Enantioselective Quantification of Amphetamine and Metabolites in Serum Samples: Forensic Evaluation and Estimation of Consumption Time
Abstract
1. Introduction
2. Results
2.1. Method Validation
2.2. Forensic Serum Samples
2.3. Cases with Self-Reported Consumption Time
3. Discussion
3.1. Method Validation
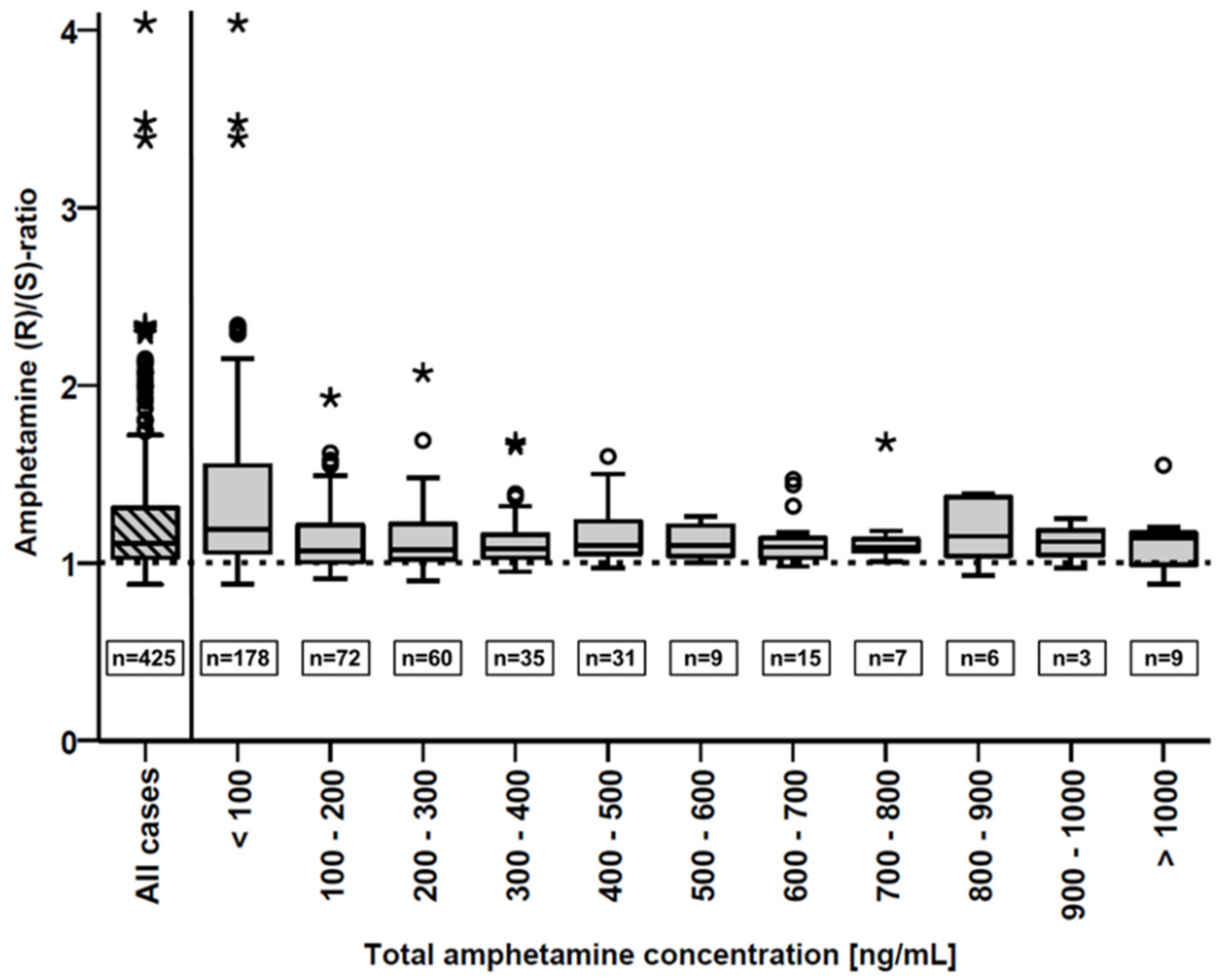
3.2. Forensic Serum Samples
3.3. Cases with Self-Reported Consumption Time
4. Materials and Methods
4.1. Material
4.2. Sample Preparation
4.3. Chiral LC-MS/MS Instrumentation and Analytical Parameters
4.4. Method Validation
4.5. Investigated Collectives
4.5.1. Forensic Serum Samples
4.5.2. Cases with Self-Reported Consumption Time
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Office on Drugs and Crime. World Drug Report 2020; United Nations: San Francisco, CA, USA, 2020. [Google Scholar]
- Musshoff, F. Illegal or legitimate use? Precursor compounds to amphetamine and methamphetamine. Drug Metab. Rev. 2000, 32, 15–44. [Google Scholar] [CrossRef]
- Musshoff, F.; Madea, B. Driving under the influence of amphetamine-like drugs. J. Forensic Sci. 2012, 57, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.; Losacker, M.; Hess, C. Chromatographic separation of R/S-enantiomers of amphetamine and methamphetamine: Pathways of methamphetamine synthesis and detection in blood samples by qualitative enantioselective LC–MS/MS analysis. Forensic Sci. Int. 2018, 291, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Rothman, R.B.; Baumann, M.H.; Dersch, C.M.; Romero, D.V.; Rice, K.C.; Carroll, F.I.; Partilla, J.S. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 2001, 39, 32–41. [Google Scholar] [CrossRef]
- Kuczenski, R.; Segal, D.S. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: Comparison with amphetamine. J. Neurochem. 1997, 68, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M.; Koller, G.; Proebstl, L.; Kamp, F.; Franke, A.; Schmidt, P.; Baumgärtner, G.; Schacht-Jablonowsky, M.; Sievert, A.; Straif, M. Prävalenz und Therapie bei Abhängigkeit von Methamphetamin (“Crystal”) [Prevalence and treatment of methamphetamine (“crystal “) dependence]. Fortschr. der Neurol. Psychiatr. 2017, 85, 92–99. [Google Scholar]
- Kalant, H.; Kalant, O.J. Death in amphetamine users: Causes and rates. Can. Med. Assoc. J. 1975, 112, 299–304. [Google Scholar] [PubMed]
- De Letter, E.A.; Piette, M.H.A.; Lambert, W.E.; Cordonnier, J.A.C.M. Amphetamines as potential inducers of fatalities: A review in the district of Ghent from 1976–2004. Med. Sci. Law 2006, 46, 37–65. [Google Scholar] [CrossRef]
- Verschraagen, M.; Maes, A.; Ruiter, B.; Bosman, I.J.; Smink, B.E.; Lusthof, K.J. Post-mortem cases involving amphetamine-based drugs in The Netherlands. Comparison with driving under the influence cases. Forensic Sci. Int. 2007, 170, 163–170. [Google Scholar] [CrossRef]
