First Report on Comparative Essential Oil Profile of Stem and Leaves of Blepharispermum hirtum Oliver and Their Antidiabetic and Anticancer Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. General Instrumentation
2.2. Collection and Identification of Plant Materials
2.3. Essential Oils Extraction
2.4. GC-MS Analysis
Identification of the Components
2.5. In Vitro α-Glucosidase Inhibitory Assay
2.6. In Vitro Cytotoxic Potential
2.7. Statistical Analysis
3. Results and Discussion
3.1. Composition of Essential Oil
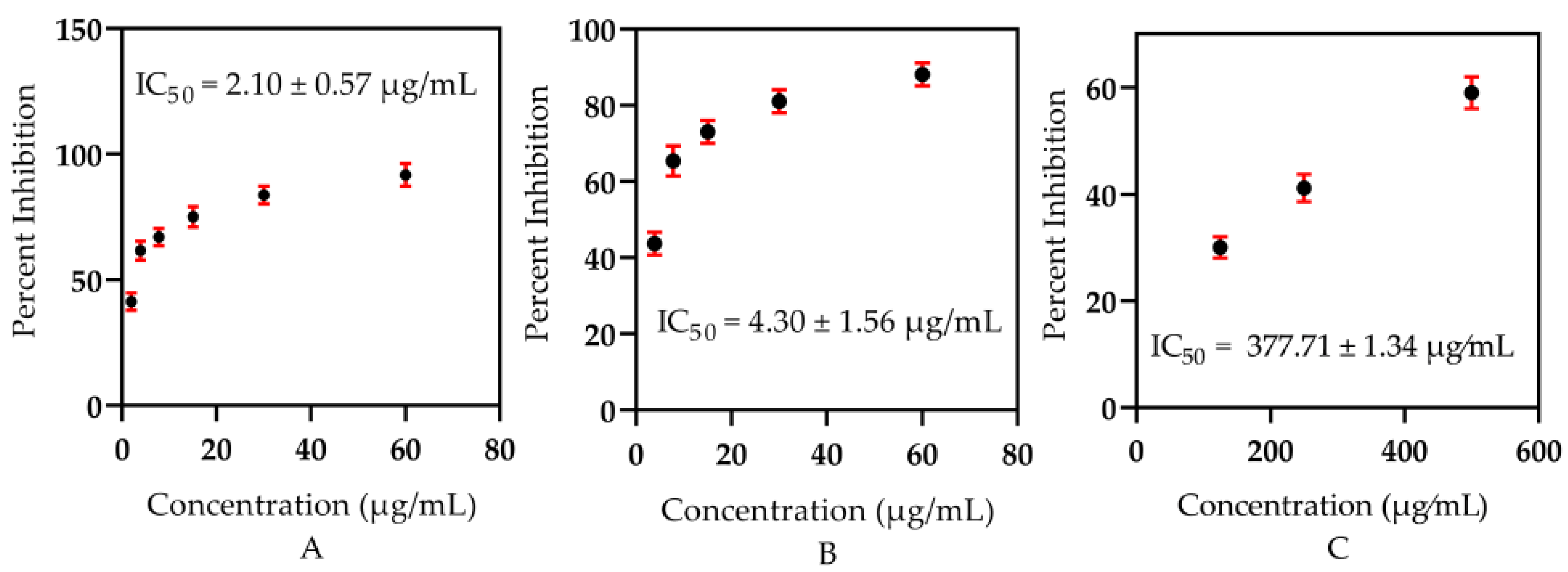
3.2. In Vitro Antidiabetic Significance
3.3. In Vitro Cytotoxicity Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes. Available online: http://www.who.int/diabetes/en/ (accessed on 28 November 2018).
