.54% to 31.50 ± 10.09% (p = 0.027). For the AIx@75, the within-group analysis showed a significant decrease only in the EXBD group, from 40.00 ± 9.44% to 32.33 ± 7.99% (p = 0.050). For the PWV, the interaction effect was significant (p = 0.023) and the within-group analysis showed that only the EXBD group had a significant increase from 8.94 ± 1.38 m/s to 9.93 ± 1.12 m/s (p = 0.015; Table S8).
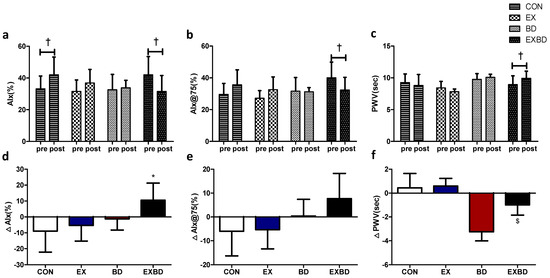
Figure 5.
Effect of 16 weeks of aqua exercise and burdock intake on arterial stiffness in older women with metabolic syndrome. Compared with the baseline values, after the 16-week intervention, (a) the AIx was decreased in the EXBD group and increased in the CON group, (b) the AIx@75 was decreased in the EXBD group, and (c) the PWV was increased in the EXBD group. One-way analysis of variance was used to compare the variations in arterial stiffness (d–f). Data are presented as the mean ± standard deviation. † p < 0.01; before vs. after intervention. * p < 0.05 vs. CON, $ p < 0.05 vs. BD. AIx, augmentation index; AIx@75, heart rate-corrected augmentation index at 75 beats per min; PWV, pulse wave velocity; CON, placebo control, EX, exercise + placebo, BD, burdock, EXBD, exercise + burdock.
The one-way ANOVA indicated that the ∆AIx showed a significant difference among groups (p = 0.026), with the post-hoc test indicating significantly higher values for the EXBD group than for the CON group (p < 0.05). The ∆AIx@75 showed no significant between-group differences. The ∆PWV showed a significant difference among groups (p = 0.023), with the post-hoc test indicating significantly higher values for the EXBD group than for the EX group (p < 0.05; Table S9).
3.6. Correlation among Variations
Pearson’s correlation analysis for the variations in the measured variables showed a positive correlation between ∆SBP and ∆DBP (r = 0.657, p < 0.001) and between ∆glucose and ∆insulin (r = 0.674, p < 0.001). A positive correlation was found with the ∆HOMA-IR (r = 0.860, p < 0.001) and ∆TXA2 (r = 0.592, p < 0.01); however, there was a negative correlation with the ∆QUICKI (r = −0.564, p < 0.01). For ∆insulin, there was a positive correlation with the ∆HOMA-IR (r = 0.892, p < 0.001), ∆AIx (r = 0.465, p < 0.05), and ∆AIx@75 (r = 0.461, p < 0.05) and a negative correlation with the ∆QUICKI (r = −0.760, p < 0.001). For the ∆HOMA-IR, there was a negative correlation with the ∆QUICKI (r = −0.608, p < 0.01) and a positive correlation with the ∆TXA2 (r = 0.427, p < 0.05). The ∆QUICKI showed a negative correlation with the ∆AIx (r = −0.411, p < 0.05), while the ∆PWV negatively correlated with the ∆AIx (r = −0.622, p < 0.001) and ∆Aix@75 (r = −0.610, p < 0.01). Finally, a positive correlation was found between ∆AIx and ∆AIx@75 (r = 0.964, p < 0.001). The results of the correlation analysis are summarized in Table S10.
4. Discussion
In this study, we hypothesized that the combined use of aquatic exercise and burdock intake would be more effective than each intervention separately for improving blood pressure, insulin resistance, vascular regulation factors, and arterial stiffness in older women with MS. Our results supported this hypothesis and revealed several novel observations. The 16-week intervention of aqua exercise decreased the levels of insulin, glucose, HOMA-IR, and TXA2, but increased the levels of QUICKI and PGI2. Independently, however, the burdock intake intervention did not show significant results for vascular function improvement. Remarkably, the combined use of burdock intake and aqua exercise had an additional effect to improve the AIx, AIx@75, and PWV.