- Holze, F.; Vizeli, P.; Müller, F.; Ley, L.; Duerig, R.; Varghese, N.; Eckert, A.; Borgwardt, S.; Liechti, M.E. Distinct acute effects of LSD, MDMA, and D-amphetamine in healthy subjects. Neuropsychopharmacol. 2020, 45, 462–471. [Google Scholar] [CrossRef]
- Holsboer, F.; Gründer, G.; Benkert, O. Handbuch der Psychopharmakotherapie [Handbook of Psychopharmacotherapy]; Springer: Berlin, Germany, 2008. [Google Scholar]
- König, F.; Kaschka, W.P. Interaktionen und Wirkmechanismen Ausgewählter Psychopharmaka [Interactions and Mechanisms of Action of Selected Psychotropic Medications]: 2. Überarbeitete und Erweiterte Auflage; Thieme: New York, NY, USA, 2003. [Google Scholar]
- Hjälmdahl, M.; Vadeby, A.; Forsman, A.; Fors, C.; Ceder, G.; Woxler, P.; Kronstrand, R. Effects of d-amphetamine on simulated driving performance before and after sleep deprivation. Psychopharmacology 2012, 222, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, A.; Neiss, C.; Kauert, G. Die Erschöpfungsreaktion nach Amphetaminkonsum und ihre Auswirkungen auf die Fahrtüchtigkeit [Fatigue symptoms after consumption of amphetamine and effects on driving suitability]. Rechtsmedizin 2000, 10, 86–89. [Google Scholar] [CrossRef]
- Kiloh, L.G.; Brandon, S. Habituation and Addiction to Amphetamines. BMJ 1962, 2, 40–43. [Google Scholar] [CrossRef][Green Version]
- Wu, D.; Otton, S.; Inaba, T.; Kalow, W.; Sellers, E.M. Interactions of amphetamine analogs with human liver CYP2D6. Biochem. Pharmacol. 1997, 53, 1605–1612. [Google Scholar] [CrossRef]
- Kroemer, H.K.; Eichelbaum, M. “It’s the genes, stupid” Molecular bases and clinical consequences of genetic cytochrome P450 2D6 polymorphism. Life Sci. 1995, 56, 2285–2298. [Google Scholar] [CrossRef]
- Varesio, E.; Veuthey, J.-L. Chiral separation of amphetamines by high-performance capillary electrophoresis. J. Chromatogr. 1995, 717, 219–228. [Google Scholar] [CrossRef]
- Iwata, Y.T.; Garcia, A.; Kanamori, T.; Inoue, H.; Kishi, T.; Lurie, I.S. The use of a highly sulfated cyclodextrin for the simultaneous chiral separation of amphetamine-type stimulants by capillary electrophoresis. Electrophoresis 2002, 23, 1328–1334. [Google Scholar] [CrossRef]
- Wang, T.; Shen, B.; Shi, Y.; Xiang, P.; Yu, Z. Chiral separation and determination of R/S-methamphetamine and its metabolite R/S-amphetamine in urine using LC–MS/MS. Forensic Sci. Int. 2015, 246, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Newmeyer, M.N.; Concheiro, M.; Huestis, M.A. Rapid quantitative chiral amphetamines liquid chromatography–tandem mass spectrometry: Method in plasma and oral fluid with a cost-effective chiral derivatizing reagent. J. Chromatogr. 2014, 1358, 68–74. [Google Scholar] [CrossRef]
