Chronic Kidney Disease Cohort Studies: A Guide to Metabolome Analyses
Abstract
1. Introduction
2. How to Get Started: A Priori Considerations for Metabolomics Cohort Studies
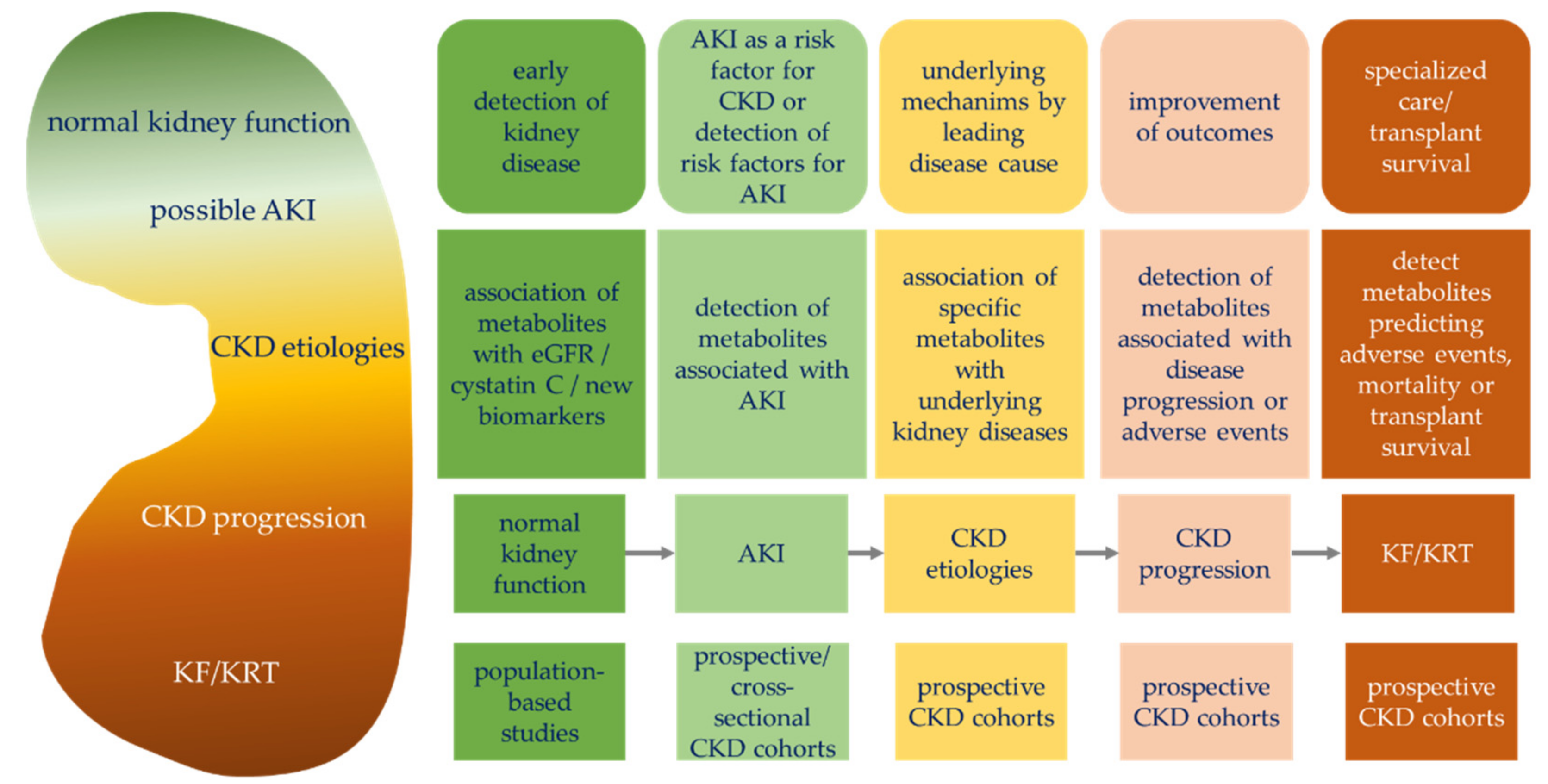
2.1. Possible Study Questions for Cohort Studies
2.2. Common Study Designs in Human Cohorts
2.2.1. Case Reports and Case Series
2.2.2. Cross-Sectional Study
2.2.3. Case–Control Study
2.2.4. Prospective Cohort Study
2.2.5. Randomized Controlled Trial
2.3. Important Considerations for Sample Collection in Metabolomics Studies
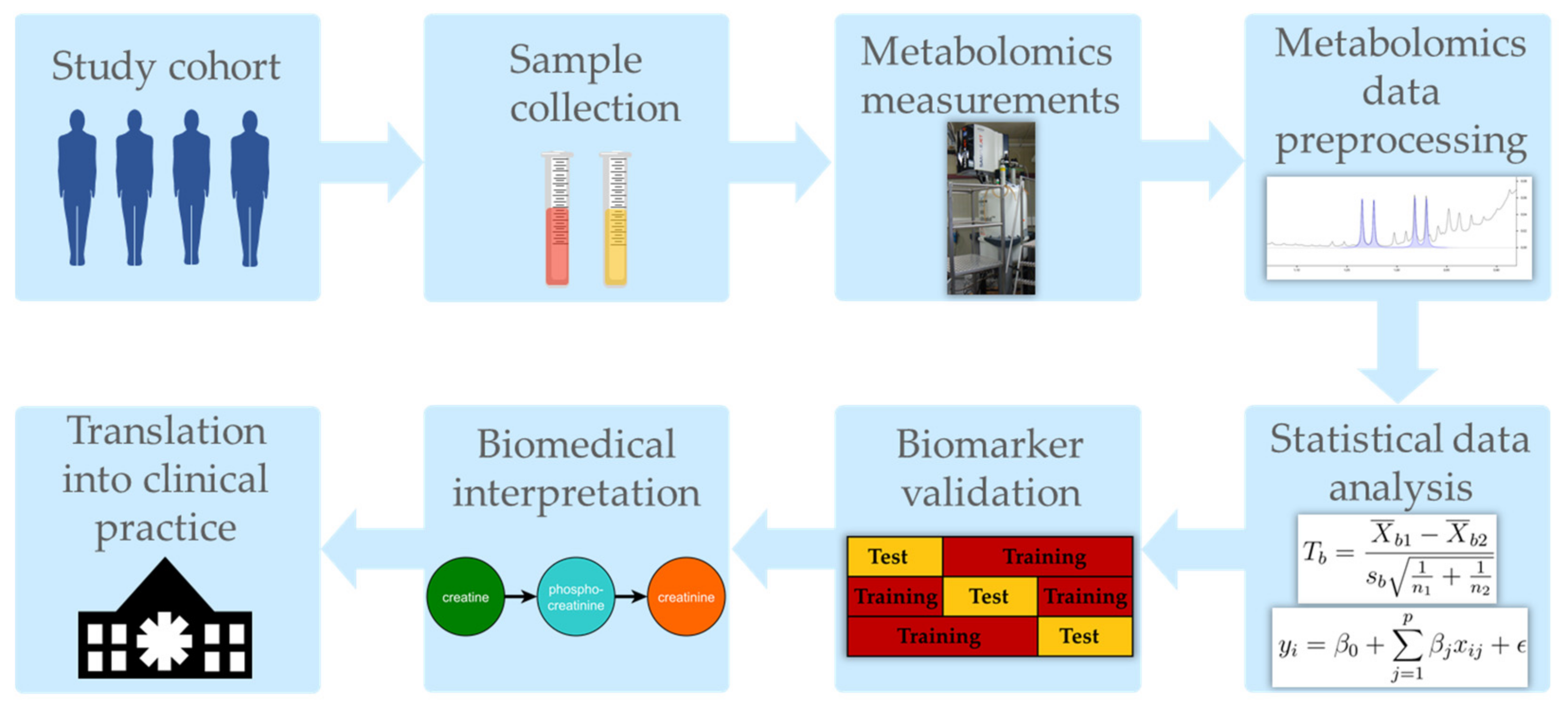
3. Metabolomics Data Acquisition
3.1. Common Analytical Platforms in Metabolomics Studies
3.2. Sample Preparation, Measurements, and Preprocessing in Metabolomics Studies
4. Statistics and Bioinformatics Data Analysis
- •
- Hypothesis testing: Univariate statistical differentiation between two or more predefined groups.
- •
- Multivariate biomarker signature detection: Generation of multivariate regression scores to predict an outcome of an unknown test sample.
- •
- Subgroup identification: Exploratory approach to identify biomedically different patient/sample subgroups.
- •
- Metabolome-wide association study: Systematic analysis of the entire measured metabolome based on regression, including appropriate confounder adjustment to identify significant associations between metabolites and an outcome. A correction for multiple testing is essential for these comparisons.
- •
- Statistical network analysis: Systematic analysis of interactions between different metabolites and/or patient parameters, other omics variables, etc., which are represented as a network. Allows a holistic view on the metabolome and its interaction with specific phenotypes, and can reveal molecular mechanisms or regulating processes.
- •
- Meta-analysis: Combination of statistical results across multiple studies to increase statistical power and to gain more robust results.
- •
- Time-to-event analysis: Time-to-event data contain information about if and when an event occurred, but typically also censored data. Survival analysis appropriately associates time-to-event data with, e.g., metabolite levels.
- •
- Time-course analysis: Analysis of metabolite concentration changes across time and typically in response to external stimuli.
- •
5. Validation, Interpretation, and Beyond
6. Conclusions
Funding
Conflicts of Interest
References
- Eckardt, K.-U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Köttgen, A.; Levey, A.S.; Levin, A. Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 2013, 382, 158–169. [Google Scholar] [CrossRef]
- Levin, A.; Lancashire, W.; Fassett, R.G. Targets, trends, excesses, and deficiencies: Refocusing clinical investigation to improve patient outcomes. Kidney Int. 2013, 83, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, M.; Li, Y.; Li, M.; Sieber, K.B.; Feitosa, M.F.; Gorski, M.; Tin, A.; Wang, L.; Chu, A.Y.; et al.; Lifelines Cohort Study A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat. Genet. 2019, 51, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.C.; Zhang, W.; Lord, G.; Van Der Harst, P.; Lawlor, D.A.; Sehmi, J.S.; Gale, D.; Wass, M.; Ahmadi, K.R.; Bakker, S.J.L.; et al. Genetic loci influencing kidney function and chronic kidney disease. Nat. Genet. 2010, 42, 373–375. [Google Scholar] [CrossRef]
- Wilson, P.C.; Ledru, N.; Humphreys, B.D. Epigenomics and the kidney. Curr. Opin. Nephrol. Hypertens. 2020, 29, 280–285. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, Z.; D’Agati, V.D.; Sun, Z.; Zhong, F.; Zhang, W.; Wen, J.; Zhou, T.; Li, Z.; He, L.; et al. Comparison of Kidney Transcriptomic Profiles of Early and Advanced Diabetic Nephropathy Reveals Potential New Mechanisms for Disease Progression. Diabetes 2019, 68, 2301–2314. [Google Scholar] [CrossRef] [PubMed]
