Detecting the Conspecific: Herbivory-Induced Olfactory Cues in the Fall Armyworm (Lepidoptera: Noctuidae)
Abstract
1. Introduction
2. Results
2.1. Two-Choice Oviposition Experiment
2.2. Feeding Trials
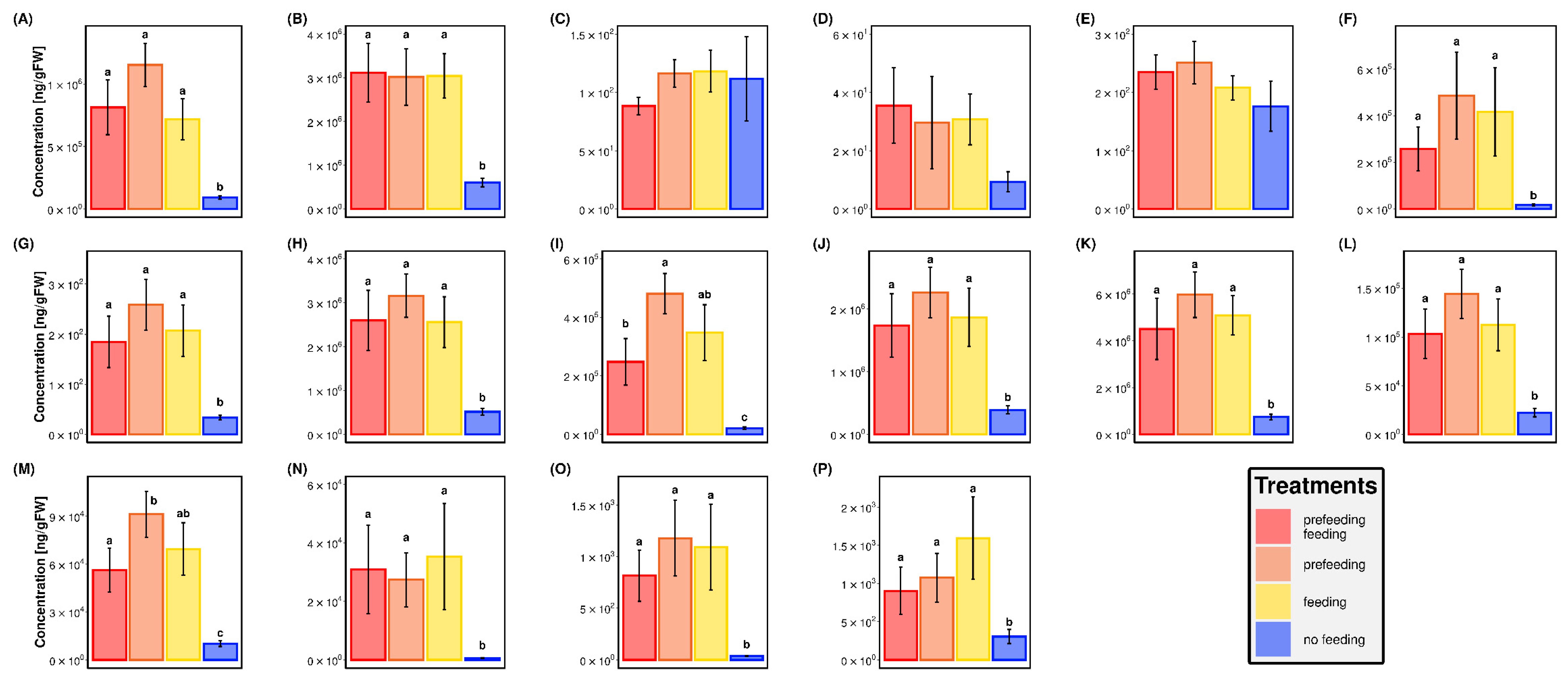
2.3. Targeted Metabolomic Analyses
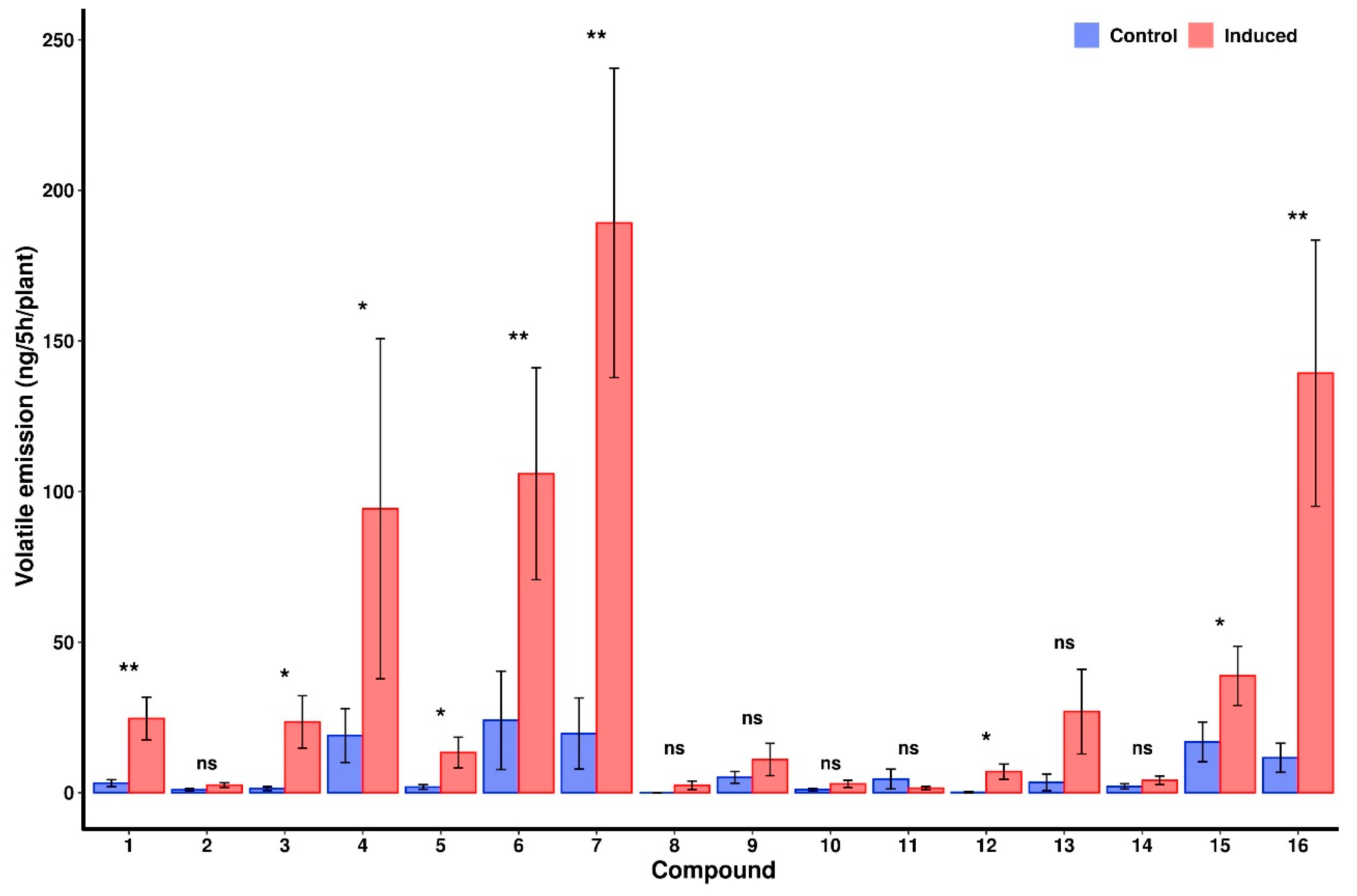
2.4. VOC Analysis
3. Discussion
4. Materials and Methods
4.1. Study Insects and Colony Rearing
4.2. Plants
4.3. Two-Choice Oviposition Experiment
4.4. Feeding Trials
4.5. Targeted Metabolomic Analyses
4.6. Volatile Organic Compound (VOC) Collection and Analysis
4.7. Data Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luginbill, P. The fall armyworm. USDA Tech. Bull. 1928, 34, 1–93. [Google Scholar]
- Sparks, A.N. A Review of the Biology of the Fall Armyworm. Fla. Entomol. 1979, 62, 82. [Google Scholar] [CrossRef]
- Pashley, D.P. Current Status of Fall Armyworm Host Strains. Fla. Entomol. 1988, 71, 227. [Google Scholar] [CrossRef]
- Young, J.R. Fall Armyworm: Control with Insecticides. Fla. Entomol. 1979, 62, 130. [Google Scholar] [CrossRef]
