Correlation between Pre-Ovulatory Follicle Diameter and Follicular Fluid Metabolome Profiles in Lactating Beef Cows
Abstract
1. Introduction
2. Results and Discussion
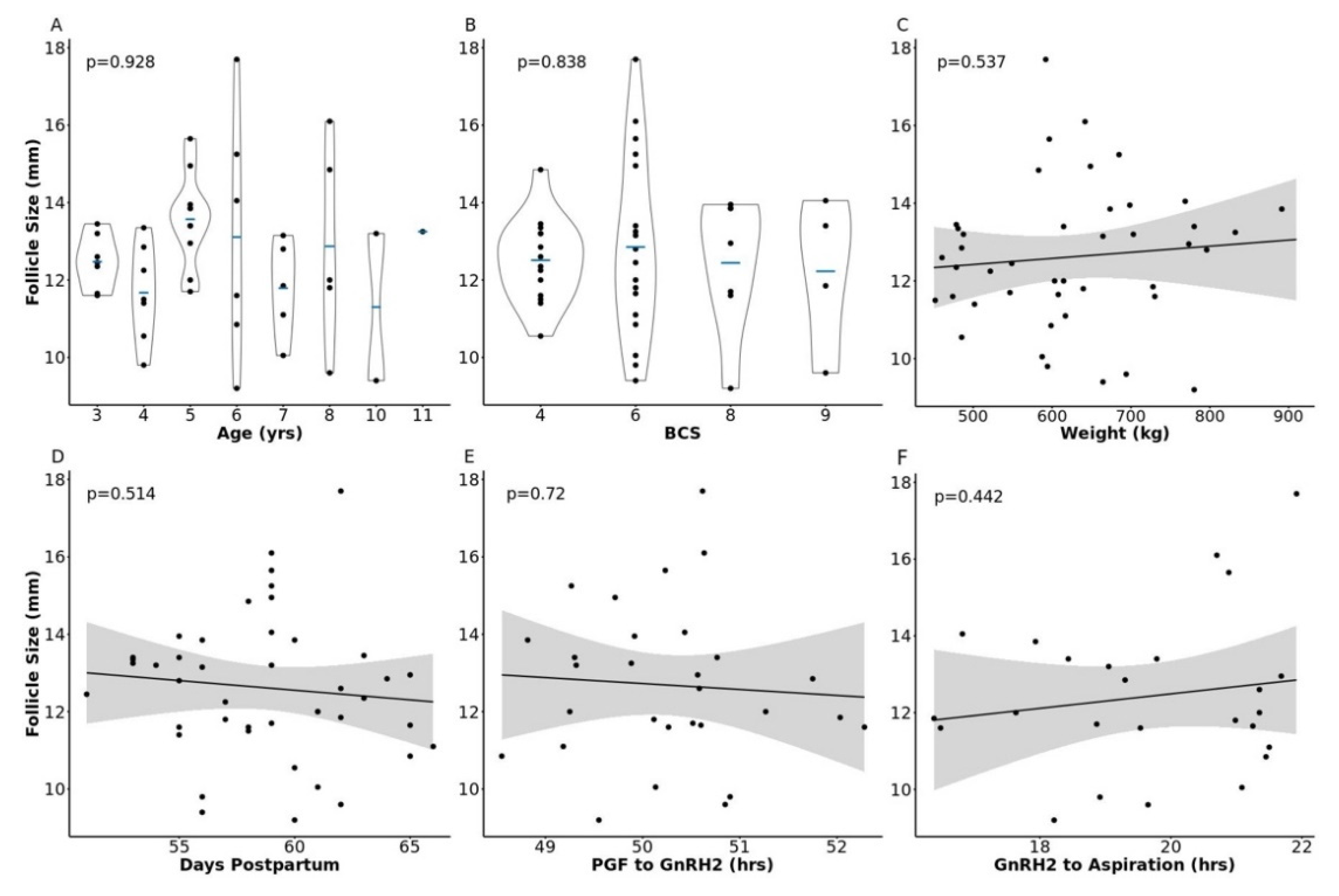
2.1. Animal Data
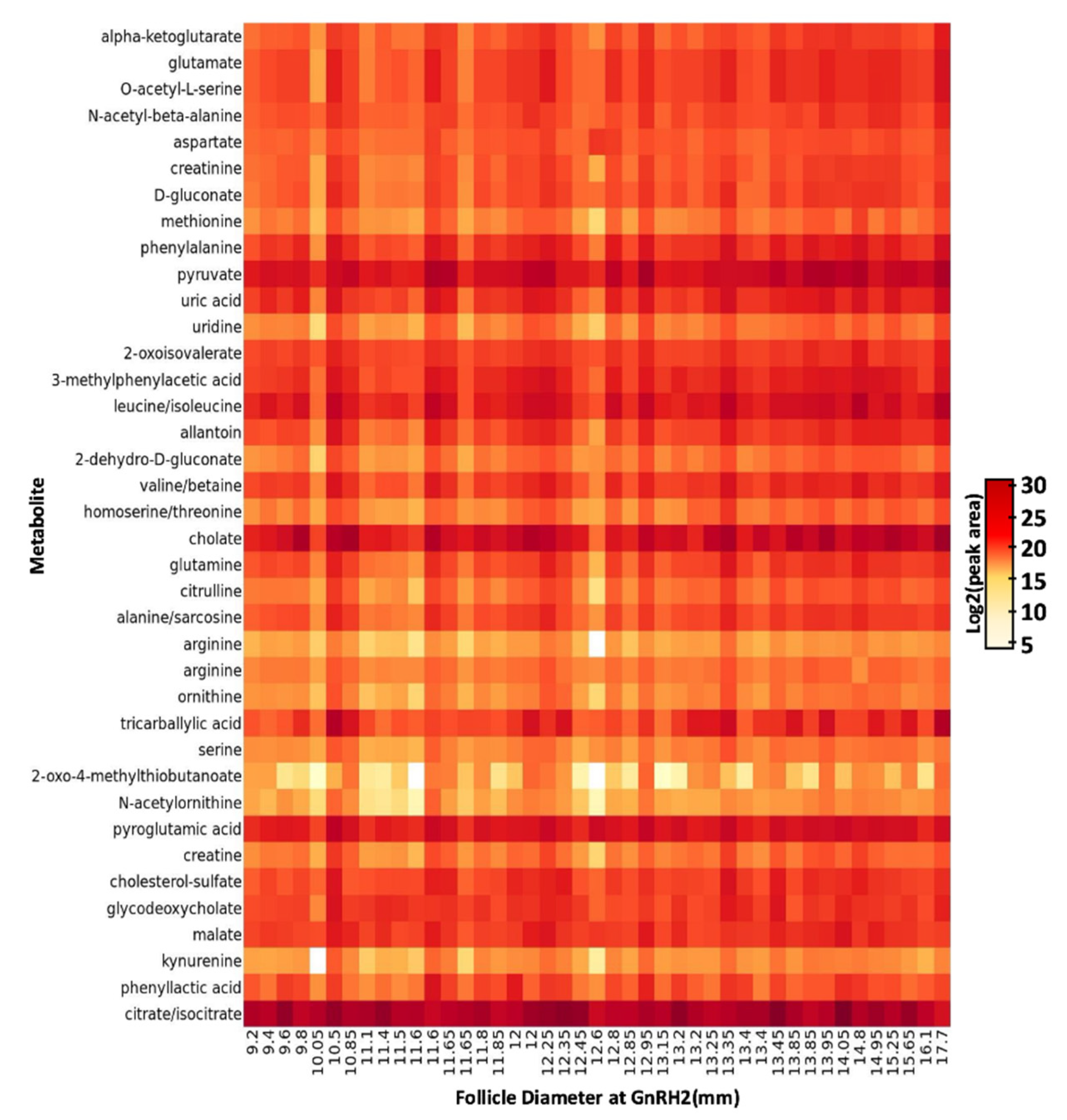
2.2. Metabolome Profiles of Follicular Fluid Collected from Pre-Ovulatory Follicles of Lactating Beef Cows
2.3. The Impact of Increasing Pre-Ovulatory Follicle Diameter on the Follicular Fluid Metabolome
3. Materials and Methods
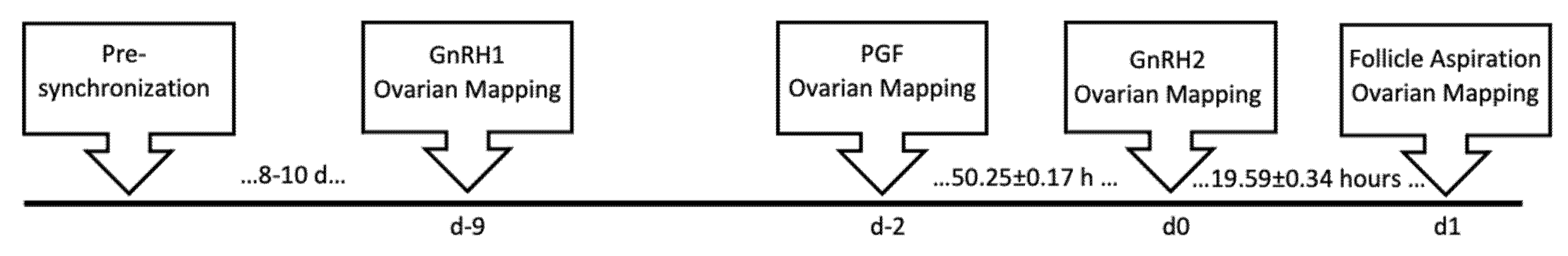
3.1. Animal Handling and Synchronization of Pre-Ovulatory Follicle Development
3.2. Transvaginal Aspiration for Collection of Follicular Fluid from the Pre-Ovulatory Follicle
3.3. Follicular Fluid Processing
3.4. UHPLC-HRMS Metabolomics
3.5. Primary Data Analysis
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perry, G.A.; Smith, M.F.; Roberts, A.J.; MacNeil, M.D.; Geary, T.W. Relationship between size of the ovulatory follicle and pregnancy success in beef heifers. J. Anim. Sci. 2007, 85, 684–689. [Google Scholar] [CrossRef]
- Perry, G.A.; Smith, M.F.; Lucy, M.C.; Green, J.A.; Parks, T.E.; MacNeil, M.D.; Roberts, A.J.; Geary, T.W. Relationship between follicle size at insemination and pregnancy success. Proc. Natl. Acad. Sci. USA 2005, 102, 5268–5273. [Google Scholar] [CrossRef] [PubMed]
- Lamb, G.C.; Stevenson, J.S.; Kesler, D.J.; Garverick, H.A.; Brown, D.R.; Salfen, B.E. Inclusion of an intravaginal progesterone insert plus GnRH and prostaglandin F2α for ovulation control in postpartum suckled beef cows. J. Anim. Sci. 2001, 79, 2253–2259. [Google Scholar] [CrossRef]
- Sá Filho, M.F.; Crespilho, A.M.; Santos, J.E.P.; Perry, G.A.; Baruselli, P.S. Ovarian follicle diameter at timed insemination and estrous response influence likelihood of ovulation and pregnancy after estrous synchronization with progesterone or progestin-based protocols in suckled Bos indicus cows. Anim. Reprod. Sci. 2010, 120, 23–30. [Google Scholar] [CrossRef]
- Ciernia, L.A.; Perry, G.A.; Smith, M.F.; Rich, J.J.; Northrop, E.J.; Perkins, S.D.; Green, J.A.; Zezeski, A.L.; Geary, T.W. Effect of estradiol preceding and progesterone subsequent to ovulation on proportion of postpartum beef cows pregnant. Anim. Reprod. Sci. 2021, 227, 106–723. [Google Scholar] [CrossRef] [PubMed]
- Jinks, E.M.; Smith, M.F.; Atkins, J.A.; Pohler, K.G.; Perry, G.A.; Macneil, M.D.; Roberts, A.J.; Waterman, R.C.; Alexander, L.J.; Geary, T.W. Preovulatory estradiol and the establishment and maintenance of pregnancy in suckled beef cows. J. Anim. Sci. 2013, 91, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.A.; Smith, M.F.; MacNeil, M.D.; Jinks, E.M.; Abreu, F.M.; Alexander, L.J.; Geary, T.W. Pregnancy establishment and maintenance in cattle. J. Anim. Sci. 2013, 91, 722–733. [Google Scholar] [CrossRef]
- Moorey, S.E.; Monnig, J.M.; Smith, M.F.; Ortega, M.S.; Green, J.A.; Pohler, K.G.; Bridges, G.A.; Behura, S.K.; Geary, T.W. Differential transcript profiles in cumulus-oocyte complexes originating from pre-ovulatory follicles of varied physiological maturity in beef cows. Genes 2021, 12, 893. [Google Scholar] [CrossRef]
- Winterhager, E.; Kidder, G.M. Gap junction connexins in female reproductive organs: Implications for women’s reproductive health. Hum. Reprod. Update 2015, 21, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef]