- Assmann, N.; Dettmer, K.; Simbuerger, J.M.B.; Broeker, C.; Nuernberger, N.; Renner, K.; Courtneidge, H.; Klootwijk, E.D.; Duerkop, A.; Hallet, A.; et al. Renal Fanconi Syndrome Is Caused by a Mistargeting-Based Mitochondriopathy. Cell Rep. 2016, 15, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, H.U.; Altenbuchinger, M.; Gronwald, W. Statistical Analysis of NMR Metabolic Fingerprints: Established Methods and Recent Advances. Metabolites 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Tofte, N.; Vogelzangs, N.; Mook-Kanamori, D.; Brahimaj, A.; Nano, J.; Ahmadizar, F.; Van Dijk, K.W.; Frimodt-Møller, M.; Arts, I.; Beulens, J.W.J.; et al. Plasma Metabolomics Identifies Markers of Impaired Renal Function: A Meta-analysis of 3089 Persons with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Z.; Liu, J.; Morningstar, J.; Heckman-Stoddard, B.; Lee, C.G.; Dagogo-Jack, S.; Ferguson, J.F.; Hamman, R.F.; Knowler, W.C.; Mather, K.J.; et al. Metabolite Profiles of Incident Diabetes and Heterogeneity of Treatment Effect in the Diabetes Prevention Program. Diabetes 2019, 68, 2337–2349. [Google Scholar] [CrossRef]
- Paynter, N.P.; Balasubramanian, R.; Giulianini, F.; Wang, D.D.; Tinker, L.F.; Gopal, S.; Deik, A.A.; Bullock, K.; Pierce, K.A.; Scott, J.; et al. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation 2018, 137, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Pinto, J.; Amaro, F.; Bastos, M.; Carvalho, M.; de Pinho, P.G. Advances and Perspectives in Prostate Cancer Biomarker Discovery in the Last 5 Years through Tissue and Urine Metabolomics. Metabolites 2021, 11, 181. [Google Scholar] [CrossRef]
- Grams, M.E.; Shafi, T.; Rhee, E.P. Metabolomics Research in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 1588–1590. [Google Scholar] [CrossRef]
- Rhee, E.; Clish, C.; Wenger, J.; Roy, J.; Elmariah, S.; Pierce, K.; Bullock, K.; Anderson, A.; Gerszten, R.; Feldman, H. Metabolomics of Chronic Kidney Disease Progression: A Case-Control Analysis in the Chronic Renal Insufficiency Cohort Study. Am. J. Nephrol. 2016, 43, 366–374. [Google Scholar] [CrossRef]
- Goek, O.-N.; Döring, A.; Gieger, C.; Heier, M.; Koenig, W.; Prehn, C.; Römisch-Margl, W.; Wang-Sattler, R.; Illig, T.; Suhre, K.; et al. Serum Metabolite Concentrations and Decreased GFR in the General Population. Am. J. Kidney Dis. 2012, 60, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zheng, Y.; Nettleton, J.A.; Alexander, D.; Coresh, J.; Boerwinkle, E. Serum Metabolomic Profiling and Incident CKD among African Americans. Clin. J. Am. Soc. Nephrol. 2014, 9, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, H.U.; Altenbuchinger, M.; Schultheiss, U.T.; Samol, C.; Kotsis, F.; Poguntke, I.; Sekula, P.; Krumsiek, J.; Köttgen, A.; Spang, R.; et al. A Novel Metabolic Signature to Predict the Requirement of Dialysis or Renal Transplantation in Patients with Chronic Kidney Disease. J. Proteome Res. 2019, 18, 1796–1805. [Google Scholar] [CrossRef]
- Zacharias, H.U.; Schley, G.; Hochrein, J.; Klein, M.S.; Köberle, C.; Eckardt, K.-U.; Willam, C.; Oefner, P.J.; Gronwald, W. Analysis of human urine reveals metabolic changes related to the development of acute kidney injury following cardiac surgery. Metabolomics 2013, 9, 697–707. [Google Scholar] [CrossRef]
- Zacharias, H.U.; Hochrein, J.; Vogl, F.C.; Schley, G.; Mayer, F.; Jeleazcov, C.; Eckardt, K.-U.; Willam, C.; Oefner, P.J.; Gronwald, W. Identification of Plasma Metabolites Prognostic of Acute Kidney Injury after Cardiac Surgery with Cardiopulmonary Bypass. J. Proteome Res. 2015, 14, 2897–2905. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shannon, M.; Ando, Y.; Schnackenberg, L.K.; Khan, N.A.; Portilla, D.; Beger, R.D. Serum metabolomic profiles from patients with acute kidney injury: A pilot study. J. Chromatogr. B 2012, 893-894, 107–113. [Google Scholar] [CrossRef]
- Gronwald, W.; Klein, M.S.; Zeltner, R.; Schulze, B.-D.; Reinhold, S.W.; Deutschmann, M.; Immervoll, A.-K.; Böger, C.A.; Banas, B.; Eckardt, K.-U.; et al. Detection of autosomal dominant polycystic kidney disease by NMR spectroscopic fingerprinting of urine. Kidney Int. 2011, 79, 1244–1253. [Google Scholar] [CrossRef]
