KLF15 Regulates Oxidative Stress Response in Cardiomyocytes through NAD+
Abstract
:1. Introduction
2. Results
2.1. KLF15 Regulates Myocardial ROS
2.2. KLF15 Depletion Increases the Susceptibility to Oxidative Stress in the Cardiomyocytes, Likely Due to Reduced ROS Clearance
2.3. Tempol Reverses the Cellular Defect of KLF15 Deficiency during Oxidative Stress
2.4. KLF15 Deficiency Resulted in NAD+ Deficiency and Subsequent Hyperacetylation of Mitochondrial Proteins and Reduced Activity of MnSOD
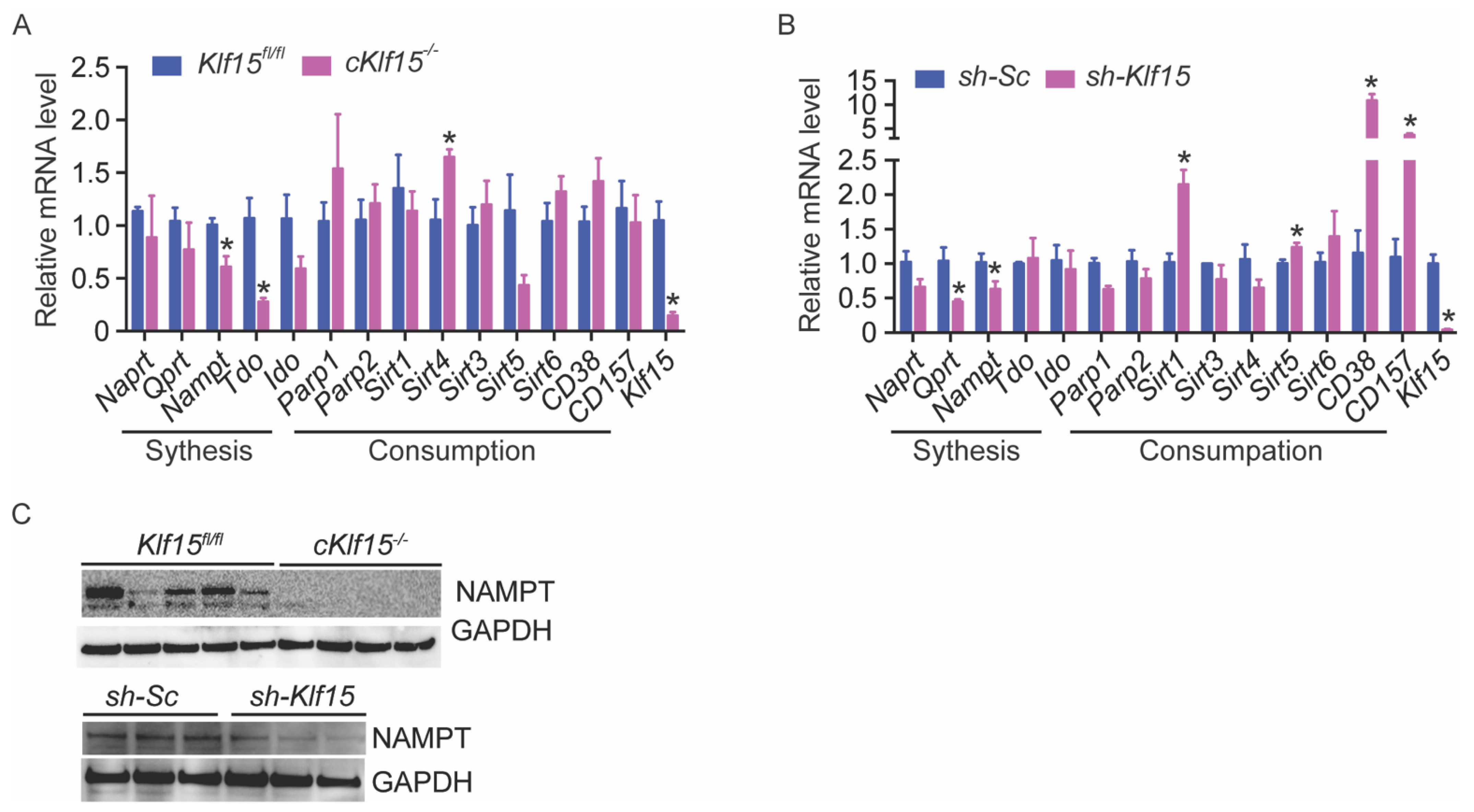
2.5. KLF15 Deficiency Leads to Reduced Nampt
2.6. NMN Rescued the KLF15 Deficiency-Associated Susceptibility to Oxidative Stress
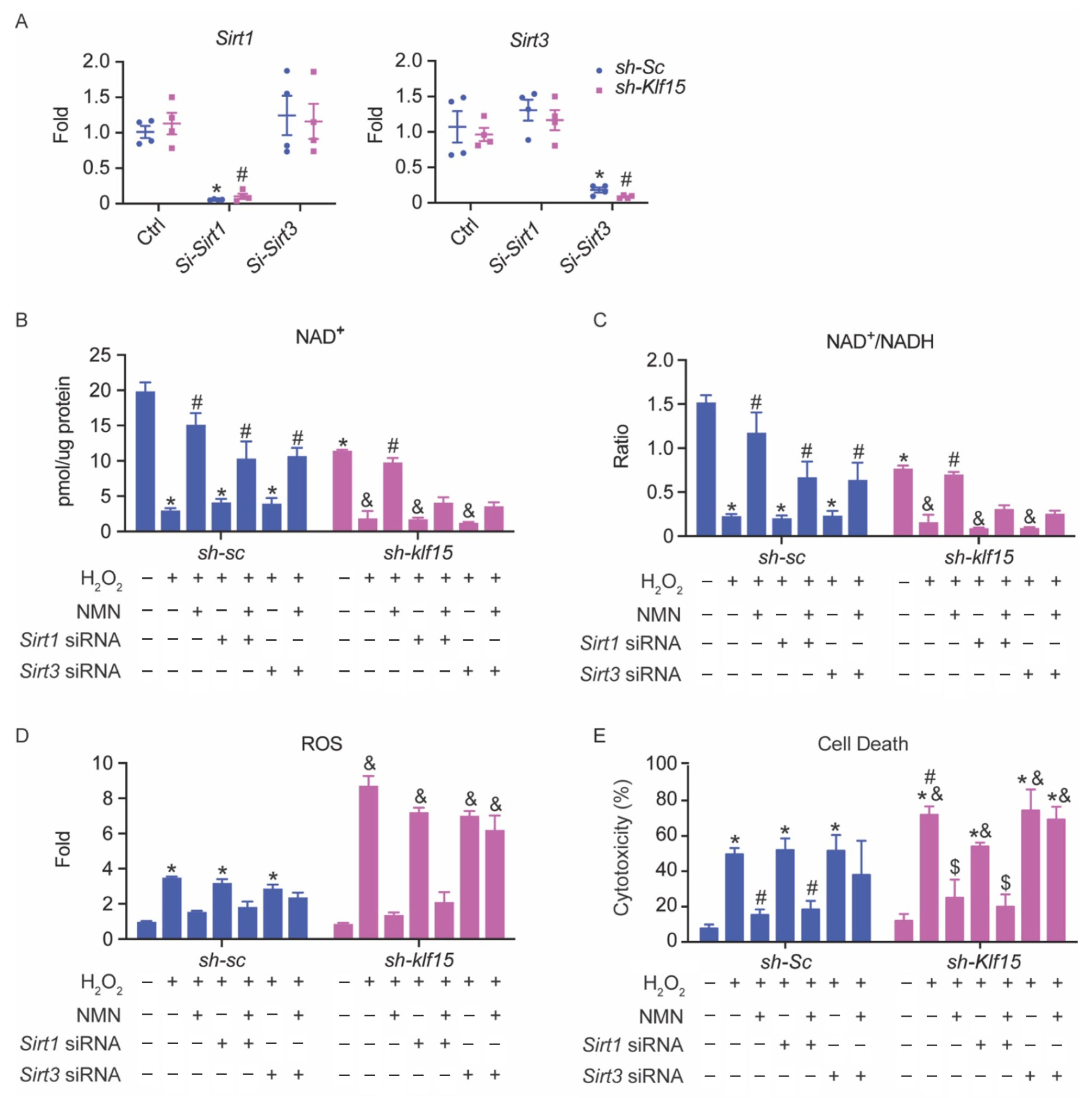
2.7. The Cardioprotective Effect of NMN in Oxidative Stress Is SIRT3-Dependent
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Cell Culture
4.3. Cytotoxicity Assay (LDH Assay)
4.4. Oxygen Consumption Assay (Seahorse Assay)
4.5. Immunoblot and Antibodies
4.6. NAD+ Measurement
4.7. DHE Staining
4.8. Quantitative RT-PCR
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neely, J.; Rovetto, M.; Oram, J. Myocardial utilization of carbohydrate and lipids. Prog. Cardiovasc. Dis. 1972, 15, 289–329. [Google Scholar] [CrossRef]
- Gray, S.; Wang, B.; Orihuela, Y.; Hong, E.-G.; Fisch, S.; Haldar, S.; Cline, G.W.; Kim, J.; Peroni, O.D.; Kahn, B.B.; et al. Regulation of Gluconeogenesis by Krüppel-like Factor 15. Cell Metab. 2007, 5, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haldar, S.M.; Jeyaraj, D.; Anand, P.; Zhu, H.; Lu, Y.; Prosdocimo, D.A.; Eapen, B.; Kawanami, D.; Okutsu, M.; Brotto, L.; et al. Kruppel-like factor 15 regulates skeletal muscle lipid flux and exercise adaptation. Proc. Natl. Acad. Sci. USA 2012, 109, 6739–6744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyaraj, D.; Scheer, F.; Ripperger, J.A.; Haldar, S.M.; Lu, Y.; Prosdocimo, D.A.; Eapen, S.J.; Eapen, B.L.; Cui, Y.; Mahabeleshwar, G.H.; et al. Klf15 Orchestrates Circadian Nitrogen Homeostasis. Cell Metab. 