- Brown, H.M.; Dunning, K.R.; Sutton-McDowall, M.; Gilchrist, R.B.; Thompson, J.G.; Russell, D.L. Failure to launch: Aberrant cumulus gene expression during oocyte in vitro maturation. Reproduction 2017, 153, R109–R120. [Google Scholar] [CrossRef]
- Coticchio, G.; Dal Canto, M.; Mignini Renzini, M.; Guglielmo, M.C.; Brambillasca, F.; Turchi, D.; Novara, P.V.; Fadini, R. Oocyte maturation: Gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 2015, 21, 427–454. [Google Scholar] [CrossRef]
- Cetica, P.; Pintos, L.; Dalvit, G.; Beconi, M. Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction 2002, 124, 675. [Google Scholar] [CrossRef] [PubMed]
- Biggers, J.; Whittingham, D.; Donahue, R. The pattern of energy metabolism in the mouse oocyte and zygote. Zoology 1967, 58, 560–567. [Google Scholar] [CrossRef]
- Johnson, M.T.; Freeman, E.A.; Gardner, D.K.; Hunt, P.A. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol. Reprod. 2007, 77, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Chappel, S. The role of mitochondria from mature oocyte to viable blastocyst. Obstet. Gynecol. Int. 2013, 2013, 183024. [Google Scholar] [CrossRef]
- Read, C.C.; Willhelm, G.; Dyce, P.W. Connexin 43 coupling in bovine cumulus cells, during the follicular growth phase, and its relationship to in vitro embryo outcomes. Mol. Reprod. Dev. 2018, 85, 579–589. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Irving-Rodgers, H.F. Formation of the ovarian follicular antrum and follicular fluid. Biol. Reprod. 2010, 82, 1021–1029. [Google Scholar] [CrossRef]
- Clarke, H.G.; Hope, S.A.; Byers, S.; Rodgers, R.J. Formation of ovarian follicular fluid may be due to the osmotic potential of large glycosaminoglycans and proteoglycans. J. Reprod. 2006, 132, 119–131. [Google Scholar] [CrossRef]
- Gosden, R.G.; Hunter, R.H.; Telfer, E.; Torrance, C.; Brown, N. Physiological factors underlying the formation of ovarian follicular fluid. J. Reprod. Fertil. 1988, 82, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.G. Follicular fluid. J. Reprod. Fertil. 1974, 37, 189–219. [Google Scholar] [CrossRef] [PubMed]
- Hennet, M.L.; Combelles, C.M. The antral follicle: A microenvironment for oocyte differentiation. Int. J. Dev. Biol. 2012, 56, 819–831. [Google Scholar] [CrossRef]
- Kidder, G.M.; Vanderhyden, B.C. Bidirectional communication between oocytes and follicle cells: Ensuring oocyte developmental competence. Can. J. Physiol. Pharmacol. 2010, 88, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Fahiminiya, S.; Gérard, N. Le liquide folliculaire chez les mammifères. Gynécologie Obstet. Fertil. 2010, 38, 402–404. [Google Scholar] [CrossRef]
- Moreno, J.M.; Núñez, M.J.; Quiñonero, A.; Martínez, S.; de la Orden, M.; Simón, C.; Pellicer, A.; Díaz-García, C.; Domínguez, F. Follicular fluid and mural granulosa cells microRNA profiles vary in in vitro fertilization patients depending on their age and oocyte maturation stage. Fertil. Steril. 2015, 104, 1037–1046.e1. [Google Scholar] [CrossRef]