- Blydt-Hansen, T.D.; Sharma, A.; Gibson, I.; Mandal, R.; Wishart, D.S. Urinary Metabolomics for Noninvasive Detection of Borderline and Acute T Cell-Mediated Rejection in Children After Kidney Transplantation. Am. J. Transplant. 2014, 14, 2339–2349. [Google Scholar] [CrossRef]
- Hallan, S.; Afkarian, M.; Zelnick, L.; Kestenbaum, B.; Sharma, S.; Saito, R.; Darshi, M.; Barding, G.; Raftery, D.; Ju, W.; et al. Metabolomics and Gene Expression Analysis Reveal Down-regulation of the Citric Acid (TCA) Cycle in Non-diabetic CKD Patients. EBioMedicine 2017, 26, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kalim, S.; Clish, C.B.; Wenger, J.; Elmariah, S.; Yeh, R.W.; Deferio, J.J.; Pierce, K.; Deik, A.; Gerszten, R.E.; Thadhani, R.; et al. A Plasma Long-Chain Acylcarnitine Predicts Cardiovascular Mortality in Incident Dialysis Patients. J. Am. Hear. Assoc. 2013, 2, e000542. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.O.; Townsend, R.R.; Feldman, H.I.; Pappan, K.L.; Kensicki, E.; Jagt, D.L.V. Plasma Metabolomic Profiles in Different Stages of CKD. Clin. J. Am. Soc. Nephrol. 2012, 8, 363–370. [Google Scholar] [CrossRef]
- Coresh, J.; Inker, L.A.; Sang, Y.; Chen, J.; Shafi, T.; Post, W.S.; Shlipak, M.G.; Ford, L.; Goodman, K.; Perichon, R.; et al. Metabolomic profiling to improve glomerular filtration rate estimation: A proof-of-concept study. Nephrol. Dial. Transplant. 2019, 34, 825–833. [Google Scholar] [CrossRef]
- Chen, D.Q.; Cao, G.; Zhao, Y.-Y. Identification of serum metabolites associating with chronic kidney disease progression and anti-fibrotic effect of 5-methoxytryptophan. Nat. Commun. 2019, 10, 1–15. [Google Scholar]
- Sekula, P.; Tin, A.; Schultheiss, U.T.; Baid-Agrawal, S.; Mohney, R.P.; Steinbrenner, I.; Yu, B.; Luo, S.; Boerwinkle, E.; Eckardt, K.-U.; et al. Urine 6-Bromotryptophan: Associations with Genetic Variants and Incident End-Stage Kidney Disease. Sci. Rep. 2020, 10, 10018. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, I.; Schultheiss, U.T.; Kotsis, F.; Schlosser, P.; Stockmann, H.; Mohney, R.P.; Schmid, M.; Oefner, P.J.; Eckardt, K.-U.; Köttgen, A.; et al. Urine Metabolite Levels, Adverse Kidney Outcomes, and Mortality in CKD Patients: A Metabolome-wide Association Study. Am. J. Kidney Dis. 2021. [Google Scholar] [CrossRef]
- Altenbuchinger, M.; Zacharias, H.U.; Solbrig, S.; Schäfer, A.; Büyüközkan, M.; Schultheiß, U.T.; Kotsis, F.; Köttgen, A.; Spang, R.; Oefner, P.J.; et al. A multi-source data integration approach reveals novel associations between metabolites and renal outcomes in the German Chronic Kidney Disease study. Sci. Rep. 2019, 9, 13954. [Google Scholar] [CrossRef]
- Schlosser, P.; Li, Y.; Sekula, P.; Raffler, J.; Grundner-Culemann, F.; Pietzner, M.; Cheng, Y.; Wuttke, M.; Steinbrenner, I.; Schultheiss, U.T.; et al. Genetic studies of urinary metabolites illuminate mechanisms of detoxification and excretion in humans. Nat. Genet. 2020, 52, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, V.-P.; Tynkkynen, T.; Soininen, P.; Peltola, T.; Kangas, A.J.; Forsblom, C.; Thorn, L.M.; Kaski, K.; Laatikainen, R.; Ala-Korpela, M.; et al. Metabolic Diversity of Progressive Kidney Disease in 325 Patients with Type 1 Diabetes (the FinnDiane Study). J. Proteome Res. 2012, 11, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Suhre, K.; Schwartz, J.E.; Sharma, V.K.; Chen, Q.; Lee, J.R.; Muthukumar, T.; Dadhania, D.M.; Ding, R.; Ikle, D.N.; Bridges, N.D.; et al. Urine metabolite profiles predictive of human kidney allograft status. J. Am. Soc. Nephrol. 2016, 27, 626–636. [Google Scholar] [CrossRef]
- Goek, O.N.; Prehn, C.; Sekula, P.; Römisch-Margl, W.; Döring, A.; Gieger, C.; Heier, M.; Koenig, W.; Wang-Sattler, R.; Illig, T. Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol. Dial. Transplant. 2013, 28, 2131–2138. [Google Scholar] [CrossRef]
- Sekula, P.; Goek, O.-N.; Quaye, L.; Barrios, C.; Levey, A.S.; Römisch-Margl, W.; Menni, C.; Yet, I.; Gieger, C.; Inker, L.A.; et al. A Metabolome-Wide Association Study of Kidney Function and Disease in the General Population. J. Am. Soc. Nephrol. 2015, 27, 1175–1188. [Google Scholar] [CrossRef]