2012, 15, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prosdocimo, D.A.; Anand, P.; Liao, X.; Zhu, H.; Shelkay, S.; Artero-Calderon, P.; Zhang, L.; Kirsh, J.; Moore, D.; Rosca, M.G.; et al. Kruppel-like Factor 15 Is a Critical Regulator of Cardiac Lipid Metabolism. J. Biol. Chem. 2014, 289, 5914–5924. [Google Scholar] [CrossRef] [Green Version]
- Matoba, K.; Lu, Y.; Zhang, R.; Chen, E.; Sangwung, P.; Wang, B.; Prosdocimo, D.A.; Jain, M.K. Adipose KLF15 Controls Lipid Handling to Adapt to Nutrient Availability. Cell Rep. 2017, 21, 3129–3140. [Google Scholar] [CrossRef]
- Sugi, K.; Hsieh, P.N.; Ilkayeva, O.; Shelkay, S.; Moroney, B.; Baadh, P.; Haynes, B.; Pophal, M.; Fan, L.; Newgard, C.B.; et al. Kruppel-like factor 15 is required for the cardiac adaptive response to fasting. PLoS ONE 2018, 13, e0192376. [Google Scholar] [CrossRef] [Green Version]
- Prosdocimo, D.A.; John, J.E.; Zhang, L.; Efraim, E.S.; Zhang, R.; Liao, X.; Jain, M.K. KLF15 and PPARalpha Cooperate to Regulate Cardiomyocyte Lipid Gene Expression and Oxidation. PPAR Res. 2015, 2015, 201625. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Prosdocimo, D.A.; Bai, X.; Fu, C.; Zhang, R.; Campbell, F.; Liao, X.; Coller, J.; Jain, M.K. KLF15 Establishes the Landscape of Diurnal Expression in the Heart. Cell Rep. 2015, 13, 2368–2375. [Google Scholar] [CrossRef] [Green Version]