- Johnson, S.J. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the western hemisphere. Int. J. Trop. Insect Sci. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Cruz, L.; Turpin, F.T. Yield impact of larval infestations of the fall armyworm (Lepidoptera: Noctuidae) to midwhorl growth stages of corn. J. Econ. Entomol. 1983, 76, 1052–1054. [Google Scholar] [CrossRef]
- Barros, E.M.; Torres, J.B.; Bueno, J.F. Oviposition, development, and reproduction of Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) fed on different hosts of economic importance. Neotrop. Entomol. 2010, 39, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Murúa, M.G.; Nagoshi, R.N.; Dos Santos, D.A.; Hay-Roe, M.M.; Meagher, R.K.; Vilardi, J.C. Demonstration using field col-lections that Argentina fall armyworm populations exhibit strain-specific host plant preferences. J. Econ. Entomol. 2015, 108, 2305–2315. [Google Scholar] [CrossRef]
- Georgen, G.; Kumar, P.L.; Sankungh, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J. E. Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar]
- Stokstad, E. New crop pest takes Africa at lightning speed. Science 2017, 356, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Sharanabasappa; Kalleshwaraswamy, C.M.; Asokan, R.; Swamy, H.W.; Maruthi, M.S.; Pavithra, H.B.; Hegde, K.; Navi, S.; Prabhu, S.T.; Goergen, G. First report of the fall armyworm, Spodoptera frugiperda (J E Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manag. Hort. Ecosys. 2018, 24, 23–29. [Google Scholar]