- Orsi, N.M.; Gopichandran, N.; Leese, H.J.; Picton, H.M.; Harris, S.E. Fluctuations in bovine ovarian follicular fluid composition throughout the oestrous cycle. Reproduction 2005, 129, 219. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.; Walsh, S.; Evans, A.C.; Fair, T.; Brennan, L. Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows. Reproduction 2010, 139, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Kafi, M.; Azari, M.; Chashnigir, O.; Gharibzadeh, S.; Aghabozorgi, Z.; Asaadi, A.; Divar, M.R. Inherent inferior quality of follicular fluid in repeat breeder heifers as evidenced by low rates of in vitro production of bovine embryos. Theriogenology 2017, 102, 29–34. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Lunn, L.A.; Kwong, W.Y.; Wonnacott, K.; Linforth, R.S.; Craigon, J. Amino acid and fatty acid composition of follicular fluid as predictors of in-vitro embryo development. Reprod. Biomed. Online 2008, 16, 859–868. [Google Scholar] [CrossRef]
- MacNeil, M.D.; Geary, T.W.; Perry, G.A.; Roberts, A.J.; Alexander, L.J. Genetic partitioning of variation in ovulatory follicle size and probability of pregnancy in beef cattle. J. Anim. Sci. 2006, 84, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- Geary, T.W.; Whittier, J.C.; Hallford, D.M.; MacNeil, M.D. Calf removal improves conception rates to the Ovsynch and CO-Synch protocols. J. Anim. Sci. 2001, 79, 1–4. [Google Scholar] [CrossRef]
- Damiran, D.; Larson, K.A.; Pearce, L.T.; Erickson, N.E.; Lardner, B.H.A. Effect of calving period on beef cow longevity and lifetime productivity in western Canada. Transl. Anim. Sci. 2018, 2 (Suppl. 1), S61–S65. [Google Scholar] [CrossRef]
- Cushman, R.A.; Kill, L.K.; Funston, R.N.; Mousel, E.M.; Perry, G.A. Heifer calving date positively influences calf weaning weights through six parturitions. J. Anim. Sci. 2013, 91, 4486–4491. [Google Scholar] [CrossRef]
- Bonacker, R.C.; Gray, K.R.; Breiner, C.A.; Anderson, J.M.; Patterson, D.J.; Spinka, C.M.; Thomas, J.M. Comparison of the 7 & 7 Synch protocol and the 7-day CO-Synch + CIDR protocol among recipient beef cows in an embryo transfer program. Theriogenology 2020, 158, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Cushman, R.A.; Allan, M.F.; Thallman, R.M.; Cundiff, L.V. Characterization of biological types of cattle (Cycle VII): Influence of postpartum interval and estrous cycle length on fertility. J. Anim. Sci. 2007, 85, 2156–2162. [Google Scholar] [CrossRef]
- Komar, C.M.; Berndtson, A.K.; Evans, A.C.; Fortune, J.E. Decline in circulating estradiol during the periovulatory period is correlated with decreases in estradiol and androgen, and in messenger RNA for p450 aromatase and p450 17alpha-hydroxylase, in bovine preovulatory follicles. Biol. Reprod. 2001, 64, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Russell, D.L.; Robker, R.L.; Dajee, M.; Alliston, T.N. Molecular mechanisms of ovulation and luteinization. Mol. Cell Endocrinol. 1998, 145, 47–54. [Google Scholar] [CrossRef]
- de Loos, F.A.M.; Bevers, M.M.; Dieleman, S.J.; Kruip, T.A.M. Morphology of preovulatory bovine follicles as related to oocyte maturation. Theriogenology 1991, 35, 527–535. [Google Scholar] [CrossRef]
- Hyttel, P.; Callesen, H.; Greve, T. Ultrastructural features of preovulatory oocyte maturation in superovulated cattle. J. Reprod. Fertil. 1986, 76, 645–656. [Google Scholar] [CrossRef]
- Hyttel, P.; Fair, T.; Callesen, H.; Greve, T. Oocyte growth, capacitation and final maturation in cattle. Theriogenology 1997, 47, 23–32. [Google Scholar] [CrossRef]
- Bernabé, B.P.; Thiele, I.; Galdones, E.; Siletz, A.; Chandrasekaran, S.; Woodruff, T.K.; Broadbelt, L.J.; Shea, L.D. Dynamic genome-scale cell-specific metabolic models reveal novel inter-cellular and intra-cellular metabolic communications during ovarian follicle development. BMC Bioinform. 2019, 20, 1–16. [Google Scholar] [CrossRef]