- Rizzi, A.; Hirz, R.; Cladrowa-Runge, S.; Jonsson, H. Enantiomeric separation of amphetamine, methamphetamine and ring substituted amphetamines by means of a β-cyclodextrin-chiral stationary phase. Chromatographia 1994, 39, 131–137. [Google Scholar] [CrossRef]
- Rasmussen, L.B.; Olsen, K.H.; Johansen, S.S. Chiral separation and quantification of R/S-amphetamine, R/S-methamphetamine, R/S-MDA, R/S-MDMA, and R/S-MDEA in whole blood by GC-EI-MS. J. Chromatogr. 2006, 842, 136–141. [Google Scholar] [CrossRef]
- Strano-Rossi, S.; Botrè, F.; Bermejo, A.M.; Tabernero, M.J. A rapid method for the extraction, enantiomeric separation and quantification of amphetamines in hair. Forensic Sci. Int. 2009, 193, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Davis, J.M. Comparative effects of d-amphetamine, l-amphetamine, and methylphenidate on mood in man. Psychopharmacology 1977, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ellinwood, E.H.; Balster, R.L. Rating the behavioral effects of amphetamine. Eur. J. Pharmacol. 1974, 28, 35–41. [Google Scholar] [CrossRef]
- Caras, S.; Sharpe, T. Pharmacokinetics of AR19, an Immediate-Release Amphetamine Sulfate Formulation Designed to Deter Manipulation for Administration Via Nonoral Routes: Bioequivalence to Reference Racemic Amphetamine Sulfate, Dose Proportionality, and Food Effect. J. Child. Adolesc. Psychopharmacol. 2020, 30, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.H.; Matin, S.B.; Azarnoff, D.L. Kinetics, salivary excretion of amphetamine isomers, and effect of urinary pH. Clin. Pharmacol. Ther. 1978, 23, 585–590. [Google Scholar] [CrossRef]
- Gunne, L.-M.; Galland, L. Stereoselective metabolism of amphetamine. Biochem. Pharmacol. 1967, 16, 1374–1377. [Google Scholar] [CrossRef]
- Caldwell, J. The metabolism of amphetamines in mammals. Drug Metab. Rev. 1976, 5, 219–280. [Google Scholar] [CrossRef]
- Caldwell, J.; Dring, L.G.; Williams, R.T. Norephedrines as metabolites of (14 C) amphetamine in urine in man. Biochem. J. 1972, 129, 23–24. [Google Scholar] [CrossRef]
- Dring, L.G.; Smith, R.L.; Williams, R.T. The metabolic fate of amphetamine in man and other species. Biochem. J. 1970, 116, 425–435. [Google Scholar] [CrossRef]
- Fallon, J.K.; Kicman, A.T.; Henry, J.A.; Milligan, P.J.; Cowan, D.A.; Hutt, A.J. Stereospecific analysis and enantiomeric disposition of 3,4-methylenedioxymethamphetamine (ecstasy) in humans. Clin. Chem. 1999, 45, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.; Losacker, M.; Maas, A. Chromatographic separation of R-(−)/S-(+)-enantiomers of amphetamine and methamphetamine: Differentiation between single methamphetamine consumption and co-consumption with amphetamine using enantioselective quantitative LC-MS/MS analysis. Int. J. Leg. Med. 2019, 133, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Losacker, M.; Toennes, S.W.; Sousa