- Haldar, S.M.; Lu, Y.; Jeyaraj, D.; Kawanami, D.; Cui, Y.; Eapen, S.J.; Hao, C.; Li, Y.; Doughman, Y.-Q.; Watanabe, M.; et al. Klf15 Deficiency Is a Molecular Link Between Heart Failure and Aortic Aneurysm Formation. Sci. Transl. Med. 2010, 2, 26ra26. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, H.; Tien, C.-L.; Jain, M.K.; Zhang, L. Kruppel-Like Factor 15 Regulates the Circadian Susceptibility to Ischemia Reperfusion Injury in the Heart. Circulation 2020, 141, 1427–1429. [Google Scholar] [CrossRef]
- Ansari, H.R.; Raghava, G.P. Identification of NAD interacting residues in proteins. BMC Bioinform. 2010, 11, 160. [Google Scholar] [CrossRef] [Green Version]
- Katsyuba, E.; Romani, M.; Hofer, D.; Auwerx, J. NAD+ homeostasis in health and disease. Nat. Metab. 2020, 2, 9–31. [Google Scholar] [CrossRef]
- Liu, L.; Su, X.; Quinn, W.J.; Hui, S.; Krukenberg, K.; Frederick, D.W.; Redpath, P.; Zhan, L.; Chellappa, K.; White, E.; et al. Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. Cell Metab. 2018, 27, 1067–1080.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, V.; Amici, A.; Mazzola, F.; Di Stefano, M.; Conforti, L.; Magni, G.; Ruggieri, S.; Raffaelli, N.; Orsomando, G. Metabolic Profiling of Alternative NAD Biosynthetic Routes in Mouse Tissues. PLoS ONE 2014, 9, e113939. [Google Scholar] [CrossRef] [Green Version]
- Ralto, K.M.; Rhee, E.P.; Parikh, S.M. NAD+ homeostasis in renal health and disease. Nat. Rev. Nephrol. 2019, 16, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Matasic, D.S.; Brenner, C.; London, B. Emerging potential benefits of modulating NAD+ metabolism in cardiovascular disease. Am. J. Physiol. Circ. Physiol. 2018, 314, H839–H852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hershberger, K.A.; Martin, A.S.; Hirschey, M.D. Role of NAD+ and mitochondrial sirtuins in cardiac and renal diseases. Nat. Rev. Nephrol. 