- Stanimirova, I.; Banasik, M.; Ząbek, A.; Dawiskiba, T.; Kościelska-Kasprzak, K.; Wojtowicz, W.; Krajewska, M.; Janczak, D.; Młynarz, P. Serum metabolomics approach to monitor the changes in metabolite profiles following renal transplantation. Sci. Rep. 2020, 10, 17223. [Google Scholar] [CrossRef]
- Luo, S.; Coresh, J.; Tin, A.; Rebholz, C.M.; Appel, L.J.; Chen, J.; Vasan, R.S.; Anderson, A.H.; Feldman, H.I.; Kimmel, P.L.; et al. Serum Metabolomic Alterations Associated with Proteinuria in CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 342–353. [Google Scholar] [CrossRef]
- Hasegawa, S.; Inagi, R. Harnessing Metabolomics to Describe the Pathophysiology Underlying Progression in Diabetic Kidney Disease. Curr. Diab. Rep. 2021, 21, 1–6. [Google Scholar] [CrossRef]
- Taherkhani, A.; Farrokhi Yekta, R.; Mohseni, M.; Saidijam, M.; Arefi Oskouie, A. Chronic kidney disease: A review of proteomic and metabolomic approaches to membranous glomerulonephritis, focal segmental glomerulosclerosis, and IgA nephropathy biomarkers. Proteome Sci. 2019, 17, 1–18. [Google Scholar] [CrossRef]
- Van Stralen, K.; Dekker, F.; Zoccali, C.; Jager, K. Case-Control Studies—An Efficient Observational Study Design. Nephron Clin. Pr. 2010, 114, c1–c4. [Google Scholar] [CrossRef] [PubMed]
- Keogh, R.H.; Cox, D.R. Case-Control Studies; Cambridge University Press (CUP): Cambridge, UK, 2014. [Google Scholar]
- Wu, I.-W.; Lee, C.-C.; Hsu, H.-J.; Sun, C.-Y.; Chen, Y.-C.; Yang, K.-J.; Yang, C.-W.; Chung, W.-H.; Lai, H.-C.; Chang, L.-C.; et al. Compositional and Functional Adaptations of Intestinal Microbiota and Related Metabolites in CKD Patients Receiving Dietary Protein Restriction. Nutrients 2020, 12, 2799. [Google Scholar] [CrossRef]
- Barrios, C.; Zierer, J.; Würtz, P.; Haller, T.; Metspalu, A.; Gieger, C.; Thorand, B.; Meisinger, C.; Waldenberger, M.; Raitakari, O.; et al. Circulating metabolic biomarkers of renal function in diabetic and non-diabetic populations. Sci. Rep. 2018, 8, 15249. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.-U.; Bärthlein, B.; Baid-Agrawal, S.; Beck, A.; Busch, M.; Eitner, F.; Ekici, A.B.; Floege, J.; Gefeller, O.; Haller, H.; et al. The German Chronic Kidney Disease (GCKD) study: Design and methods. Nephrol. Dial. Transplant. 2011, 27, 1454–1460. [Google Scholar] [CrossRef]
- Dienemann, T.; Fujii, N.; Orlandi, P.; Nessel, L.; Furth, S.L.; Hoy, W.E.; Matsuo, S.; Mayer, G.; Methven, S.; Schaefer, F.; et al. International Network of Chronic Kidney Disease cohort studies (iNET-CKD): A global network of chronic kidney disease cohorts. BMC Nephrol. 2016, 17, 1–9. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Palygin, O.; Guijas, C.; Palermo, A.; Palacio-Escat, N.; Domingo-Almenara, X.; Montenegro-Burke, R.; Saez-Rodriguez, J.; Staruschenko, A.; Siuzdak, G. Metabolic rewiring of the hypertensive kidney. Sci. Signal. 2019, 12, eaax9760. [Google Scholar] [CrossRef] [PubMed]
- Winkvist, A.; Bärebring, L.; Gjertsson, I.; Ellegård, L.; Lindqvist, H.M. A randomized controlled cross-over trial investigating the effect of anti-inflammatory diet on disease activity and quality of life in rheumatoid arthritis: The Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA) study protocol. Nutr. J. 2018, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Adamska-Patruno, E.; Samczuk, P.; Ciborowski, M.; Godzien, J.; Pietrowska, K.; Bauer, W.; Gorska, M.; Barbas, C.; Kretowski, A. Metabolomics Reveal Altered Postprandial Lipid Metabolism After a High-Carbohydrate Meal in Men at High Genetic Risk of Diabetes. J. Nutr. 2019, 149, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Beuchel, C.; Becker, S.; Dittrich, J.; Kirsten, H.; Toenjes, A.; Stumvoll, M.; Loeffler, M.; Thiele, H.; Beutner, F.; Thiery, J.; et al. Clinical and lifestyle related factors influencing whole blood metabolite levels—A comparative analysis of three large cohorts. Mol. Metab. 2019, 29, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Altmaier, E.; Fobo, G.; Heier, M.; Thorand, B.; Meisinger, C.; Römisch-Margl, W.; Waldenberger, M.; Gieger, C.; Illig, T.; Adamski, J.; et al. Metabolomics approach reveals effects of antihypertensives and lipid-lowering drugs on the human metabolism. Eur. J. Epidemiol. 2014, 29, 325–336. [Google Scholar] [CrossRef]