- Bertoldo, M.J.; Nadal-Desbarats, L.; Gérard, N.; Dubois, A.; Holyoake, P.K.; Grupen, C.G. Differences in the metabolomic signatures of porcine follicular fluid collected from environments associated with good and poor oocyte quality. Reproduction 2013, 146, 221–231. [Google Scholar] [CrossRef]
- Blaschka, C.; Schuler, G.; Sánchez-Guijo, A.; Zimmer, B.; Feller, S.; Kotarski, F.; Wudy, S.A.; Wrenzycki, C. Occurrence of sulfonated steroids and ovarian expression of steroid sulfatase and SULT1E1 in cyclic cows. J. Steroid Biochem. Mol. Biol. 2018, 179, 79–87. [Google Scholar] [CrossRef]
- Bódis, J.; Sulyok, E.; Koppán, M.; Prémusz, V.; Gödöny, K.; Rascher, W.; Rauh, M. Tryptophan catabolism to serotonin and kynurenine in women undergoing in-vitro fertilization. Physiol. Res. 2020, 69, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.S.; Jones, J.D.; Ellefson, R.D.; Ryan, R.J. The porcine ovarian follicle: I. selected chemical analysis of follicular fluid at different developmental stages. Biol. Reprod. 1976, 15, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Forde, N.; O’Gorman, A.; Whelan, H.; Duffy, P.; O’Hara, L.; Kelly, A.K.; Havlicek, V.; Besenfelder, U.; Brennan, L.; Lonergan, P. Lactation-induced changes in metabolic status and follicular-fluid metabolomic profile in postpartum dairy cows. Reprod. Fertil. Dev. 2016, 28, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Gérard, N.; Loiseau, S.; Duchamp, G.; Seguin, F. Analysis of the variations of follicular fluid composition during follicular growth and maturation in the mare using proton nuclear magnetic resonance (1H NMR). Reproduction 2002, 124, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Grimek, H.J.; Bellin, M.E.; Ax, R.L. Characteristics of proteoglycans isolated from small and large bovine ovarian follicles. Biol. Reprod. 1984, 30, 397–409. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Di, R.; Liu, Q.; Hu, W.; He, X.; Yu, J.; Zhang, X.; Zhang, J.; Broniowska, K.; et al. Metabolic effects of FecB gene on follicular fluid and ovarian vein serum in sheep (Ovis aries). Int. J. Mol. Sci. 2018, 19, 539. [Google Scholar] [CrossRef]
- Hou, E.; Zhao, Y.; Hang, J.; Qiao, J. Metabolomics and correlation network analysis of follicular fluid reveals associations between l-tryptophan, l-tyrosine and polycystic ovary syndrome. Biomed. Chromatog. 2021, 35, e4993. [Google Scholar] [CrossRef] [PubMed]
- Jóźwik, M.; Jóźwik, M.; Milewska, A.J.; Battaglia, F.C.; Jóźwik, M. Competitive inhibition of amino acid transport in human preovulatory ovarian follicles. Syst. Biol. Reprod. Med. 2017, 63, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Luti, S.; Fiaschi, T.; Magherini, F.; Modesti, P.A.; Piomboni, P.; Governini, L.; Luddi, A.; Amoresano, A.; Illiano, A.; Pinto, G.; et al. Relationship between the metabolic and lipid profile in follicular fluid of women undergoing in vitro fertilization. Mol. Reprod. Dev. 2020, 87, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Sun, L.; Cheng, J.; Lu, Y.; Wei, Y.; Qin, G.; Liang, J.; Lan, G. Non-targeted metabolomics reveals metabolic characteristics of porcine atretic follicles. Front. Vet. Sci. 2021, 8, 679947. [Google Scholar] [CrossRef]