- Kalleshwaraswamy, C.M.; Asokan, R.; Mahadevaswamy, H.M.; Sharanabasappa. First record of invasive fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) on rice (Oryza sativa) from India. J. Entomol. Zool. Stud. 2019, 7, 332–337. [Google Scholar]
- Guo, J.; Zhao, J.; He, K.; Zhang, F.; Wang, Z. Potential invasion of the crop-devastating insect pest fall armyworm Spodoptera frugiperda to China. Plant Prot. 2018, 44, 1–10. [Google Scholar]
- Sun, X.-X.; Hu, C.-X.; Jia, H.-R.; Wu, Q.-L.; Shen, X.-J.; Zhao, S.-Y.; Jiang, Y.-Y.; Wu, K.-M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Jing, D.; Guo, J.; Jiang, Y.; Zhao, J.; Sethi, A.; He, K.; Wang, S. Initial detections and spread of invasive Spodoptera frugiperda in Chin and comparisons with other noctuid larvae in cornfield using molecular techniques. Insect Sci. 2020, 27, 780–790. [Google Scholar] [CrossRef]
- ADAWE (Australian Department of Agriculture, Water, and the Environment). Fall Armyworm Detected in Torres Strait. 2020. Available online: https://www.awe.gov.au/news/media-releases/fall-armyworm-detected-torres-strait (accessed on 1 June 2021).