Insulin resistance is the most common risk factor for MS. As it reduces the response of target cells to insulin owing to a decline in their sensitivity to insulin secretion, insulin resistance has a negative effect on the overall metabolism [36,37]. Characteristic outcomes caused by insulin resistance include metabolic disorders, such as type 2 diabetes, obesity, glucose intolerance, and dyslipidemia. Insulin resistance is also recognized as a CVD-related factor in atherosclerosis and hypertension and is characterized by endothelial dysfunction [38].
In older women, the degree of insulin resistance increases with a distinct reduction in physical activity because the hormonal imbalance after menopause results in increased adipose tissue accumulation, as well as increased fasting insulin and glucose levels [39]. Physical exercise is recommended as a solution to these problems and it is associated with minimal side effects. Physical exercise is effective in treating insulin resistance [40], with the levels of insulin resistance and physical exercise being inversely related [41]. In addition, participation in regular exercise not only improves antioxidation in the body but also facilitates glucose absorption in peripheral tissues and enhances insulin sensitivity by increasing the number of insulin receptors [42,43]. In a previous study in patients with type 2 diabetes, an 8-week intervention of aqua exercise significantly reduced insulin resistance [44].
The results of the present study showed a significant decrease in insulin, glucose, and HOMA-IR levels and a significant increase in QUICKI values in the EX group, suggesting that continuous participation in the aqua exercise had a positive effect on insulin resistance. Thus, additional studies should be conducted to further evaluate the preparation and composition of burdock extract for improving insulin resistance in older women with MS.
The risk factors for MS are closely associated with the progression of atherosclerosis, with cytokine secretion from adipocytes exerting a negative effect on insulin sensitivity, thereby resulting in endothelial dysfunction [8,45]. In turn, endothelial dysfunction increases the prevalence of atherosclerosis, while arterial tension is controlled by vasodilators, such as NO and PGI2, and vasoconstrictors, such as TXA2 [46].
PGI2 is known for its powerful role in the induction of vasodilation and in preventing platelet coagulation [47,48]. Gamez-Mendez et al. [49] reported a decline in PGI2 levels in obese mice, suggesting a role for PGI2 in endothelial dysfunction. Conversely, TXA2, which exerts antagonistic effects to those of PGI2, is a powerful vasoconstrictor with additional roles in inducing platelet coagulation and various physiological responses, such as facilitated thrombosis and endothelial inflammation [50]. Interestingly, a close association was observed between increased platelet activation and coagulation and CVD complications [51].
Regular exercise induces endovascular shear stress and subsequent activation of calcium ion channels and phospholipases, leading to the release of PGI2 [52]. In a previous study in patients with hypertension, a 16-week exercise intervention was shown to increase the levels of PGI2 metabolites and decrease those of TXA2 metabolites [53]. In addition, in a human red blood cell count experiment with burdock extract, an anti-thrombotic effect was reported [54]. Furthermore, in a study in high-fat/cholesterol-diet rats, Lee et al. [55] identified a positive effect of burdock intake on vascular dysfunction.
The results of this study revealed a significant increase in PGI2 levels in the EX group and a significant decrease in TXA2 levels in the EX and EXBD groups, indicating a positive effect of exercise on insulin resistance, by enhancing endothelial function and reducing the risk of CVD in patients with MS. However, no significant effect was shown in the BD group, which highlights the need for further studies to investigate potential markers associated with endothelial dysfunction.
Insulin resistance and hyperglycemia are reported to increase cytokine production and oxidative stress, thereby compromising vascular endothelial function [56,57]. Endothelial dysfunction caused by damage to endothelial cells through structural or functional changes in vascular walls promotes or exacerbates atherosclerosis, an independent predictor of mortality due to coronary artery disease and CVD [58,59]. To detect atherosclerosis, the carotid–femoral PWV is used, whereas the Aix is used for evaluating systemic arterial stiffness [33,60]. Patients with MS are characterized by high PWV values [61].