Fernandes Perna, E.B.d.; Ramaekers, J.G.; Roehrich, J.; Hess, C. Chiral Serum Pharmacokinetics of 4-Fluoroamphetamine after Controlled Oral Administration: Can (R)/(S) Concentration Ratios Help in Interpreting Forensic Cases? J. Anal. Toxicol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Holler, J.M.; Vorce, S.P.; Bosy, T.Z.; Jacobs, A. Quantitative and isomeric determination of amphetamine and methamphetamine from urine using a nonprotic elution solvent and R (−)-α-methoxy-α-trifluoromethylphenylacetic acid chloride derivatization. J. Anal. Toxicol 2005, 29, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lin, Z.; Huang, Z.; Liang, H.; Jiang, Y.; Ye, Y.; Wu, Z.; Zhang, R.; Zhang, Y.; Rao, Y. Simple and rapid analysis of four amphetamines in human whole blood and urine using liquid–liquid extraction without evaporation/derivatization and gas chromatography–mass spectrometry. Forensic Toxicol. 2015, 33, 104–111. [Google Scholar] [CrossRef]
- Peters, F.T.; Kraemer, T.; Maurer, H.H. Drug Testing in Blood: Validated Negative-Ion Chemical Ionization Gas Chromatographic–Mass Spectrometric Assay for Determination of Amphetamine and Methamphetamine Enantiomers and Its Application to Toxicology Cases. Clin. Chem. 2002, 48, 1472–1485. [Google Scholar] [CrossRef]
- Slawson, M.H.; Taccogno, J.L.; Foltz, R.L.; Moody, D.E. Quantitative analysis of selegiline and three metabolites (N-desmethylselegiline, methamphetamine, and amphetamine) in human plasma by high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J. Anal. Toxicol. 2002, 26, 430–437. [Google Scholar] [CrossRef][Green Version]
- Leis, H.-J.; Rechberger, G.N.; Fauler, G.; Windischhofer, W. Enantioselective trace analysis of amphetamine in human plasma by gas chromatography/negative ion chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Peters, F.T.; Kraemer, T.; Maurer, H.H. Detection and validated quantification of nine herbal phenalkylamines and methcathinone in human blood plasma by LC-MS/MS with electrospray ionization. J. Mass Spectrom. 2007, 42, 150–160. [Google Scholar] [CrossRef]
- Losacker, M.; Zoerntlein, S.; Schwarze, B.; Staudt, S.; Roehrich, J.; Hess, C. Determination of the enantiomeric composition of amphetamine, methamphetamine and 3,4-methylendioxy-N-methylamphetamine (MDMA) in seized street drug samples from southern Germany. Drug Test. Anal. 2021. [Google Scholar] [CrossRef]
- United Nations: International Narcotics Control Board. Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances 2019; United Nations: San Francisco, CA, USA, 2019. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. Amphetamine Drug Profile. Available online: https://www.emcdda.europa.eu/publications/drug-profiles/amphetamine_en (accessed on 8 June 2021).