- Yu, S. Insecticide resistance in the fall armyworm, Spodoptera frugiperda (J. E. Smith). Pestic. Biochem. Physiol. 1991, 39, 84–91. [Google Scholar] [CrossRef]
- Ríos-Díez, J.D.; Saldamando-Benjumea, C.I. Susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) strains from central Colombia to two insecticides, methomyl and lambda-cyhalothrin: A study of the genetic basis of resistance. J. Econ. Entomol. 2011, 104, 1698–1705. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Omoto, C.; Field, L.M.; Williamson, M.S.; Bass, C. Investigating the molecular mechanisms of organo-phosphate and pyrethroid resistance in the fall armyworm, Spodoptera frugiperda. PLoS ONE 2013, 8, e62268. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Blanco, C.; Portilla, M.; Adamczyk, J.J.; Lutrell, R.; Huang, F. Evidence of multiple/cross resistance to Bt and or-ganophosphate insecticides in Puerto Rico populations of the fall armyworm, Spodoptera frugiperda. Pestic. Biochem. Physiol. 2014, 122, 15–21. [Google Scholar] [CrossRef]
- Storer, N.P.; Babcock, J.M.; Schlenz, M.; Meade, T.; Thompson, G.T.; Bing, J.W.; Huckaba, R.M. Discovery and characteri-zation of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol. 2010, 103, 1031–1038. [Google Scholar] [CrossRef]
- Signoretti, A.G.C.; Peñaflor, M.F.G.V.; Bento, J.M.S. Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), Female Moths Respond to Herbivore-Induced Corn Volatiles. Neotropical Entomol. 2012, 41, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Rodríguez, P.; Raymond, B.; Morán-Bertot, I.; Rodríguez-Cabrera, L.; Wright, D.J.; Borroto, C.G.; Ayra-Pardo, C. Strong oviposition preference for Bt over non-Bt maize in Spodoptera frugiperda and its implications for the evolution of resistance. BMC Biol. 2014, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Hardke, J.T.; Leonard, B.R.; Huang, F.; Jackson, R. Damage and survivorship of fall armyworm (Lepidoptera: Noctuidae) on transgenic field corn expressing Bacillus thuringiensis Cry proteins. Crop. Prot. 2011, 30, 168–172. [Google Scholar] [CrossRef]
- A Ingber, D.; McDonald, J.H.; E Mason, C.; Flexner, L. Oviposition preferences, Bt susceptibilities, and tissue feeding of fall armyworm (Lepidoptera: Noctuidae) host strains. Pest Manag. Sci. 2021, 77, 4091–4099. [Google Scholar] [CrossRef] [PubMed]
- He, L.-M.; Zhao, S.Y.; Gao, X.-W.; Wu, K.-M. Ovipositional responses of Spodoptera frugiperda on host plants provide a basis for using Bt-transgenic maize as a trap crop in China. J. Integr. Agric. 2021, 20, 804–814. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rodrigues, J.V.C.; Amaya, O.F.S.; Paula-Moraes, S.V.; Pereira, E.J.G. The oviposition behavior of fall armyworm moths is unlikely to compromise the refuge strategy in genetically modified Bt crops. J. Pest. Sci. 2020, 93, 965–977. [Google Scholar] [CrossRef]
- Acevedo, F.E.; Peiffer, M.; Ray, S.; Tan, C.-W.; Felton, G.W. Silicon-mediated enhancement of herbivore resistance in agri-cultural crops. Front. Plant Sci. 2021, 12, 116–133. [Google Scholar] [CrossRef]
- Ray, S.; Alves, P.C.; Ahmad, I.; Gaffoor, I.; Acevedo, F.E.; Peiffer, M.; Jin, S.; Han, Y.; Shakeel, S.; Felton, G.W.; et al. Turnabout Is Fair Play: Herbivory-Induced Plant Chitinases Excreted in Fall Armyworm Frass Suppress Herbivore Defenses in Maize. Plant Physiol. 2016, 171, 694–706. [Google Scholar] [CrossRef]