- Shah, M.; Bibi, S.; Kamal, Z.; Al-Sabahi, J.N.; Alam, T.; Ullah, O.; Murad, W.; Rehman, N.U.; Al-Harrasi, A. Bridging the Chemical Profile and Biomedical Effects of Scutellaria edelbergii Essential Oils. Antioxidants 2022, 11, 1723. [Google Scholar] [CrossRef]
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, K.F.; Landau, B.R.; et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Fitsiou, E.; Pappa, A. Anticancer activity of essential oils and other extracts from aromatic plants grown in Greece. Antioxidants 2019, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Eriksson, T. The genus Blepharispermum (Asteraceae, Heliantheae). Plant Syst. Evol. 1992, 182, 149–227. [Google Scholar] [CrossRef]
- Jadhav, A.; Acharya, R.; Harisha, C.R.; Shukla, V.J.; Chandola, H. Pharmacognostical and preliminary physico-chemical profiles of Blepharispermum subsessile DC. root. Ayu 2015, 36, 73. [Google Scholar]
- Fatope, M.O.; Varma, G.B.; Alzri, N.M.; Marwah, R.G.; Nair, R.S. ent-Kaurene Diterpenoids from Blepharispermum hirtum. Chem. Biodivers. 2010, 7, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Verma, S.; Singh, S.S.; Tripathi, A.; Khan, Z.; Kumar, S. Antifeedant and antifungal activity of chromene compounds isolated from Blepharispermum subsessile. J. Ethnopharmacol. 2000, 71, 231–234. [Google Scholar] [CrossRef]
- Ahamad, J.; Uthirapathy, S.; Mohammed Ameen, M.S.; Anwer, E.T. Essential oil composition and antidiabetic, anticancer activity of Rosmarinus officinalis L. leaves from Erbil (Iraq). J. Essent. Oil Bear. Plants 2019, 22, 1544–1553. [Google Scholar] [CrossRef]
- Rashan, L.; Hakkim, F.L.; Idrees, M.; Essa, M.; Velusamy, T.; Al-Baloshi, M.; Al-Bulushi, B.; Al Jabri, A.; Alrizeiki, M.; Guillemin, G. Boswellia gum resin and essential oils: Potential health benefits—An evidence based review. Int. J. Nutr. Pharmacol. Neurol. Dis. 2019, 9, 53–71. [Google Scholar] [CrossRef]
- Ahamad, J.; Naquvi, K.J.; Mir, S.R.; Ali, M.; Shuaib, M. Review on role of natural αlpha-glucosidase inhibitors for management of diabetes mellitus. Int. J. Biomed. Res. 2011, 2, 374–380. [Google Scholar]