- Havnen, H.; Hansen, M.; Spigset, O.; Hegstad, S. Enantiomeric separation and quantification of R/S-amphetamine in serum using semi-automated liquid-liquid extraction and ultra-high performance supercritical fluid chromatography-tandem mass spectrometry. Drug Test. Anal. 2020, 12, 1344–1353. [Google Scholar] [CrossRef]
- Musshoff, F.; Skopp, G.; Madea, B.; Maas, A. Amphetamine und das Medikamentenprivileg im Straßenverkehr [Amphetamines and the medication privilege in driving]. Blutalkohol 2020, 58, 260–269. [Google Scholar]
- Cody, J.T.; Valtier, S.; Nelson, S.L. Amphetamine enantiomer excretion profile following administration of Adderall. J. Anal. Toxicol. 2003, 27, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Segal, D.S.; Kuczenski, R. An Escalating Dose “Binge” Model of Amphetamine Psychosis: Behavioral and Neurochemical Characteristics. J. Neurosci. 1997, 17, 2551–2566. [Google Scholar] [CrossRef]
- Verstraete, A.G. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther. Drug Monit. 2004, 26, 200–205. [Google Scholar] [CrossRef]
- Peters, F.T.; Samyn, N.; Wahl, M.; Kraemer, T.; De Boeck, G.; Maurer, H.H. Concentrations and Ratios of Amphetamine, Methamphetamine, MDA, MDMA, and MDEA Enantiomers Determined in Plasma Samples from Clinical Toxicology and Driving Under the Influence of Drugs Cases by GC-NICI-MS*. J. Anal. Toxicol. 2003, 27, 552–559. [Google Scholar] [CrossRef]
- Steuer, A.E.; Schmidhauser, C.; Schmid, Y.; Rickli, A.; Liechti, M.E.; Kraemer, T. Chiral plasma pharmacokinetics of 3, 4-methylenedioxymethamphetamine and its phase I and II metabolites following controlled administration to humans. Drug Metab. Dispos. 2015, 43, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Baggot, J.D.; Davis, L.E. A Comparative Study of the Pharmacokinetics of Amphetamine. Res. Vet. Sci. 1973, 14, 207–215. [Google Scholar] [CrossRef]
- Stolk, J.M.; Rech, R.H. Enhanced stimulant effects of d-amphetamine on the spontaneous locomotor activity of rats treated with reserpine. J. Pharmacol. Exp. Ther. 1967, 158, 140–149. [Google Scholar] [PubMed]