- Chua, E.C.P.; Shui, G.; Tian-Guang Lee, I.; Lau, P.; Tan, L.-C.; Yeo, S.-C.; Lam, B.D.; Bulchand, S.; Summers, S.A.; Puvanendran, K.; et al. Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 14468–14473. [Google Scholar] [CrossRef] [PubMed]
- Suhre, K.; Shin, S.Y.; Petersen, A.K.; Mohney, R.P.; Meredith, D.; Wagele, B.; Altmaier, E.; Gram, C.; Deloukas, P.; Erdmann, J.; et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011, 477, 54–60. [Google Scholar] [CrossRef]
- Smith, L.; Villaret-Cazadamont, J.; Claus, S.P.; Canlet, C.; Guillou, H.; Cabaton, N.J.; Ellero-Simatos, S. Important Considerations for Sample Collection in Metabolomics Studies with a Special Focus on Applications to Liver Functions. Metabolites 2020, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Barton, R.H.; Waterman, D.S.; Bonner, F.W.; Holmes, E.; Clarke, R.; Nicholson, J.; Lindon, J.; the PROCARDIS Consortium. The influence of EDTA and citrate anticoagulant addition to human plasma on information recovery from NMR-based metabolic profiling studies. Mol. BioSyst. 2009, 6, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, B.; Holmes, E.; Heude, C.; Tolson, R.F.; Harvey, N.; Lodge, S.L.; Chetwynd, A.J.; Cannet, C.; Fang, F.; Pearce, J.T.M.; et al. Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by 1H NMR Spectroscopy in a Multilaboratory Trial. Anal. Chem. 2018, 90, 11962–11971. [Google Scholar] [CrossRef]
- Von Schlippenbach, T.; Oefner, P.J.; Gronwald, W. Systematic Evaluation of Non-Uniform Sampling Parameters in the Targeted Analysis of Urine Metabolites by 1H,1H 2D NMR Spectroscopy. Sci. Rep. 2018, 8, 4249. [Google Scholar] [CrossRef] [PubMed]
- Soininen, P.; Kangas, A.; Würtz, P.; Suna, T.; Ala-Korpela, M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Cardiovascular Epidemiology and Genetics. Circ. Cardiovasc. Genet. 2015, 8, 192–206. [Google Scholar] [CrossRef]
- Percival, B.C.; Grootveld, M.; Gibson, M.; Osman, Y.; Molinari, M.; Jafari, F.; Sahota, T.; Martin, M.; Casanova, F.; Mather, M.L.; et al. Low-field, benchtop NMR spectroscopy as a potential tool for point-of-care diagnostics of metabolic conditions: Validation, protocols and computational models. High Throughput 2019, 8, 2. [Google Scholar] [CrossRef]
- Leenders, J.; Grootveld, M.; Percival, B.; Gibson, M.; Casanova, F.; Wilson, P.B. Benchtop Low-Frequency 60 MHz NMR Analysis of Urine: A Comparative Metabolomics Investigation. Metabolites 2020, 10, 155. [Google Scholar] [CrossRef]
- Edgar, M.; Percival, B.C.; Gibson, M.; Jafari, F.; Grootveld, M. Low-field benchtop NMR spectroscopy as a potential non-stationary tool for point-of-care urinary metabolite tracking in diabetic conditions. Diabetes Res. Clin. Pr. 2021, 171, 108554. [Google Scholar] [CrossRef]
- Dona, A.C.; Jiménez, B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision High-Throughput Proton NMR Spectroscopy of Human Urine, Serum, and Plasma for Large-Scale Metabolic Phenotyping. Anal Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef]
- Lodge, S.; Nitschke, P.; Leng Loo, R.; Kimhofer, T.; Bong, S.-H.; Richards, T.; Begum, S.; Spraul, M.; Schaefer, H.; Lindon, J.C.; et al. Low Volume in Vitro Diagnostic Proton NMR Spectroscopy of Human Blood Plasma for Lipoprotein and Metabolite Analysis: Application to SARS-CoV-2 Biomarkers. J. Proteome Res. 2021, 20, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Tveite Bjerrum, J.T. Metabonomics: Methods and protocols. Methods Mol. Biol. 2015, 1277. Available online: https://mosys.univie.ac.at/publications/books/metabolomics-methods-and-protocols/ (accessed on 15 July 2021).