- Nagy, R.A.; van Montfoort, A.P.A.; Dikkers, A.; van Echten-Arends, J.; Homminga, I.; Land, J.A.; Hoek, A.; Tietge, U.J.F. Presence of bile acids in human follicular fluid and their relation with embryo development in modified natural cycle IVF. Hum. Reprod. 2015, 30, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Kumar, V.G.; Manjunatha, B.M.; Gupta, P.S.P. Biochemical composition of ovine follicular fluid in relation to follicle size. Dev. Growth Differ. 2007, 49, 61–66. [Google Scholar] [CrossRef]
- Piñero-Sagredo, E.; Nunes, S.; de los Santos, M.J.; Celda, B.; Esteve, V. NMR metabolic profile of human follicular fluid. NMR Biomed. 2010, 23, 485–495. [Google Scholar] [CrossRef]
- Song, J.-Y.; Xiang, S.; Yang, Y.; Sun, Z. Assessment of follicular fluid metabolomics of polycystic ovary syndrome in kidney yang deficiency syndrome. Eur. J. Integr. Med. 2019, 30, 100944. [Google Scholar] [CrossRef]
- Song, Y.X.; Hu, P.; Bai, Y.L.; Zhao, C.; Xia, C.; Xu, C. Plasma metabolic characterisation of dairy cows with inactive ovaries and oestrus during the peak of lactation. J. Vet. Res. 2019, 63, 359–367. [Google Scholar] [CrossRef]
- Tabatabaei, S.; Mamoei, M.; Aghaei, A. Dynamics of ovarian follicular fluid in cattle. Comp. Clin. Path 2011, 20, 591–595. [Google Scholar] [CrossRef]
- Xia, L.; Zhao, X.; Sun, Y.; Hong, Y.; Gao, Y.; Hu, S. Metabolomic profiling of human follicular fluid from patients with repeated failure of in vitro fertilization using gas chromatography/mass spectrometry. Int. J. Clin. Exp. Pathol. 2014, 7, 7220. [Google Scholar] [PubMed]
- Zhang, Z.; He, C.; Gao, Y.; Zhang, L.; Song, Y.; Zhu, T.; Zhu, K.; Lv, D.; Wang, J.; Tian, X.; et al. α-ketoglutarate delays age-related fertility decline in mammals. Aging Cell 2021, 20, e13291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, Y.; Li, T.; Li, M.; Li, J.; Li, R.; Liu, P.; Yu, Y.; Qiao, J. Metabolism alteration in follicular niche: The nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic. Biol. Med. 2015, 86, 295–307. [Google Scholar] [CrossRef]
- Harris, S.E.; Adriaens, I.; Leese, H.J.; Gosden, R.G.; Picton, H.M. Carbohydrate metabolism by murine ovarian follicles and oocytes grown in vitro. Reproduction 2007, 134, 415–424. [Google Scholar] [CrossRef]
- Thompson, J.G.; Gilchrist, R.B.; Sutton-McDowall, M.L. The metabolism of the ruminant cumulus-oocyte complex revisted. Reprod. Dom. Rum. 2014, 311–326. [Google Scholar] [CrossRef][Green Version]
- Rieger, D.; Loskutoff, N.M. Changes in the metabolism of glucose, pyruvate, glutamine and glycine during maturation of cattle oocytes in vitro. Reproduction 1994, 100, 257. [Google Scholar] [CrossRef]
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. Cumulus expansion and glucose utilisation by bovine cumulus–oocyte complexes during in vitro maturation: The influence of glucosamine and follicle-stimulating hormone. Reproduction 2004, 128, 313–319. [Google Scholar] [CrossRef]
- Uhde, K.; van Tol, H.T.A.; Stout, T.A.E.; Roelen, B.A.J. Metabolomic profiles of bovine cumulus cells and cumulus-oocyte-complex-conditioned medium during maturation in vitro. Sci. Rep. 2018, 8, 9477. [Google Scholar] [CrossRef]
- Warzych, E.; Lipinska, P. Energy metabolism of follicular environment during oocyte growth and maturation. J. Reprod. Dev. 2020, 66, 1–7. [Google Scholar] [CrossRef]