- Rehman, N.U.; Alsabahi, J.N.; Alam, T.; Khan, A.; Rafiq, K.; Khan, M.; Al-Harrasi, A. Chemical Constituents and Carbonic Anhydrase II Activity of Essential Oil of Acridocarpus orientalis A. Juss. in Comparison with Stem and Leaves. J. Essent. Oil Bear. Plants 2021, 24, 68–74. [Google Scholar] [CrossRef]
- Rehman, N.U.; Alsabahi, J.N.; Alam, T.; Rafiq, K.; Khan, A.; Hidayatullah; Khan, N.A.; Khan, A.L.; Al Ruqaishi, H.; Al-Harrasi, A. Chemical Composition and Biological Activities of Essential Oil from Aerial Parts of Frankenia pulverulenta L. and Boerhavia elegans Choisy from Northern Oman. J. Essent. Oil Bear.Plants 2021, 24, 1180–1191. [Google Scholar] [CrossRef]
- Sarma, N.; Gogoi, R.; Loying, R.; Begum, T.; Munda, S.; Pandey, S.; Lal, M. Phytochemical composition and biological activities of essential oils extracted from leaves and flower parts of Corymbia citriodora (Hook.). J. Environ. Biol. 2021, 42, 552–562. [Google Scholar]
- Welsh, J. Animal models for studying prevention and treatment of breast cancer. Anim. Mod. Hum. Dis. 2013, 3, 997–1018. [Google Scholar]
- Sakhi, M.; Khan, A.; Iqbal, Z.; Khan, I.; Raza, A.; Ullah, A.; Nasir, F.; Khan, S.A. Design and Characterization of Paclitaxel-Loaded Polymeric Nanoparticles Decorated with Trastuzumab for the Effective Treatment of Breast Cancer. Front. Pharmacol. 2022, 13, 85529. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.; Kassab, A.E.; Zaher, A.F.; Mahmoud, Z. Novel pyrazolo [3,4-d] pyrimidines: Design, synthesis, anticancer activity, dual EGFR/ErbB2 receptor tyrosine kinases inhibitory activity, effects on cell cycle profile and caspase-3-mediated apoptosis. J. Enzy. Inhib. Med. Chem. 2019, 34, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.; Matsuo, A.L.; Travassos, L.R. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophy. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Chen, Y.; Han, X.; Zhan, Z.; Tian, S.; Cui, Q.; Wang, Y. Chemical composition of essential oils of Litsea cubeba harvested from its distribution areas in China. Molecules 2012, 17, 7057–7066. [Google Scholar] [CrossRef] [PubMed]