- Zacharias, H.U.; Hochrein, J.; Klein, M.; Samol, C.; Oefner, P.; Gronwald, W. Current Experimental, Bioinformatic and Statistical Methods used in NMR Based Metabolomics. Curr. Metab. 2013, 1, 253–268. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2006, 26, 51–78. [Google Scholar] [CrossRef] [PubMed]
- Suhre, K.; Meisinger, C.; Döring, A.; Altmaier, E.; Belcredi, P.; Gieger, C.; Chang, D.; Milburn, M.V.; Gall, W.E.; Weinberger, K.; et al. Metabolic Footprint of Diabetes: A Multiplatform Metabolomics Study in an Epidemiological Setting. PLoS ONE 2010, 5, e13953. [Google Scholar] [CrossRef]
- Almstetter, M.F.; Oefner, P.J.; Dettmer, K. Comprehensive two-dimensional gas chromatography in metabolomics. Anal. Bioanal. Chem. 2012, 402, 1993–2013. [Google Scholar] [CrossRef]
- François, I.; Sandra, K.; Sandra, P. Comprehensive liquid chromatography: Fundamental aspects and practical considerations—A review. Anal. Chim. Acta 2009, 641, 14–31. [Google Scholar] [CrossRef]
- Zhang, X.W.; Li, Q.H.; Di Xu, Z.; Dou, J.J. Mass spectrometry-based metabolomics in health and medical science: A systematic review. RSC Adv. 2020, 10, 3092–3104. [Google Scholar] [CrossRef]
- Amberg, A.; Riefke, B.; Schlotterbeck, G.; Ross, A.; Senn, H.; Dieterle, F.; Keck, M. NMR and MS Methods for Metabolomics BT—Drug Safety Evaluation: Methods and Protocols. Methods Mol. Biol. 2017, 1641, 229–258. [Google Scholar]
- Beckonert, O.; Keun, H.C.; Ebbels, T.; Bundy, J.; Holmes, E.; Lindon, J.; Nicholson, J. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef]
- Vettukattil, R. Preprocessing of Raw Metabonomic Data. Adv. Struct. Saf. Stud. 2015, 1277, 123–136. [Google Scholar] [CrossRef]
- Tian, H.; Li, B.; Shui, G. Untargeted LC–MS Data Preprocessing in Metabolomics. J. Anal. Test. 2017, 1, 187–192. [Google Scholar] [CrossRef]
- Wallmeier, J.; Samol, C.; Ellmann, L.; Zacharias, H.U.; Vogl, F.C.; Garcia, M.; Dettmer, K.; Oefner, P.J.; Gronwald, W.; GCKD Study Investigators. Quantification of Metabolites by NMR Spectroscopy in the Presence of Protein. J. Proteome Res. 2017, 16, 1784–1796. [Google Scholar] [CrossRef]
- McHugh, C.E.; Flott, T.L.; Schooff, C.R.; Smiley, Z.; Puskarich, M.; Myers, D.D.; Younger, J.G.; Jones, A.E.; Stringer, K.A. Rapid, Reproducible, Quantifiable NMR Metabolomics: Methanol and Methanol: Chloroform Precipitation for Removal of Macromolecules in Serum and Whole Blood. Metabolites 2018, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, H.U.; Rehberg, T.; Mehrl, S.; Richtmann, D.; Wettig, T.; Oefner, P.J.; Spang, R.; Gronwald, W.; Altenbuchinger, M. Scale-Invariant Biomarker Discovery in Urine and Plasma Metabolite Fingerprints. J. Proteome Res. 2017, 16, 3596–3605. [Google Scholar] [CrossRef] [PubMed]
- Altenbuchinger, M.; Rehberg, T.; Zacharias, H.U.; Stämmler, F.; Dettmer, K.; Weber, D.; Hiergeist, A.; Gessner, A.; Holler, E.; Oefner, P.J.; et al. Reference point insensitive molecular data analysis. Bioinformatics 2017, 33, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Shi, P.; Feng, R.; Li, H. Variable selection in regression with compositional covariates. Biometrika 2014, 101, 785–797. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Allison, P.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Vogl, F.C.; GCKD Study Investigators; Mehrl, S.; Heizinger, L.; Schlecht, I.; Zacharias, H.U.; Ellmann, L.; Nürnberger, N.; Gronwald, W.; Leitzmann, M.F.; et al. Evaluation of dilution and normalization strategies to correct for urinary output in HPLC-HRTOFMS metabolomics. Anal. Bioanal. Chem. 2016, 408, 8483–8493. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.S.; Sabbisetti, V.S.; Bonventre, J.V. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010, 78, 486–494. [Google Scholar] [CrossRef]
- Stevens, L.A.; Levey, A.S. Measured GFR as a Confirmatory Test for Estimated GFR. J. Am. Soc. Nephrol. 2009, 20, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Rabassa, M.; Cherubini, A.; Urpi-Sarda, M.; Llorach, R.; Bandinelli, S.; Ferrucci, L.; Andres-Lacueva, C. Comparison of 24-h volume and creatinine-corrected total urinary polyphenol as a biomarker of total dietary polyphenols in the Invecchiare InCHIANTI study. Anal. Chim. Acta 2011, 704, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G. Cystatin C: A Marker of Renal Function or Something More? Clin. Chem. 2005, 51, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Warrack, B.M.; Hnatyshyn, S.; Ott, K.-H.; Reily, M.; Sanders, M.; Zhang, H.; Drexler, D.M. Normalization strategies for metabonomic analysis of urine samples. J. Chromatogr. B 2009, 877, 547–552. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, G.; Zhang, R.; He, J.; Zhang, Y.; Xu, J.; Yang, W.; Chen, X.; Song, Y.; Abliz, Z. Combination of Injection Volume Calibration by Creatinine and MS Signals’ Normalization to Overcome Urine Variability in LC-MS-Based Metabolomics Studies. Anal. Chem. 2013, 85, 7659–7665. [Google Scholar] [CrossRef]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 1995, 6, 277–293. [Google Scholar] [CrossRef]