2017, 13, 213–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.I.; Nazarewicz, R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: Potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013, 19, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- McCommis, K.S.; Douglas, D.L.; Krenz, M.; Baines, C.P. Cardiac-specific hexokinase 2 overexpression attenuates hypertrophy by increasing pentose phosphate pathway flux. J. Am. Heart Assoc. 2013, 2, e000355. [Google Scholar] [CrossRef] [PubMed]
- Lombard, D.B.; Alt, F.W.; Cheng, H.-L.; Bunkenborg, J.; Streeper, R.S.; Mostoslavsky, R.; Kim, J.; Yancopoulos, G.; Valenzuela, D.; Murphy, A.; et al. Mammalian Sir2 Homolog SIRT3 Regulates Global Mitochondrial Lysine Acetylation. Mol. Cell. Biol. 2007, 27, 8807–8814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Byun, J.; Zhai, P.; Ikeda, Y.; Oka, S.; Sadoshima, J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE 2014, 9, e98972. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Haldar, S.M.; Lu, Y.; Jeyaraj, D.; Paruchuri, K.; Nahori, M.; Cui, Y.; Kaestner, K.H.; Jain, M.K. Krüppel-like factor 4 regulates pressure-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 2010, 49, 334–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Xu, W.; Zhang, L. KLF15 Regulates Oxidative Stress Response in Cardiomyocytes through NAD+. Metabolites 2021, 11, 620. https://doi.org/10.3390/metabo11090620
Li L, Xu W, Zhang L. KLF15 Regulates Oxidative Stress Response in Cardiomyocytes through NAD+. Metabolites. 2021; 11(9):620. https://doi.org/10.3390/metabo11090620
Chicago/Turabian StyleLi, Le, Weiyi Xu, and Lilei Zhang. 2021. "KLF15 Regulates Oxidative Stress Response in Cardiomyocytes through NAD+" Metabolites 11, no. 9: 620. https://doi.org/10.3390/metabo11090620
APA StyleLi, L., Xu, W., & Zhang, L. (2021). KLF15 Regulates Oxidative Stress Response in Cardiomyocytes through NAD+. Metabolites, 11(9), 620. https://doi.org/10.3390/metabo11090620