- Sarma, N.; Begum, T.; Pandey, S.K.; Gogoi, R.; Munda, S.; Lal, M. Chemical profiling of leaf essential oil of Lantana camara Linn. from North-East India. J. Essent. Oil Bear. Plants 2020, 23, 1035–1041. [Google Scholar] [CrossRef]
- Quassinti, L.; Lupidi, G.; Maggi, F.; Sagratini, G.; Papa, F.; Vittori, S.; Bianco, A.; Bramucci, M. Antioxidant and antiproliferative activity of Hypericum hircinum L. subsp. majus (Aiton) N. Robson essential oil. Nat. Prod. Res. 2013, 27, 862–868. [Google Scholar] [CrossRef]
- Sajjadi, S.; Shokoohinia, Y.; Jamali, M. Chemical composition of essential oil of Ferulago macrocarpa (Fenzl) Boiss. fruits. Res. Pharm. Sci. 2012, 7, 197. [Google Scholar]
- Akpulat, H.A.; Tepe, B.; Sokmen, A.; Daferera, D.; Polissiou, M. Composition of the essential oils of Tanacetum argyrophyllum (C. Koch) Tvzel. var. argyrophyllum and Tanacetum parthenium (L.) Schultz Bip.(Asteraceae) from Turkey. Biochem. Syst. Ecol. 2005, 33, 511–516. [Google Scholar] [CrossRef]
- Hulley, I.; Özek, G.; Sadgrove, N.; Tilney, P.; Özek, T.; Başer, K.; Van Wyk, B.-E. Essential oil composition of a medicinally important Cape species: Pentzia punctata (Asteraceae). S. Afr. J. Bot. 2019, 127, 208–212. [Google Scholar] [CrossRef]
- Mejia-Garibay, B.; Palou, E.; López-Malo, A. Composition, diffusion, and antifungal activity of black mustard (Brassica nigra) essential oil when applied by direct addition or vapor phase contact. J. Food Prot. 2015, 78, 843–848. [Google Scholar] [CrossRef]
- Oroojalian, F.; Kasra-Kermanshahi, R.; Azizi, M.; Bassami, M.R. Phytochemical composition of the essential oils from three Apiaceae species and their antibacterial effects on food-borne pathogens. Food Chem. 2010, 120, 765–770. [Google Scholar] [CrossRef]
- Mubin, S.; Rehman, N.U.; Murad, W.; Shah, M.; Al-Harrasi, A.; Afza, R. Scutellaria petiolata Hemsl. ex Lace & Prain (Lamiaceae).: A New Insight in Biomedical Therapies. Antioxidants 2022, 11, 1446. [Google Scholar]
- Heghes, S.C.; Filip, L.; Vostinaru, O.; Mogosan, C.; Miere, D.; Iuga, C.A.; Moldovan, M. Essential oil-bearing plants from Balkan Peninsula: Promising sources for new drug candidates for the prevention and treatment of diabetes mellitus and dyslipidemia. Front. Pharmacol. 2020, 11, 989–996. [Google Scholar] [CrossRef]