- Ravanbakhsh, S.; Liu, P.; Bjordahl, T.C.; Mandal, R.; Grant, J.R.; Wilson, M.; Eisner, R.; Sinelnikov, I.; Hu, X.; Luchinat, C.; et al. Accurate, fully-automated NMR spectral profiling for metabolomics. PLoS ONE 2015, 10, e0124219. [Google Scholar] [CrossRef]
- Madrid-Gambin, F.; Oller-Moreno, S.; Fernandez, L.; Bartova, S.; Giner, M.P.; Joyce, C.; Ferraro, F.; Montoliu, I.; Moco, S.; Marco, S. AlpsNMR: An R package for signal processing of fully untargeted NMR-based metabolomics. Bioinformatics 2020, 36, 2943–2945. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.N.; Valkenborg, D.; Smets, K.; Verwaest, K.A.; Dommisse, R.; Lemière, F.; Verschoren, A.; Goethals, B.; Laukens, K. An integrated workflow for robust alignment and simplified quantitative analysis of NMR spectrometry data. BMC Bioinform. 2011, 12, 405. [Google Scholar] [CrossRef]
- Hoffmann, N.; Stoye, J. ChromA: Signal-based retention time alignment for chromatography–mass spectrometry data. Bioinformatics 2009, 25, 2080–2081. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Ho, T.-J.; Kuo, C.-H.; Tseng, Y.J. Chromaligner: A web server for chromatogram alignment. Bioinformatics 2010, 26, 2338–2339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lommen, A. MetAlign: Interface-Driven, Versatile Metabolomics Tool for Hyphenated Full-Scan Mass Spectrometry Data Preprocessing. Anal. Chem. 2009, 81, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Katajamaa, M.; Oresic, M. Processing methods for differential analysis of LC/MS profile data. BMC Bioinform. 2005, 6, 179. [Google Scholar] [CrossRef]
- Katajamaa, M.; Miettinen, J.; Orešič, M. MZmine: Toolbox for processing and visualization of mass spectrometry based molecular profile data. Bioinformatics 2006, 22, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Röst, H.; Sachsenberg, T.; Aiche, S.; Bielow, C.; Weisser, H.; Aicheler, F.; Andreotti, S.; Ehrlich, H.-C.; Gutenbrunner, P.; Kenar, E.; et al. OpenMS: A flexible open-source software platform for mass spectrometry data analysis. Nat. Methods 2016, 13, 741–748. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Benton, H.P.; Wong, D.M.; Trauger, S.; Siuzdak, G. XCMS2: Processing Tandem Mass Spectrometry Data for Metabolite Identification and Structural Characterization. Anal. Chem. 2008, 80, 6382–6389. [Google Scholar] [CrossRef]
- Melamud, E.; Vastag, L.; Rabinowitz, J.D. Metabolomic Analysis and Visualization Engine for LC−MS Data. Anal. Chem. 2010, 82, 9818–9826. [Google Scholar] [CrossRef]
- Domingo-Almenara, X.; Brezmes, J.; Vinaixa, M.; Samino, S.; Ramirez, N.; Ramon-Krauel, M.; Lerin, C.; Díaz, M.; Ibáñez, L.; Correig, X.; et al. eRah: A Computational Tool Integrating Spectral Deconvolution and Alignment with Quantification and Identification of Metabolites in GC/MS-Based Metabolomics. Anal. Chem. 2016, 88, 9821–9829. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.E.; Reo, N.V.; Delraso, N.J.; Doom, T.E.; Raymer, M.L. Gaussian binning: A new kernel-based method for processing NMR spectroscopic data for metabolomics. Metabolomics 2008, 4, 261–272. [Google Scholar] [CrossRef]
- Davis, R.A.; Charlton, A.J.; Godward, J.; Jones, S.A.; Harrison, M.; Wilson, J.C. Adaptive binning: An improved binning method for metabolomics data using the undecimated wavelet transform. Chemom. Intell. Lab. Syst. 2007, 85, 144–154. [Google Scholar] [CrossRef]
- De Meyer, T.; Sinnaeve, D.; Van Gasse, B.; Tsiporkova, E.; Rietzschel, E.R.; De Buyzere, M.L.; Gillebert, T.; Bekaert, S.; Martins, J.; Van Criekinge, W. NMR-Based Characterization of Metabolic Alterations in Hypertension Using an Adaptive, Intelligent Binning Algorithm. Anal. Chem. 2008, 80, 3783–3790. [Google Scholar] [CrossRef] [PubMed]
- AndersonDeirdre, P.E.; Mahle, D.A.; Doom, T.E.; Reo, N.V.; Delraso, N.J.; Raymer, M.L. Dynamic adaptive binning: An improved quantification technique for NMR spectroscopic data. Metabolomics 2011, 7, 179–190. [Google Scholar] [CrossRef]