The increase in shear stress during exercise increases the bioavailability of NO and activates sympathetic nerves to enhance vascular endothelial function [62]. Donley et al. [63] reported that an 8-week intervention of aerobic exercise in patients with MS was associated with a reduction in carotid–femoral PWV levels [64]. Burdock has antioxidant effects due to its caffeoylquinic acid content. This enhanced antioxidation capacity may reduce the content of free radicals and promote endothelial NO synthase expression, thereby increasing NO bioavailability [65]. In addition, Lee et al. [66] performed a principal component analysis of burdock and reported the presence of L-arginine, an NO precursor.
In this study, we found a significant decrease in the AIx and AIx@75 only in the EXBD group after the intervention. In addition, the largest variations in AIx and AIx@75 values (∆AIx and ∆AIx@75) were observed in the EXBD group. Furthermore, only the BD group exhibited a decline in SBP, although there was no difference in the other variables. These findings indicated that aqua exercise improved insulin resistance and vascular regulation factors in older women with MS, while burdock intake resulted in SBP reduction, with no confirmed effects on other vascular function-related variables. Therefore, it remains unclear whether burdock intake affects vascular function and insulin resistance indicators in older women with MS. However, it was reaffirmed that aquatic exercise can be effective for improving insulin resistance and vascular regulation factors in this population. Moreover, this study revealed that combined burdock intake and aqua exercise can reduce arterial stiffness in individuals with MS.
A potential limitation of this study is that direct markers of antioxidation and NO levels were not measured in response to burdock intake. Thus, additional studies are warranted to investigate the relationship between burdock intake and direct markers of antioxidation and NO. Additionally, there are some limitations pertaining to the generalizability of our findings. First, as the focus of the study was on older women with MS, it is difficult to generalize the results to men or women of other ages. Second, owing to the small sample size, the effect size in our study was limited to 70% of the power. Therefore, future studies should be performed on a larger number of study participants to support our findings.
5. Conclusions
In summary, burdock intake alone cannot be expected to have a significant effect in older women with MS. However, its combined use with aqua exercise, which is effective in decreasing insulin resistance and vascular regulation factors, can additionally improve arterial stiffness.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo12100970/s1, Table S1: Composition of the burdock root extract; Table S2: Details of the 16-week aqua exercise program for older women with metabolic syndrome; Table S3: Descriptive characteristics of the study participants; Table S4: Effects of the 16-week aqua exercise on blood pressure and insulin resistance in older women with metabolic syndrome; Table S5: Changes in blood pressure and insulin resistance after the 16-week aqua exercise in older women with metabolic syndrome; Table S6: Effects of the 16-week aqua exercise on vascular regulation factors in older women with metabolic syndrome; Table S7: Changes in the vascular regulation factors after the 16-week aqua exercise in older women with metabolic syndrome; Table S8: Effects of the 16-week aqua exercise on arterial stiffness in older women with metabolic syndrome; Table S9: Changes in arterial stiffness after the 16-week aqua exercise in older women with metabolic syndrome; Table S10: Correlation between delta (∆) values.
Author Contributions
Conceptualization, W.H.S. and M.-S.H.; methodology, W.-M.J. and M.-S.H.; software, J.-H.L.; validation, M.-S.H.; formal analysis, J.-H.L. and H.R.K.; data curation, J.-H.L.; writing—original draft preparation, W.H.S., J.-H.L. and M.-S.H.; writing—review and editing, M.-S.H.; visualization, J.-H.L.; supervision, M.-S.H.; project administration, W.-M.J. and M.-S.H.; funding acquisition, M.-S.H. All authors critically revised the manuscript for important intellectual content and approved the final version for publication. All authors significantly contributed to the research. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2019S1A5B5A07106826).
Institutional Review Board Statement
All procedures and protocols performed in this study involving human participants adhered to the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and were approved by the National Bioethics Committee and Institutional Review Board of Dongguk University (DUIRB-202009-07). This trial was retrospectively registered in the Clinical Research Information Service (CRIS) (Republic of Korea, KCT0007627).