- Mishra, C.; Code, Q. Comparative anti-diabetic study of three phytochemicals on high-fat diet and streptozotocin-induced diabetic dyslipidemic rats. Int. J. Biomed. Adv. Res. 2018, 9, 8–21. [Google Scholar]
- Hachlafi, N.E.; Aanniz, T.; Menyiy, N.E.; Baaboua, A.E.; Omari, N.E.; Balahbib, A.; Shariati, M.A.; Zengin, G.; Fikri-Benbrahim, K.; Bouyahya, A. In vitro and in vivo biological investigations of camphene and its mechanism insights: A review. Food Rev. Int. 2021, 1–28. [Google Scholar] [CrossRef]
- Majouli, K.; Hlila, M.B.; Hamdi, A.; Flamini, G.; Jannet, H.B.; Kenani, A. Antioxidant activity and α-glucosidase inhibition by essential oils from Hertia cheirifolia (L.). Ind. Crops Prod. 2016, 82, 23–28. [Google Scholar] [CrossRef]
- Ceylan, R.; Zengin, G.; Uysal, S.; Ilhan, V.; Aktumsek, A.; Kandemir, A.; Anwar, F. GC-MS analysis and in vitro antioxidant and enzyme inhibitory activities of essential oil from aerial parts of endemic Thymus spathulifolius Hausskn. et Velen. J. Enzym. Inhib. Med. Chem. 2016, 31, 983–990. [Google Scholar] [CrossRef]
- Ahamad, J. Aroma profile and α-glucosidase inhibitory activity of essential oil of Mentha spicata leaves. J. Essent. Oil Bear. Plants 2021, 24, 1042–1048. [Google Scholar] [CrossRef]
- Basak, S.S.; Candan, F. Effect of Laurus nobilis L. essential oil and its main components on α-glucosidase and reactive oxygen species scavenging activity. Iran. J. Pharm. Res. 2013, 12, 367–382. [Google Scholar]
- Shah, M.; Murad, W.; Ur Rehman, N.; Halim, S.A.; Ahmed, M.; Rehman, H.; Zahoor, M.; Mubin, S.; Khan, A.; Nassan, M.A. Biomedical applications of Scutellaria edelbergii Rech. f.: In vitro and in vivo approach. Molecules 2021, 26, 3740. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.; Morales, L.; Sastre, M.; Haskins, W.E.; Matta, J. Cytotoxicity and genotoxicity assessment of sandalwood essential oil in human breast cell lines MCF-7 and MCF-10A. Evid. Based Comp. Altern. Med. 2016, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Furtado, F.B.; Borges, B.C.; Teixeira, T.L.; Garces, H.G.; Almeida Junior, L.D.d.; Alves, F.C.B.; Silva, C.V.d.; Fernandes Junior, A. Chemical composition and bioactivity of essential oil from Blepharocalyx salicifolius. Int. J. Mol. Sci. 2018, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res. 2007, 27, 3293–3299. [Google Scholar] [PubMed]
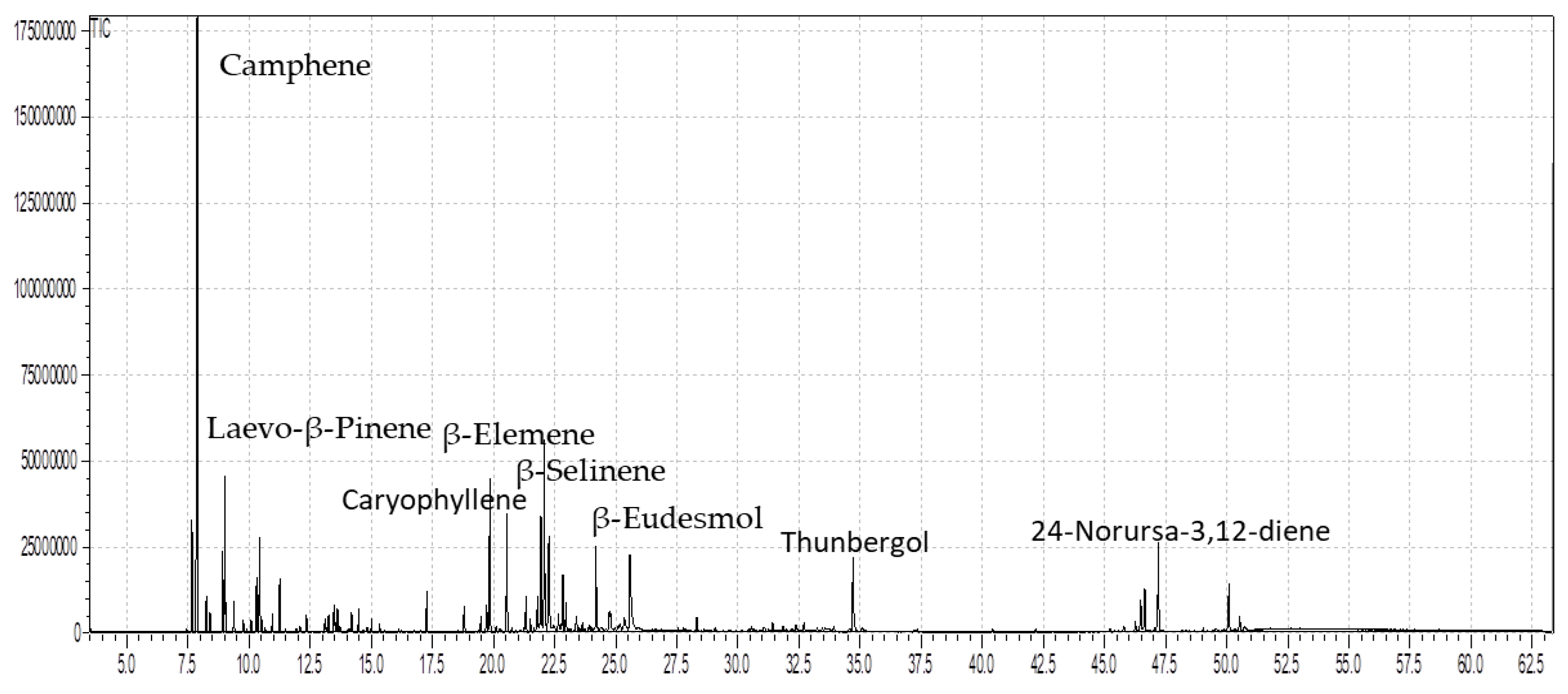
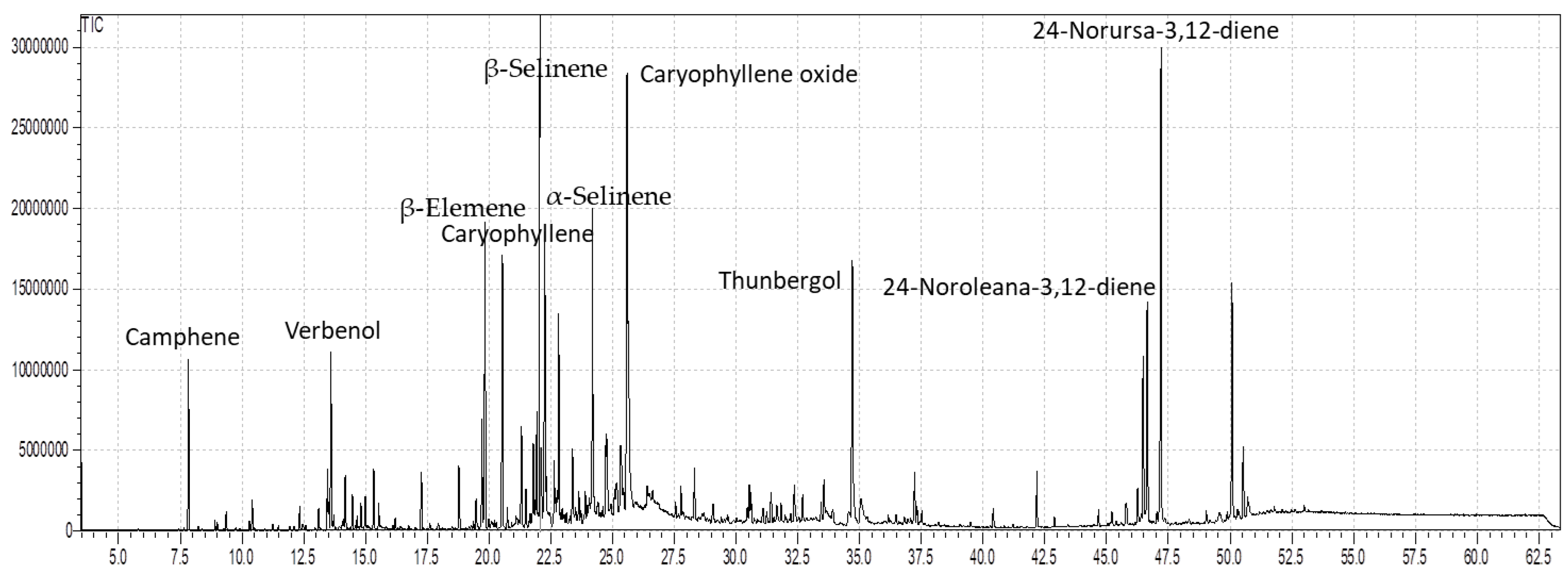
| S. No. | Compounds | RTmin | RIcal | RIrep | % Stem | % Leaves |
|---|---|---|---|---|---|---|
| 1 | 5,5-Dimethyl-1-vinylbicyclo [2.1.1] hexane | 7.44 | 927 | 920 | 0.12 | 0.03 |
| 2 | 3-Thujene | 7.65 | 935 | 928 | 3.11 | 0.06 |
| 3 | Camphene | 7.88 | 944 | 935 | 23.63 | 2.19 |
| 4 | 2,4(10)-Thujadiene | 8.39 | 963 | 957 | 0.53 | 0.03 |
| 5 | Sabinene | 8.91 | 982 | 964 | 2.21 | 0.14 |
| 6 | Laevo-β-Pinene | 9.01 | 986 | 978 | 4.38 | 0.12 |
| 7 | β-Myrcene | 9.37 | 999 | 981 | 0.91 | 0.25 |
| 8 | α-Phellandrene | 9.75 | 1013 | 997 | 0.39 | 0.05 |
| 9 | 3-Carene | 9.92 | 1019 | 1005 | 0.11 | 0.04 |
| 10 | p-Cymene | 10.30 | 1033 | 1011 | 1.46 | 0.14 |
| 11 | D-Limonene | 10.42 | 1037 | 1018 | 2.79 | 0.46 |
| 12 | γ-Terpinene | 11.25 | 1067 | 1047 | 1.51 | 0.11 |
| 13 | Linalool | 12.34 | 1106 | 1082 | 0.49 | 0.32 |
| 14 | Perillen | 12.40 | 1108 | 1086 | 0.04 | 0.08 |
| 15 | α-Campholenal | 13.10 | 1134 | 1102 | 0.42 | 0.31 |
| 16 | 2,9-Dimethyl-5-decyne | 13.10 | 1136 | 1103 | 0.31 | 0.03 |
| 17 | L-Pinocarveol | 13.46 | 1147 | 1108 | 0.85 | 0.84 |
| 18 | cis-Verbenol | 13.52 | 1149 | 1110 | 0.25 | 0.36 |
| 19 | trans-Verbenol | 13.61 | 1153 | 1128 | 0.71 | 2.51 |
| 20 | p-Mentha-1,5-dien-8-ol | 14.18 | 1174 | 1148 | 0.56 | 0.69 |
| 21 | Terpinen-4-ol | 14.47 | 1185 | 1175 | 0.67 | 0.46 |
| 22 | Myrtenol | 15.00 | 1205 | 1174 | 0.45 | 0.49 |
| 23 | Levoverbenone | 15.34 | 1218 | 1191 | 0.26 | 0.86 |
| 24 | cis-Carveol | 15.54 | 1226 | 1208 | 0.08 | 0.37 |
| 25 | Bornyl acetate | 17.26 | 1292 | 1269 | 1.23 | 0.83 |
| 26 | α-Terpinyl acetate | 18.78 | 1354 | 1322 | 0.91 | 0.97 |
| 27 | Copaene | 19.48 | 1383 | 1376 | 0.47 | 0.44 |