- Figueroa, R.; Camino, M.; Pérez-Amador, M.C.; Muñoz, V.; Bratoeff, E.; Labastida, C. Fatty acid composition and toxic actiity of the acetonic extract of Carica papaya L. (Caricaceae) seeds. Phyton 2002, 51, 97–99. [Google Scholar]
- Ramos-López, M.A.; González-Chávez, M.M.; Cárdenas-Ortega, N.C.; Zavala-Sánchez, M.A.; Pérez, S. Activity of the main fatty acid components of the hexane leaf extract of Ricinus communis against Spodoptera frugiperda. Afr. J. Biotechnol. 2012, 11, 4274–4278. [Google Scholar] [CrossRef]
- Barakat, A.A.; El-Mahy, S.A.; Moustafa, O.K.; Mansour, A.F.; El-Hadek, M.K. Biological effect of Cassia fistula (L.) seeds against the cotton leafworm Spodoptera littoralis (Boisd.) With special reference to chemical constituents. Bull. Ent. Soc. Egypt. Econ. Ser. 2004, 30, 1–14. [Google Scholar]
- Heba, Y.; El Akwah, S.F.; El Sayed, Y.A. Insecticidal activitiy of linoleic acid against Spodoptera littoralis (Boisd). Egypt J. Agric. 2013, 91, 573–579. [Google Scholar]
- Vatanparast, M.; Ahmed, S.; Lee, D.-H.; Hwang, S.H.; Hammock, B.; Kim, Y. EpOME’s act as immune suppressors in a lepidopteran insect, Spodoptera exigua. Sci. Rep. 2020, 10, 20183. [Google Scholar] [CrossRef] [PubMed]
- Urry, L.A.; Michael, C.L.; Wasserman, S.A.; Alexander, S.; Minorsky, P.V.; Orr, R.B.; Campbell, N.A. Cellular Respiration and Fermentation. Campbell Biology, 12th ed.; Pearson: New York, NY, USA, 2021. [Google Scholar]
- Pastor, V.; Balmer, A.; Gamir, J.; Flors, V.; Mauch-Mani, B. Preparing to fight back: Generation and storage of priming compounds. Front. Plant Sci. 2014, 5, 295. [Google Scholar] [CrossRef]
- Balmer, A.; Pastor, V.; Glauser, G.; Mauch-Mani, B. Tricarboxylates Induce Defense Priming Against Bacteria in Arabidopsis thaliana. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.W.; Pickett, J.A. Wild potato repels aphids by release of aphid alarm pheromone. Nature 1983, 302, 608–609. [Google Scholar] [CrossRef]
- Ray, S.; Helms, A.M.; Matulis, N.L.; Davidson-Lowe, E.; Grisales, W.; Ali, J.G. Asymmetry in Herbivore Effector Responses: Caterpillar Frass Effectors Reduce Performance of a Subsequent Herbivore. J. Chem. Ecol. 2019, 46, 76–83. [Google Scholar] [CrossRef]
- Pinto-Zevallos, D.M.; Strapasson, P.; Zarbin, P.H.G. Herbivore-induced volatile organic compounds emitted by maize: Elec-trophysiological responses in Spodoptera frugiperda females. Phytochem. Lett. 2016, 16, 70–74. [Google Scholar] [CrossRef]
- Gouinguené, S.; Degen, T.; Turlings, T.C.J. Variability in herbivore-induced odour emissions among maize cultivars and their wild ancestors (teosinte). Chemoecology 2001, 11, 9–16. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2143. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; Giri, A.P.; Deuerlein, S.; Griepink, F.; de Kogel, W.J.; Verstappen, F.W.A.; Verhoeven, H.A.; Jongsma, M.; Schwab, W.; Bouwmeester, H. Terpenoid Metabolism in Wild-Type and Transgenic Arabidopsis Plants. Plant Cell 2003, 15, 2866–2884. [Google Scholar] [CrossRef]