Informed Consent Statement
Written informed consent for participation was obtained from all study participants prior to enrollment. Additionally, written informed consent was obtained from all participants for the publication of this paper.
Data Availability Statement
The authors declare that all data and materials are available to be shared upon formal request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vega, G.L. Cardiovascular Outcomes for Obesity and Metabolic Syndrome. Obes. Res. 2002, 10, 27S–32S. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Tillin, T.; Forouhi, N.; Johnston, D.G.; McKeigue, P.M.; Chaturvedi, N.; Godsland, I.F. Metabolic Syndrome and Coronary Heart Disease in South Asians, African-Caribbeans and White Europeans: A UK Population-Based Cross-Sectional Study. Diabetologia 2005, 48, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Mykkänen, L.; Kuusisto, J.; Pyörälä, K.; Laakso, M. Cardiovascular Disease Risk Factors as Predictors of Type 2 (Non-Insulin-Dependent) Diabetes Mellitus in Elderly Subjects. Diabetologia 1993, 36, 553–559. [Google Scholar] [CrossRef]
- Foright, R.M.; Presby, D.M.; Sherk, V.D.; Kahn, D.; Checkley, L.A.; Giles, E.D.; Bergouignan, A.; Higgins, J.A.; Jackman, M.R.; Hill, J.O.; et al. Is Regular Exercise an Effective Strategy for Weight Loss Maintenance? Physiol. Behav. 2018, 188, 86–93. [Google Scholar] [CrossRef]
- Sypniewska, G. Pro-Inflammatory and Prothrombotic Factors and Metabolic Syndrome. EJIFCC 2007, 18, 39–46. [Google Scholar] [PubMed]
- Brook, R.D.; Bard, R.L.; Rubenfire, M.; Ridker, P.M.; Rajagopalan, S. Usefulness of Visceral Obesity (Waist/Hip Ratio) in Predicting Vascular Endothelial Function in Healthy Overweight Adults. Am. J. Cardiol. 2001, 88, 1264–1269. [Google Scholar] [CrossRef]
- McVeigh, G.E.; Brennan, G.M.; Johnston, G.D.; McDermott, B.J.; McGrath, L.T.; Henry, W.R.; Andrews, J.W.; Hayes, J.R. Impaired Endothelium-Dependent and Independent Vasodilation in Patients with Type 2 (Non-Insulin-Dependent) Diabetes Mellitus. Diabetologia 1992, 35, 771–776. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The Obligatory Role of Endothelial Cells in the Relaxation of Arterial Smooth Muscle by Acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Riddell, D.R.; Owen, J.S. Nitric Oxide and Platelet Aggregation. Vitam. Horm. 1997, 57, 25–48. [Google Scholar] [CrossRef]
- Nicosia, S.; Oliva, D.; Bernini, F.; Fumagalli, R. Prostacyclin-sensitive Adenylate Cyclase and Prostacyclin Binding Sites in Platelets and Smooth Muscle Cells. Adv. Cycl. Nucleotide Protein Phosphorylation Res. 1984, 17, 593–599. [Google Scholar] [PubMed]
- Fournier, S.B.; Reger, B.L.; Donley, D.A.; Bonner, D.E.; Warden, B.E.; Gharib, W.; Failinger, C.F.; Olfert, M.D.; Frisbee, J.C.; Olfert, I.M.; et al. Exercise Reveals Impairments in Left Ventricular Systolic Function in Patients with Metabolic Syndrome. Exp. Physiol. 2014, 99, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Najjar, S.S.; Orru’, M.; Usala, G.; Piras, M.G.; Ferrucci, L.; Cao, A.; Schlessinger, D.; Uda, M.; Lakatta, E.G. The Central Arterial Burden of the Metabolic Syndrome Is Similar in Men and Women: The SardiNIA Study. Eur. Heart J. 2010, 31, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; Thomas, F.; Blacher, J.; Nzietchueng, R.; Bureau, J.M.; Pannier, B.; Benetos, A. Metabolic Syndrome and Age-Related Progression of Aortic Stiffness. J. Am. Coll. Cardiol. 2006, 47, 72–75. [Google Scholar] [CrossRef]