- Meyer, M.R.; Peters, F.T.; Maurer, H.H. The role of human hepatic cytochrome P450 isozymes in the metabolism of racemic 3, 4-methylenedioxy-methamphetamine and its enantiomers. Drug Metab. Dispos. 2008, 36, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Law, M.Y.; Slawson, M.H.; Moody, D.E. Selective involvement of cytochrome P450 2D subfamily in in vivo 4-hydroxylation of amphetamine in rat. Drug Metab. Dispos. 2000, 28, 348–353. [Google Scholar] [PubMed]
- Weiß, J.A.; Kadkhodaei, K.; Schmid, M.G. Indirect chiral separation of 8 novel amphetamine derivatives as potential new psychoactive compounds by GC-MS and HPLC. Sci. Justice 2017, 57, 6–12. [Google Scholar] [CrossRef]
- Cody, J.T.; Schwarzhoff, R. Interpretation of methamphetamine and amphetamine enantiomer data. J. Anal. Toxicol. 1993, 17, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.T.; Drummer, O.H.; Musshoff, F. Validation of new methods. Forensic Sci. Int. 2007, 165, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
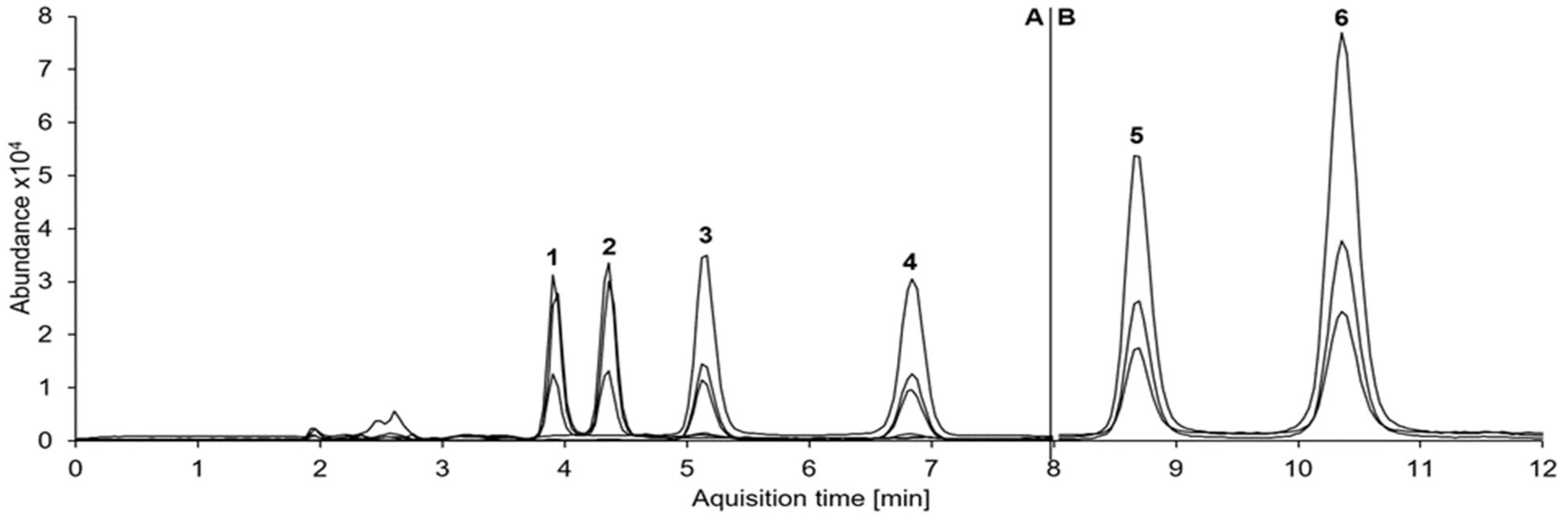




| Concentration | Analyte | Accuracy (Bias) n = 6 | Interday Precision n = 6 | Recovery n = 6 | Matrix Effects n = 6 |
|---|---|---|---|---|---|
| (ng/mL) | (%) | (%) | (%) | (%) | |
| 20 | (R)-AM | −1.7 | 0.4 | 28.1 ± 3.2 | 86.5 ± 6.5 |
| 20 | (S)-AM | 4.5 | 4.3 | 28.4 ± 3.9 | 87.3 ± 6.7 |
| 125 | (R)-AM | 3.3 | 2.7 | 30.3 ± 3.3 | 106 ± 7.3 |
| 125 | (S)-AM | 7.3 | 2.5 | 32.3 ± 3.6 | 105 ± 7.7 |
| 20 | (1S,2R)-NE | 3.4 | 4.6 | 10.3 ± 1.2 | 99.4 ± 8.4 |
| 20 | (1R,2S)-NE | −1.2 | 1.1 | 10.7 ± 1.3 | 97.8 ± 8.9 |
| 125 | (1S,2R)-NE | 5.2 | 6.0 | 13.4 ± 1.6 | 112.2 ± 9.3 |
| 125 | (1R,2S)-NE | −0.5 | 8.4 | 13.5 ± 1.7 | 112.5 ± 8.4 |
| 20 | (R)-4OH-AM | 1.8 | 12.8 | 10.0 ± 0.9 | 78.7 ± 10.3 |
| 20 | (S)-4OH-AM | −0.3 | 17.6 | 10.4 ± 1.2 | 98.4 ± 7.7 |
| 125 | (R)-4OH-AM | 0.2 | 9.7 | 14.5 ± 3.4 | 85.5 ± 4.9 |
| 125 | (S)-4OH-AM | 1.4 | 13.7 | 14.6 ± 1.8 | 114.8 ± 6.7 |
| Case | Binge | Δt | (R)-AM | (S)-AM | Σ AM | (R)/(S) | (1R,2S)-NE |
|---|---|---|---|---|---|---|---|
| (ng/mL) | (h) | (ng/mL) | (ng/mL) | (ng/mL) | (ng/mL) | ||
| 1 | 12.0 | 12.3 | 13.9 | 26.3 | 0.88 | ||
| 2 | 5.5 | 32.4 | 35.2 | 67.6 | 0.92 | ||
| 3 | * | 0.8 | 114 | 124 | 238 | 0.93 | 0.6 |
| 4 | * | 20.5 | 98.3 | 102 | 200 | 0.97 | |
| 5 | * | 7.0 | 128 | 125 | 253 | 1.02 | 1.2 |
| 6 | 7.0 | 25.5 | 24.7 | 50.2 | 1.03 | ||
| 7 | 22.0 | 41.9 | 40.1 | 81.9 | 1.04 | ||
| 8 | * | 4.0 | 112 | 107 | 219 | 1.05 | 0.6 |
| 9 | 11.5 | 42.1 | 39.3 | 81.4 | 1.07 | ||
| 10 | * | 6.3 | 111 | 103 | 214 | 1.08 | 1.4 |