- Zhang, M.-X.; Ling, B.; Chen, S.; Liang, G.-W.; Pang, X.-F. Repellent and oviposition deterrent activities of the essential oil from mikania micrantha and its compounds on plutella xylostella. Insect Sci. 2004, 11, 37–45. [Google Scholar] [CrossRef]
- Kostić, M.; Popović, Z.; Brkić, D.; Milanović, S.; Sivčev, I.; Stanković, S. Larvicidal and antifeedant activity of some plant-derived compounds to Lymantria dispar L. (Lepidoptera: Limantriidae). Bioresour. Technol. 2008, 99, 7897–7901. [Google Scholar] [CrossRef] [PubMed]
- McCallum, E.J.; Cunningham, J.P.; Lucker, J.; Zalucki, M.P.; De Voss, J.J.; Botella, J.R. Increased plant volatile production affects oviposition, but not larval development in the moth Helicopverpa armigera. J. Exp. Biol. 2011, 214, 3672–3677. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Stoopen, G.; Thoen, M.; Wiegers, G.; Jongsma, M.A. Chrysanthemum expressing a linalool synthase gene ‘smells good’, but ‘tastes bad’ to western flower thrips. Plant Biotechnol. J. 2013, 11, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.-W.; Liu, F.-H.; Zhang, Z.-F.; Tian, H.-G.; Liu, T.-X. Volatile β-Ocimene Can Regulate Developmental Performance of Peach Aphid Myzus persicae Through Activation of Defense Responses in Chinese Cabbage Brassica pekinensis. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Papanastasiou, S.A.; Ioannou, C.S.; Papadopoulos, N.T. Oviposition-deterrent effect of linalool—A compound of citrus es-sential oils—On female Mediterranean fruit flies, Ceratitis capitata (Diptera: Tephritidae). Pest Manag. Sci. 2020, 76, 3066–3077. [Google Scholar] [CrossRef]
- Binder, B.F.; Robbins, J.C. Effect of Terpenoids and Related Compounds on the Oviposition Behavior of the European Corn Borer, Ostrinia nubilalis(Lepidoptera: Pyralidae). J. Agric. Food Chem. 1997, 45, 980–984. [Google Scholar] [CrossRef]
- Hatano, E.; Saveer, A.M.; Borrero-Echeverry, F.; Strauch, M.; Zakir, A.; Bengtsson, M.; Ignell, R.; Anderson, P.; Becher, P.G.; Witzgall, P.; et al. A herbivore-induced plant volatile interferes with host plant and mate location in moths through suppression of olfactory signalling pathways. BMC Biol. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Dewer, Y.; Li, D.; Qu, C.; Luo, C. Functional and evolutionary characterization of chemosensory proteinCSP2 in the whitefly, Bemisia tabaci. Pest Manag. Sci. 2020, 77, 378–388. [Google Scholar] [CrossRef]
- Markheiser, A.; Rid, M.; Biancu, S.; Gross, J.; Hoffmann, C. Tracking Short-Range Attraction and Oviposition of European Grapevine Moths Affected by Volatile Organic Compounds in a Four-Chamber Olfactometer. Insects 2020, 11, 45. [Google Scholar] [CrossRef]
- Turlings, T.C.; Loughrin, J.; McCall, P.; Rose, U.S.; Lewis, W.J.; Tumlinson, J.H. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 4169–4174. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Lengwiler, U.B.; Bernasconi, M.L.; Wechsler, D. Timing of induced volatile emissions in maize seedlings. Planta 1998, 207, 146–152. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green Leaf Volatiles: A Plant’s Multifunctional Weapon against Herbivores and Pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, C.; López, M.G.; Délano-Frier, J.P. Reduced levels of volatile emissions in jasmoate-deficient spr2 mutants favour oviposition by insect herbivores. Plant Cell Environ. 