- Malik, S.; Wong, N.D.; Franklin, S.S.; Kamath, T.V.; L’Italien, G.J.; Pio, J.R.; Williams, G.R. Impact of the Metabolic Syndrome on Mortality from Coronary Heart Disease, Cardiovascular Disease, and All Causes in United States Adults. Circulation 2004, 110, 1245–1250. [Google Scholar] [CrossRef]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic Syndrome and Risk of Incident Cardiovascular Events and Death. A Systematic Review and Meta-Analysis of Longitudinal Studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef]
- Stachowiak, G.; Pertyński, T.; Pertyńska-Marczewska, M. Metabolic Disorders in Menopause. Prz. Menopauzalny 2015, 14, 59–64. [Google Scholar] [CrossRef]
- Golbidi, S.; Mesdaghinia, A.; Laher, I. Exercise in the Metabolic Syndrome. Oxid. Med. Cell. Longev. 2012, 2012, 349710. [Google Scholar] [CrossRef]
- Barbosa, T.M.; Garrido, M.F.; Bragada, J. Physiological Adaptations to Head-out Aquatic Exercises with Different Levels of Body Immersion. J. Strength Cond. Res. 2007, 21, 1255–1259. [Google Scholar] [CrossRef]
- Ha, M.-S.; Kim, J.-H.; Ha, S.-M.; Kim, Y.-S.; Kim, D.-Y. Positive Influence of Aqua Exercise and Burdock Extract Intake on Fitness Factors and Vascular Regulation Substances in Elderly. J. Clin. Biochem. Nutr. 2019, 64, 73–78. [Google Scholar] [CrossRef]
- Ha, M.S.; Yook, J.S.; Lee, M.; Suwabe, K.; Jeong, W.M.; Kwak, J.-J.; Soya, H. Exercise Training and Burdock Root (Arctium Lappa L.) Extract Independently Improve Abdominal Obesity and Sex Hormones in Elderly Women with Metabolic Syndrome. Sci. Rep. 2021, 11, 5175. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.M.; Macedo, C.B.; Araújo, S.F.M.; Santos, J.C.; Borges, V.S.; Soares, A.A.; Ayres, F.; Pfrimer, L.M. Subacute Blood Pressure Response in Elderly Hypertensive Women after a Water Exercise Session: A Controlled Clinical Trial. High Blood Press. Cardiovasc. Prev. 2012, 19, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ha, M.S.; Ha, S.M.; Kim, D.Y. Aquatic Exercise Positively Affects Physiological Frailty among Postmenopausal Women: A Randomized Controlled Clinical Trial. Healthcare 2021, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, C.; Zhang, P.; Cao, X.; Huang, T.; Bai, Y.; Chen, K. Antidiabetic Effect of Burdock (Arctium Lappa L.) Root Ethanolic Extract on Streptozotocin-induced Diabetic Rats. African J. Biotechnol. 2012, 11, 9079–9085. [Google Scholar] [CrossRef]
- Ha, M.S.; Kim, J.H.; Kim, Y.S.; Kim, D.Y. Effects of Aquarobic Exercise and Burdock Intake on Serum Blood Lipids and Vascular Elasticity in Korean Elderly Women. Exp. Gerontol. 2018, 101, 63–68. [Google Scholar] [CrossRef]
- Wang, B.-S.; Yen, G.C.; Chang, L.W.; Yen, W.J.; Duh, P. Protective Effects of Burdock (Arctium Lappa Linne) on Oxidation of Low-Density Lipoprotein and Oxidative Stress in RAW 264.7 Macrophages. Food Chem. 2007, 101, 729–738. [Google Scholar] [CrossRef]
- Kim, B.Y.; Kang, S.M.; Kang, J.H.; Kang, S.Y.; Kim, K.K.; Kim, K.B.; Kim, B.; Kim, S.J.; Kim, Y.H.; Kim, J.H.; et al. 2020 Korean Society for the Study of Obesity Guidelines for the Management of Obesity in Korea. J. Obes. Metab. Syndr. 2021, 30, 81–92. [Google Scholar] [CrossRef]
- Borg, G. Perceived Exertion as an Indicator of Somatic Stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar]