- D’Aniello, G.; Grieco, N.; Di Filippo, M.A.; Cappiello, F.; Topo, E.; D’Aniello, E.; Ronsini, S. Reproductive implication of D-aspartic acid in human pre-ovulatory follicular fluid. Hum. Reprod. 2007, 22, 3178–3183. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau-Goeseels, S. Effects of culture media and energy sources on the inhibition of nuclear maturation in bovine oocytes. Theriogenology 2006, 66, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, L.; Yao, K. The antioxidative function of alpha-ketoglutarate and its applications. Biomed. Res. Int. 2018, 2018, 3408467. [Google Scholar] [CrossRef] [PubMed]
- Rathod, P.K.; Fellman, J.H. Regulation of mammalian aspartate-4-decarboxylase: Its possible role in oxaloacetate and energy metabolism. Arch. Biochem. Biophys. 1985, 238, 447–451. [Google Scholar] [CrossRef]
- Colonna, R.; Mangia, F. Mechanisms of amino acid uptake in cumulus-enclosed mouse oocytes. Biol. Reprod. 1983, 28, 797–803. [Google Scholar] [CrossRef]
- Thompson, J.G.; Lane, M.; Gilchrist, R.B. Metabolism of the bovine cumulus-oocyte complex and influence on subsequent developmental competence. Soc. Reprod. Fertil. Suppl. 2007, 64, 179–190. [Google Scholar] [CrossRef]
- Edson, M.A.; Nagaraja, A.K.; Matzuk, M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009, 30, 624–712. [Google Scholar] [CrossRef]
- Revelli, A.; Delle Piane, L.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef]
- Sendžikaitė, G.; Kelsey, G. The role and mechanisms of DNA methylation in the oocyte. Essays Biochem. 2019, 63, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef]
- Kand’ár, R.; Záková, P.; Muzáková, V. Monitoring of antioxidant properties of uric acid in humans for a consideration measuring of levels of allantoin in plasma by liquid chromatography. Clin. Chim. Acta 2006, 365, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Cassano, E.; Tosto, L.; Balestrieri, M.; Zicarelli, L.; Abrescia, P. Antioxidant defense in the follicular fluid of water buffalo. Cell Physiol. Biochem. 1999, 9, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Bou Nemer, L.; Shi, H.; Carr, B.R.; Word, R.A.; Bukulmez, O. Effect of body weight on metabolic hormones and fatty acid metabolism in follicular fluid of women undergoing in vitro fertilization: A pilot study. Reprod. Sci. 2019, 26, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Whitman, R.W. Weight Change, Body Condition and Beef-Cow Reproduction. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 1975. [Google Scholar]
- Lu, W.; Clasquin, M.F.; Melamud, E.; Amador-Noguez, D.; Caudy, A.A.; Rabinowitz, J.D. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal. Chem. 2010, 82, 3212–3221. [Google Scholar] [CrossRef]
- Greene, E.; Cauble, R.; Dhamad, A.E.; Kidd, M.T.; Kong, B.; Howard, S.M.; Castro, H.F.; Campagna, S.R.; Bedford, M.; Dridi, S. Muscle metabolome profiles in woody breast-(un)affected broilers: Effects of quantum blue phytase-enriched diet. Front. Vet. Sci. 2020, 7, 458. [Google Scholar] [CrossRef] [PubMed]
- Martens, L.; Chambers, M.; Sturm, M.; Kessner, D.; Levander, F.; Shofstahl, J.; Tang, W.H.; Römpp, A.; Neumann, S.; Pizarro, A.D.; et al. mzML—A community standard for mass spectrometry data. Mol. Cell Proteom. 2011, 10, R110.000133. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS data processing with MAVEN: A metabolomic analysis and visualization engine. Curr. Protoc. Bioinform. 2012, 37. [Google Scholar] [CrossRef]
- Melamud, E.; Vastag, L.; Rabinowitz, J.D. Metabolomic analysis and visualization engine for LC-MS data. Anal. Chem. 2010, 82, 9818–9826. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 30 August 2021).