2006, 26, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Allmann, S.; Späthe, A.; Bisch-Knaden, S.; Kallenbach, M.; Reinecke, A.; Sachse, S.; Baldwin, I.T.; Hansson, B.S. Feed-ing-induced rearrangement of green leaf volatiles reduces moth oviposition. eLife 2013, 2, e00421. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.P.; Johnson, S.J.; Sparks, A.N. Genetic Population Structure of Migratory Moths: The Fall Armyworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1985, 78, 756–762. [Google Scholar] [CrossRef]
- Pashley, D. Host-associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae): A sibling species complex? Ann. Entomol. Soc. Am. 1986, 79, 898–904. [Google Scholar] [CrossRef]
- Adamczyk, J.J.; Holloway, J.W.; Leonard, B.R.; Graves, J.B. Susceptibility of fall armyworm collected from different plant hosts to selected insecticides and transgenic Bt cotton. J. Cotton Sci. 1997, 1, 21–28. [Google Scholar]
- Luttrell, R.G.; Mink, J.S. Damage to cotton fruiting structures by the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Cotton Sci. 1999, 3, 35–44. [Google Scholar]
- Prowell, D.P.; McMichael, M.; Silvain, J.-F. Multilocus Genetic Analysis of Host Use, Introgression, and Speciation in Host Strains of Fall Armyworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 2004, 97, 1034–1044. [Google Scholar] [CrossRef]
- Groot, A.T.; Marr, M.; Heckel, D.; SchÖfl, G. The roles and interactions of reproductive isolation mechanisms in fall armyworm (Lepidoptera: Noctuidae) host strains. Ecol. Entomol. 2010, 35, 105–118. [Google Scholar] [CrossRef]
- Pashley, D.; Sparks, T.C.; Quisenberry, S.W.; Jamjanya, T.; Dowd, P.F. Two fall armyworm strains feed on corn, rice, and Bermuda grass. Louisiana Agric. 1987, 30, 8–9. [Google Scholar]
- A Ingber, D.; E Mason, C.; Flexner, L. Cry1 Bt Susceptibilities of Fall Armyworm (Lepidoptera: Noctuidae) Host Strains. J. Econ. Entomol. 2017, 111, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Virla, E.G.; Álvarez, A.; Loto, F.; Pera, L.M.; Baigori, M.D. Fall Armyworm Strains (Lepidoptera: Noctuidae) in Argentina, Their Associate Host Plants and Response to Different Mortality Factors in Laboratory. Fla. Entomol. 2008, 91, 63–69. [Google Scholar] [CrossRef]
- Ríos-Díez, J.D.; Siegfried, B.; Saldamando-Benjumea, C.I. Susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) Strains from Central Colombia to Cry1Ab and Cry1Ac Entotoxins ofBacillus thuringiensis. Southwest. Entomol. 2012, 37, 281–293. [Google Scholar] [CrossRef]
- Gouin, A.; Bretaudeau, A.; Nam, K.; Gimenez, S.; MarcAury, J.; Duvic, B.; Hilliou, F.; Durand, N.; Montagné, N.; Darboux, I.; et al. Two genomes of highly polyphagous lepidopteran pests (Spodoptera frugiperda, Noctuidae) with different host-plant ranges. Sci. Repor. 2017, 7, 11816. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.E.; Peiffer, M.; Ray, S.; Meagher, R.; Luthe, D.S.; Felton, G.W. Intraspecific differences in plant defense induction by fall armyworm strains. New Phytol. 2018, 218, 310–321. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Silvie, P.; Meagher, R.L. Comparison of haplotype frequencies differentiate fall armyworm (Lepidoptera: Noctuidae) corn-strain populations from Florida and Brazil. J. Econ. Entomol. 2007, 100, 654–961. [Google Scholar] [CrossRef]