- Ha, M.-S.; Baek, H. Floor Exercise Improves on Senior Fitness Test, Blood Lipids and Arterial Stiffness in Elderly Women with Metabolic Syndrome. J. Korean Appl. Sci. Technol. 2017, 34, 899–907. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity in Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, L.M.; Duprez, D.; Starmans-Kool, M.J.; Safar, M.E.; Giannattasio, C.; Cockcroft, J.; Kaiser, D.R.; Thuillez, C. Clinical Applications of Arterial Stiffness, Task Force III: Recommendations for User Procedures. Am. J. Hypertens. 2002, 15, 445–452. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert Consensus Document on Arterial Stiffness: Methodological Issues and Clinical Applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Kelly, R.; Hayward, C.; Avolio, A.; O’Rourke, M. Noninvasive Determination of Age-Related Changes in the Human Arterial Pulse. Circulation 1989, 80, 1652–1659. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Morino, K.; Petersen, K.F.; Shulman, G.I. Molecular Mechanisms of Insulin Resistance in Humans and Their Potential Links with Mitochondrial Dysfunction. Diabetes 2006, 55, S9–S15. [Google Scholar] [CrossRef]
- McLaughlin, T.; Abbasi, F.; Cheal, K.; Chu, J.; Lamendola, C.; Reaven, G. Use of Metabolic Markers to Identify Overweight Individuals Who Are Insulin Resistant. Ann. Intern. Med. 2003, 139, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Montagnani, M.; Kwang, K.K.; Quon, M.J. Reciprocal Relationships between Insulin Resistance and Endothelial Dysfunction: Molecular and Pathophysiological Mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.C. The Emergence of the Metabolic Syndrome with Menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Trabert, B.; Katki, H.A.; Chaturvedi, A.K.; Kemp, T.J.; Pinto, L.A.; Moore, S.C.; Purdue, M.P.; Wentzensen, N.; Hildesheim, A.; et al. Body Mass Index, Physical Activity, and Serum Markers of Inflammation, Immunity, and Insulin Resistance. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2840–2849. [Google Scholar] [CrossRef]
- Balkau, B.; Mhamdi, L.; Oppert, J.M.; Nolan, J.; Golay, A.; Porcellati, F.; Laakso, M.; Ferrannini, E. Physical Activity and Insulin Sensitivity: The RISC Study. Diabetes 2008, 57, 2613–2618. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program Working Group Report on Hypertension in the Elderly. National High Blood Pressure Education Program Working Group. Hypertension 1994, 23, 275–285. [Google Scholar] [CrossRef]
- Sanz, C.; Gautier, J.-F.; Hanaire, H. Physical Exercise for the Prevention and Treatment of Type 2 Diabetes. Diabetes Metab. 2010, 36, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Rezaeimanesh, D.; Farsani, P.A. The Effect of an 8-Week Selected Aquatic Aerobic Training Period on Plasma Leptin and Insulin Resistance in Men with Type 2 Diabetes. J. Adv. Pharm. Educ. Res. 2019, 9, 121–124. [Google Scholar]
- Lupattelli, G.; Lombardini, R.; Schillaci, G.; Ciuffetti, G.; Marchesi, S.; Siepi, D.; Mannarino, E. Flow-mediated Vasoactivity and Circulating Adhesion Molecules in Hypertriglyceridemia: Association with Small, Dense LDL Cholesterol Particles. Am. Heart J. 2000, 140, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.C.; Feletou, M. Endothelial Dysfunction and Vascular Disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef]
- Bunting, S.; Moncada, S.; Vane, J.R.; Gryglewski, R. Arterial Walls Generate from Prostaglandin Endoperoxides a Substance (Prostaglandin X) Which Relaxes Strips of Mesenteric and Coeliac Arteries and Inhibits Platelet Aggregation. Prostaglandins 1976, 12, 897–913. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, E.A.; Vane, J.R. Human Arterial and Venous Tissues Generate Prostacyclin (Prostaglandin X), a Potent Inhibitor of Platelet Aggregation. Lancet 1977, 309, 18–21. [Google Scholar] [CrossRef]