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
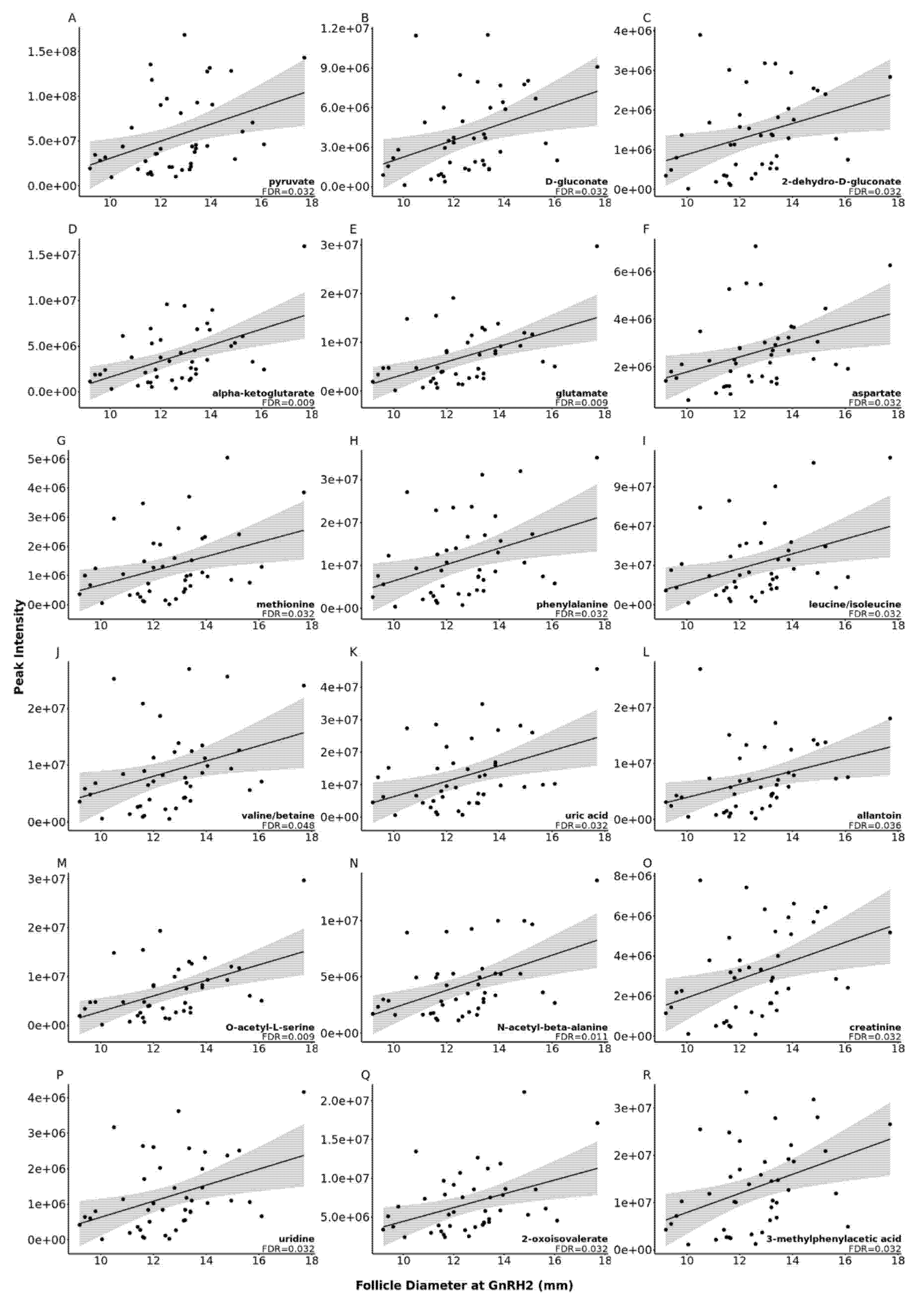
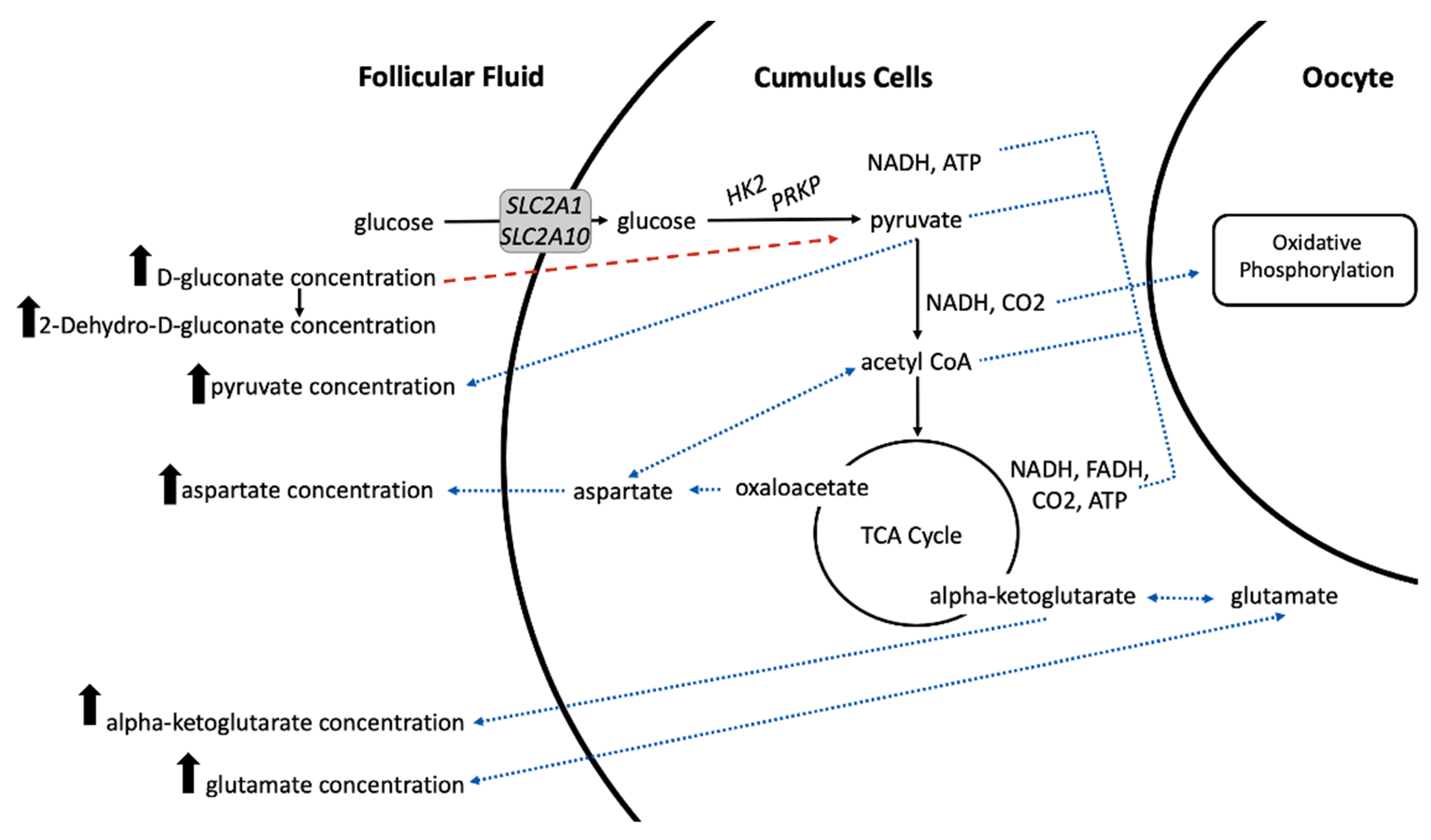
| Pathway | Pathway Name | Match Status * | FDR | Differentially Abundant Metabolites in Pathway † |
|---|---|---|---|---|
| bta00250 | alanine, aspartate and glutamate metabolism | 4/28 | 0.009 | C00026 (Alpha-Ketoglutarate) C00049 (Aspartate) C00025 (Glutamate) C00022 (Pyruvate) |
| bta00220 | arginine biosynthesis | 3/14 | 0.011 | C00026 (Alpha-Ketoglutarate) C00049 (Aspartate) C00025 (Glutamate) |
| bta00970 | aminoacyl-tRNA biosynthesis | 4/48 | 0.019 | C00049 (Aspartate) C00025 (Glutamate) C00073 (Methionine) C00079 (Phenylalanine) |
| bta00471 | d-glutamine and d-glutamate metabolism | 2/5 | 0.020 | C00026 (Alpha-Ketoglutarate) C00025 (Glutamate) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Read, C.C.; Edwards, L.; Schrick, N.; Rhinehart, J.D.; Payton, R.R.; Campagna, S.R.; Castro, H.F.; Klabnik, J.L.; Horn, E.J.; Moorey, S.E. Correlation between Pre-Ovulatory Follicle Diameter and Follicular Fluid Metabolome Profiles in Lactating Beef Cows. Metabolites 2021, 11, 623. https://doi.org/10.3390/metabo11090623