| 11 | * | 3.0 | 239 | 219 | 457 | 1.09 | 7.0 |
| 12 | 3.0 | 40.9 | 37.4 | 78.3 | 1.09 | 1.4 | |
| 13 | 34.2 | 18.0 | 16.0 | 24.0 | 1.13 | ||
| 14 | 13.5 | 261 | 226 | 487 | 1.15 | 1.8 | |
| 15 | 18.0 | 100 | 82.4 | 182 | 1.21 | 1.4 | |
| 16 | * | 33.0 | 8.9 | 7.2 | 16.1 | 1.23 | |
| 17 | 28.8 | 8.7 | 7.1 | 15.8 | 1.23 | ||
| 18 | * | 25.0 | 28.5 | 23.1 | 51.5 | 1.23 | 0.9 |
| 19 | 18.5 | 34.3 | 27.2 | 61.6 | 1.23 | 1.0 | |
| 20 | 20.0 | 105 | 82.4 | 188 | 1.28 | ||
| 21 | * | 3.0 | 201 | 157 | 358 | 1.28 | |
| 22 | * | 31.0 | 18.6 | 14.5 | 33.1 | 1.29 | |
| 23 | * | 7.3 | 239 | 181 | 420 | 1.32 | |
| 24 | 17.5 | 114 | 85.1 | 199 | 1.34 | 0.7 | |
| 25 | 38.5 | 43.8 | 31.5 | 75.3 | 1.39 | 0.9 | |
| 26 | 40.0 | 6.2 | 3.6 | 9.8 | 1.72 | ||
| 27 | * | 47.5 | 14.2 | 7.3 | 21.5 | 1.93 | |
| 28 | 42.0 | 1.2 | 0.6 | 1.8 | 1.96 | ||
| 29 | 40.5 | 115 | 53.8 | 169 | 2.14 | ||
| 30 | 77.5 | 10.3 | 3.6 | 13.9 | 2.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losacker, M.; Kraemer, M.; Philipsen, A.; Duecker, K.; Dreimueller, N.; Engelmann, J.; Roehrich, J.; Hess, C. Enantioselective Quantification of Amphetamine and Metabolites in Serum Samples: Forensic Evaluation and Estimation of Consumption Time. Metabolites 2021, 11, 521. https://doi.org/10.3390/metabo11080521
Losacker M, Kraemer M, Philipsen A, Duecker K, Dreimueller N, Engelmann J, Roehrich J, Hess C. Enantioselective Quantification of Amphetamine and Metabolites in Serum Samples: Forensic Evaluation and Estimation of Consumption Time. Metabolites. 2021; 11(8):521. https://doi.org/10.3390/metabo11080521
Chicago/Turabian StyleLosacker, Moritz, Michael Kraemer, Alexandra Philipsen, Kristina Duecker, Nadine Dreimueller, Jan Engelmann, Joerg Roehrich, and Cornelius Hess. 2021. "Enantioselective Quantification of Amphetamine and Metabolites in Serum Samples: Forensic Evaluation and Estimation of Consumption Time" Metabolites 11, no. 8: 521. https://doi.org/10.3390/metabo11080521
APA StyleLosacker, M., Kraemer, M., Philipsen, A., Duecker, K., Dreimueller, N., Engelmann, J., Roehrich, J., & Hess, C. (2021). Enantioselective Quantification of Amphetamine and Metabolites in Serum Samples: Forensic Evaluation and Estimation of Consumption Time. Metabolites, 11(8), 521. https://doi.org/10.3390/metabo11080521