- Gamez-Mendez, A.M.; Vargas-Robles, H.; Ríos, A.; Escalante, B. Oxidative Stress-dependent Coronary Endothelial Dysfunction in Obese Mice. PLoS ONE 2015, 10, e0138609. [Google Scholar] [CrossRef]
- Nakahata, N. Thromboxane A2: Physiology/Pathophysiology, Cellular Signal Transduction and Pharmacology. Pharmacol. Ther. 2008, 118, 18–35. [Google Scholar] [CrossRef]
- Erhart, S.; Beer, J.H.; Reinhart, W.H. Influence of Aspirin on Platelet Count and Volume in Humans. Acta Haematol. 1999, 101, 140–144. [Google Scholar] [CrossRef]
- Pahakis, M.Y.; Kosky, J.R.; Dull, R.O.; Tarbell, J.M. The Role of Endothelial Glycocalyx Components in Mechanotransduction of Fluid Shear Stress. Biochem. Biophys. Res. Commun. 2007, 355, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.H.; Nyberg, M.; Bangsbo, J.; Saltin, B.; Hellsten, Y. Exercise Training Alters the Balance between Vasoactive Compounds in Skeletal Muscle of Individuals with Essential Hypertension. Hypertension 2011, 58, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, Y.; Sohn, H. Anti-Thrombosis and Anti-Oxidative Activity of the Root of Arctium Lappa L. Korean J. Food Preserv. 2014, 21, 727–734. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, D.H.; Cho, G.H.; Kim, J.S.; Kang, D.G.; Lee, H.S. Arctium Lappa Ameliorates Endothelial Dysfunction in Rats Fed with High Fat/Cholesterol Diets. BMC Complement. Altern. Med. 2012, 12, 116. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J.F. Role of Oxidative Modifications in Atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- MalaisseI, W.J.; Conget, I.; Sener, A.; Rorsman, P. Insulinotropic Action of AICA Riboside. II. Secretory, Metabolic and Cationic Aspects. Diabetes Res. 1994, 25, 25–37. [Google Scholar] [PubMed]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cífková, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The Role of Vascular Biomarkers for Primary and Secondary Prevention. A Position Paper from the European Society of Cardiology Working Group on Peripheral Circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic Stiffness Is an Independent Predictor of All-cause and Cardiovascular Mortality in Hypertensive Patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.J.; Cho, B.M.; Lee, S. Metabolic Syndrome and Arterial Pulse Wave Velocity. Acta Cardiol. 2010, 65, 315–321. [Google Scholar] [CrossRef]
- Padilla, J.; Harris, R.A.; Wallace, J.P. Can the Measurement of Brachial Artery Flow-mediated Dilation Be Applied to the Acute Exercise Model? Cardiovasc. Ultrasound 2007, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Donley, D.A.; Fournier, S.B.; Reger, B.L.; DeVallance, E.; Bonner, D.E.; Olfert, I.M.; Frisbee, J.C.; Chantler, P.D. Aerobic Exercise Training Reduces Arterial Stiffness in Metabolic Syndrome. J. Appl. Physiol. 2014, 116, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.A.; Wu, A.B.; Chen, C.Y. The Influence of Different Treatments on the Free Radical Scavenging Activity of Burdock and Variations of Its Active Components. Food Chem. 2004, 86, 479–484. [Google Scholar] [CrossRef]
- Wang, P.; Zweier, J.L. Measurement of Nitric Oxide and Peroxynitrite Generation in the Postischemic Heart: Evidence for Peroxynitrite-mediated Reperfusion Injury. J. Biol. Chem. 1996, 271, 29223–29230. [Google Scholar] [CrossRef]
- Lee, J.; Ha, S.J.; Park, J.; Kim, Y.H.; Lee, N.H.; Kim, Y.E.; Hong, Y.S.; Song, K.M. Arctium Lappa Root Extract Containing L-Arginine Prevents TNF-α-induced Early Atherosclerosis in Vitro and in Vivo. Nutr. Res. 2020, 77, 85–96. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).