Read CC, Edwards L, Schrick N, Rhinehart JD, Payton RR, Campagna SR, Castro HF, Klabnik JL, Horn EJ, Moorey SE. Correlation between Pre-Ovulatory Follicle Diameter and Follicular Fluid Metabolome Profiles in Lactating Beef Cows. Metabolites. 2021; 11(9):623. https://doi.org/10.3390/metabo11090623
Chicago/Turabian StyleRead, Casey C., Lannett Edwards, Neal Schrick, Justin D. Rhinehart, Rebecca R. Payton, Shawn R. Campagna, Hector F. Castro, Jessica L. Klabnik, Emma J. Horn, and Sarah E. Moorey. 2021. "Correlation between Pre-Ovulatory Follicle Diameter and Follicular Fluid Metabolome Profiles in Lactating Beef Cows" Metabolites 11, no. 9: 623. https://doi.org/10.3390/metabo11090623
APA StyleRead, C. C., Edwards, L., Schrick, N., Rhinehart, J. D., Payton, R. R., Campagna, S. R., Castro, H. F., Klabnik, J. L., Horn, E. J., & Moorey, S. E. (2021). Correlation between Pre-Ovulatory Follicle Diameter and Follicular Fluid Metabolome Profiles in Lactating Beef Cows. Metabolites, 11(9), 623. https://doi.org/10.3390/metabo11090623