| 28 | β-Bourbonene | 19.71 | 1392 | 1386 | 0.84 | 1.57 |
| 29 | β-Elemene | 19.84 | 1398 | 1398 | 4.66 | 4.52 |
| 30 | Caryophyllene | 20.54 | 1428 | 1421 | 3.73 | 4.35 |
| 31 | Humulene | 21.32 | 1462 | 1454 | 1.31 | 1.55 |
| 32 | Alloaromadendrene | 21.49 | 1469 | 1459 | 0.39 | 0.59 |
| 33 | γ-Muurolene | 21.80 | 1483 | 1471 | 1.05 | 1.01 |
| 34 | Germacrene D | 21.94 | 1489 | 1480 | 3.26 | 1.31 |
| 35 | β-Selinene | 22.08 | 1495 | 1509 | 5.33 | 7.26 |
| 36 | α-Selinene | 22.26 | 1503 | 1500 | 2.92 | 4.63 |
| 37 | Cubebol | 22.64 | 1521 | 1512 | 0.33 | 0.99 |
| 38 | δ-Cadinene | 22.82 | 1529 | 1514 | 1.59 | 2.93 |
| 39 | Elemol | 23.38 | 1554 | 1535 | 0.63 | 1.42 |
| 40 | Germacrene D-4-ol | 23.99 | 1582 | 1570 | 0.09 | 0.31 |
| 41 | Caryophyllene oxide | 24.19 | 1991 | 1575 | 2.89 | 5.62 |
| 42 | Humulene 1,2-epoxide | 24.743 | 1617 | 1596 | 0.61 | 1.16 |
| 43 | γ-Eudesmol | 24.786 | 1619 | 1627 | 0.58 | 1.18 |
| 44 | Cubenol | 25.09 | 1634 | 1631 | 0.13 | 0.38 |
| 45 | tau-Cadinol | 25.341 | 1946 | 1637 | 0.77 | 1.78 |
| 46 | β-Eudesmol | 25.57 | 1657 | 1644 | 2.73 | 7.81 |
| 47 | Benzyl Benzoate | 27.77 | 1767 | 1765 | 0.12 | 0.54 |
| 48 | α-Phellandrene, dimer | 28.32 | 1794 | 1801 | 0.43 | 0.76 |
| 49 | m-Camphorene | 31.11 | 1945 | 1960 | 0.09 | 0.31 |
| 50 | Cembrene A | 31.41 | 1961 | 1970 | 0.32 | 0.58 |
| 51 | p-Camphorene | 31.68 | 1978 | 1977 | 0.08 | 0.41 |
| 52 | Geranyl-α-terpinene | 32.21 | 2007 | 1990 | 0.05 | 0.13 |
| 53 | Verticillol | 32.70 | 2036 | 2036 | 0.29 | 0.38 |
| 54 | Cembrenol | 34.55 | 2046 | 2161 | 0.25 | 0.29 |
| 55 | Thunbergol | 34.71 | 2156 | 2173 | 3.32 | 5.84 |
| 56 | 24-Norursa-3,9(11),12-triene | 46.48 | 2156 | 3042 | 1.23 | 3.17 |
| 57 | 24-Noroleana-3,12-diene | 46.645 | 3013 | 3057 | 1.55 | 4.03 |
| 58 | 24-Norursa-3,12-diene | 47.198 | 3060 | 3105 | 3.46 | 9.08 |
| Total % of the identified compounds | 93.88 | 89.07 |
| Tested Samples | Conc. (μg/mL) | % Viability | % Inhibition | IC50 (μg/mL) |
|---|---|---|---|---|
| Leaves | 3 | 94.33 | 5.66 | 88.4 ± 0.5 |
| 10 | 82.41 | 17.58 | ||
| 30 | 71.09 | 28.90 | ||
| 100 | 47.85 | 52.14 | ||