- Perkins, W.D. Laboratory Rearing of the Fall Armyworm. Fla. Entomol. 1979, 62, 87. [Google Scholar] [CrossRef]
- Vélez, A.M.; Spencer, T.A.; Alves, A.P.; Moellenbeck, D.; Meagher, R.L.; Chirakkal, H.; Siegfried, B.D. Inheritance of Cry1F resistance, cross-resistance and frequency of resistant alleles in Spodoptera frugiperda (Lepidoptera: Noctuidae). Entomol. Res. 2013, 103, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Engelberth, J.; Tumlinson, J.H.; Block, A.; Alborn, H.T. The use of vapor phase extraction in metabolic pro-filing of phytohormones and other metabolites. Plant J. 2004, 39, 790–808. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Davison, A.C.; Tamò, C. A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol. Entomol. 2004, 29, 45–55. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Brunner, V.; von Mérey, G.; Turlings, T.C.J. Strong attraction of the parasitoid Cotesia marginiventris to-wards minor volatile compounds of maize. J. Chem. Ecol. 2009, 35, 999–1008. [Google Scholar] [CrossRef][Green Version]
- Ramsey, F.L.; Schafer, D.W. The Statistical Sleuth, 2nd ed.; Log-Linear Regression for Poisson Counts; Cengage Learning: Belmont, CA, USA, 2002. [Google Scholar]
- Kergunteuil, A.; Röder, G.; Rasmann, S. Environmental gradients and the evolution of tri-trophic interactions. Ecol. Lett. 2018, 22, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J.; Hiltpold, I.; Köllner, T.G.; Frey, M.; Gierl, A.; Gershenzon, J.; Hibbard, B.E.; Ellersieck, M.R.; Turlings, T.C.J. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc. Natl. Acad. Sci. USA 2009, 106, 13213–13218. [Google Scholar] [CrossRef]

| Parameter | Control a | Induced a | df b | t-Value | p-Value |
|---|---|---|---|---|---|
| Egg Masses c | 0.47 ± 0.15 | 0.09 ± 0.06 | 92 | 2.17 | 0.0325 |
| Hatch Rate | 0.87 ± 0.03 | 0.93 ± 0.01 | 24 | −0.98 | 0.3373 |
| Live Weight d | 8.01 ± 1.17 | 4.68 ± 1.09 | 25 | 1.61 | 0.1201 |
| Dry Weight d | 1.01 ± 0.14 | 0.67 ± 0.15 | 25 | 1.65 | 0.1112 |
| HCW e,f | 1.00 ± 0.06 | 0.77 ± 0.05 | 25 | 3.02 | 0.0057 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingber, D.A.; Christensen, S.A.; Alborn, H.T.; Hiltpold, I. Detecting the Conspecific: Herbivory-Induced Olfactory Cues in the Fall Armyworm (Lepidoptera: Noctuidae). Metabolites 2021, 11, 583. https://doi.org/10.3390/metabo11090583
Ingber DA, Christensen SA, Alborn HT, Hiltpold I. Detecting the Conspecific: Herbivory-Induced Olfactory Cues in the Fall Armyworm (Lepidoptera: Noctuidae). Metabolites. 2021; 11(9):583. https://doi.org/10.3390/metabo11090583
Chicago/Turabian StyleIngber, David A., Shawn A. Christensen, Hans T. Alborn, and Ivan Hiltpold. 2021. "Detecting the Conspecific: Herbivory-Induced Olfactory Cues in the Fall Armyworm (Lepidoptera: Noctuidae)" Metabolites 11, no. 9: 583. https://doi.org/10.3390/metabo11090583
APA StyleIngber, D. A., Christensen, S. A., Alborn, H. T., & Hiltpold, I. (2021). Detecting the Conspecific: Herbivory-Induced Olfactory Cues in the Fall Armyworm (Lepidoptera: Noctuidae). Metabolites, 11(9), 583. https://doi.org/10.3390/metabo11090583