| 300 | 26.26 | 73.73 | ||
| Stem | 3 | 96.49 | 3.50 | 123.6 ± 0.8 |
| 10 | 85.43 | 14.56 | ||
| 30 | 72.65 | 27.34 | ||
| 100 | 53.04 | 46.95 | ||
| 300 | 37.05 | 62.94 |
| Tested Samples | Conc (μg/mL) | % Viability | % Inhibition | IC50 (μg/mL) |
|---|---|---|---|---|
| Leaves | 3 | 96.29 | 3.70 | >300 |
| 10 | 93.04 | 6.99 | ||
| 30 | 89.34 | 10.65 | ||
| 100 | 86.96 | 13.03 | ||
| 300 | 78.07 | 23.99 | ||
| Stem | 3 | 97.50 | 2.49 | >300 |
| 10 | 95.28 | 4.71 | ||
| 30 | 89.93 | 10.06 | ||
| 100 | 85.10 | 14.89 | ||
| 300 | 79.22 | 20.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, M.; Al-Housni, S.K.; Khan, F.; Ullah, S.; Al-Sabahi, J.N.; Khan, A.; Al-Yahyaei, B.E.M.; Al-Ruqaishi, H.; Rehman, N.U.; Al-Harrasi, A. First Report on Comparative Essential Oil Profile of Stem and Leaves of Blepharispermum hirtum Oliver and Their Antidiabetic and Anticancer Effects. Metabolites 2022, 12, 907. https://doi.org/10.3390/metabo12100907
Shah M, Al-Housni SK, Khan F, Ullah S, Al-Sabahi JN, Khan A, Al-Yahyaei BEM, Al-Ruqaishi H, Rehman NU, Al-Harrasi A. First Report on Comparative Essential Oil Profile of Stem and Leaves of Blepharispermum hirtum Oliver and Their Antidiabetic and Anticancer Effects. Metabolites. 2022; 12(10):907. https://doi.org/10.3390/metabo12100907
Chicago/Turabian StyleShah, Muddaser, Saif Khalfan Al-Housni, Faizullah Khan, Saeed Ullah, Jamal Nasser Al-Sabahi, Ajmal Khan, Balqees Essa Mohammed Al-Yahyaei, Houda Al-Ruqaishi, Najeeb Ur Rehman, and Ahmed Al-Harrasi. 2022. "First Report on Comparative Essential Oil Profile of Stem and Leaves of Blepharispermum hirtum Oliver and Their Antidiabetic and Anticancer Effects" Metabolites 12, no. 10: 907. https://doi.org/10.3390/metabo12100907
APA StyleShah, M., Al-Housni, S. K., Khan, F., Ullah, S., Al-Sabahi, J. N., Khan, A., Al-Yahyaei, B. E. M., Al-Ruqaishi, H., Rehman, N. U., & Al-Harrasi, A. (2022). First Report on Comparative Essential Oil Profile of Stem and Leaves of Blepharispermum hirtum Oliver and Their Antidiabetic and Anticancer Effects. Metabolites, 12(10), 907. https://doi.org/10.3390/metabo12100907