- Blaise, B.J.; Shintu, L.; Elena-Herrmann, B.; Emsley, L.; Dumas, M.-E.; Toulhoat, P. Statistical Recoupling Prior to Significance Testing in Nuclear Magnetic Resonance Based Metabonomics. Anal. Chem. 2009, 81, 6242–6251. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, A.; Ayala, R.; Posma, J.M.; Harvey, N.; Jiménez, B.; Sonomura, K.; Sato, T.-A.; Matsuda, F.; Zalloua, P.; Gauguier, D.; et al. pJRES Binning Algorithm (JBA): A new method to facilitate the recovery of metabolic information from pJRES 1H NMR spectra. Bioinformatics 2018, 35, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Weljie, A.M.; Newton, J.; Mercier, P.; Carlson, E.; Slupsky, C.M. Targeted profiling: Quantitative analysis of 1H NMR metabolomics data. Anal. Chem. 2006, 78, 4430–4442. [Google Scholar] [CrossRef]
- Hedjazi, L.; Gauguier, D.; Zalloua, P.A.; Nicholson, J.K.; Dumas, M.-E.; Cazier, J.-B. mQTL.NMR: An Integrated Suite for Genetic Mapping of Quantitative Variations of 1H NMR-Based Metabolic Profiles. Anal. Chem. 2015, 87, 4377–4384. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, A.; Posma, J.M.; Ayala, R.; Neves, A.L.; Anwar, M.; Petretto, E.; Emanueli, C.; Gauguier, D.; Nicholson, J.; Dumas, M.-E. MWASTools: An R/bioconductor package for metabolome-wide association studies. Bioinformatics 2017, 34, 890–892. [Google Scholar] [CrossRef]
- Beirnaert, C.; Meysman, P.; Vu, T.N.; Hermans, N.; Apers, S.; Pieters, L.; Covaci, A.; Laukens, K. speaq 2.0: A complete workflow for high-throughput 1D NMR spectra processing and quantification. PLoS Comput. Biol. 2018, 14, e1006018. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Tikunov, Y.M.; Laptenok, S.; Hall, R.D.; Bovy, A.; De Vos, R.C.H. MSClust: A tool for unsupervised mass spectra extraction of chromatography-mass spectrometry ion-wise aligned data. Metabolomics 2012, 8, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Gorrochategui, E.; Jaumot, J.; Tauler, R. ROIMCR: A powerful analysis strategy for LC-MS metabolomic datasets. BMC Bioinform. 2019, 20, 256. [Google Scholar] [CrossRef] [PubMed]
- Tauler, R. Multivariate curve resolution applied to second order data. Chemom. Intell. Lab. Syst. 1995, 30, 133–146. [Google Scholar] [CrossRef]
- Hao, J.; Liebeke, M.; Astle, W.; De Iorio, M.; Bundy, J.G.; Ebbels, T. Bayesian deconvolution and quantification of metabolites in complex 1D NMR spectra using BATMAN. Nat. Protoc. 2014, 9, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Astle, W.; De Iorio, M.; Ebbels, T.M.D. BATMAN—An R package for the automated quantification of metabolites from nuclear magnetic resonance spectra using a Bayesian model. Bioinformatics 2012, 28, 2088–2090. [Google Scholar] [CrossRef]
- Hughes, T.; Wilson, H.D.; De Vera, I.M.S.; Kojetin, D.J. Deconvolution of Complex 1D NMR Spectra Using Objective Model Selection. PLoS ONE 2015, 10, e0134474. [Google Scholar] [CrossRef] [PubMed]
- Häckl, M.; Tauber, P.; Schweda, F.; Zacharias, H.U.; Altenbuchinger, M.; Oefner, P.J.; Gronwald, W. An R-package for the Deconvolution and Integration of 1D NMR data: MetaboDecon1D. Metabolites 2021, 11, 452. [Google Scholar] [CrossRef]
- Haslauer, K.E.; Schmitt-Kopplin, P.; Heinzmann, S.S. Data processing optimization in untargeted metabolomics of urine using voigt lineshape model non-linear regression analysis. Metabolites 2021, 11, 285. [Google Scholar] [CrossRef]
- Wei, X.; Shi, X.; Kim, S.; Zhang, L.; Patrick, J.S.; Binkley, J.; McClain, C.; Zhang, X. Data preprocessing method for liquid chromatography-mass spectrometry based metabolomics. Anal. Chem. 2012, 84, 7963–7971. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, R.; Cai, Y.; Wang, Z.; Zhu, Z.-J. DecoMetDIA: Deconvolution of Multiplexed MS/MS Spectra for Metabolite Identification in SWATH-MS-Based Untargeted Metabolomics. Anal. Chem. 2019, 91, 11897–11904. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Kowarik, A.; Templ, M. Imputation with the R Package VIM. J. Stat. Softw. 2016, 74, 1–16. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Zhang, F.; Robinette, S.L.; Bruschweiler-Li, L.; Brüschweiler, R. Web server suite for complex mixture analysis by covariance NMR. Magn. Reson. Chem. 2009, 47, S118–S122. [Google Scholar] [CrossRef]
- Tulpan, D.; Léger, S.; Belliveau, L.; Culf, A.; Čuperlović-Culf, M. MetaboHunter: An automatic approach for identification of metabolites from 1H-NMR spectra of complex mixtures. BMC Bioinform. 2011, 12, 1–22. [Google Scholar] [CrossRef]
- Xia, J.; Bjorndahl, T.C.; Tang, P.; Wishart, D.S. MetaboMiner—Semi-automated identification of metabolites from 2D NMR spectra of complex biofluids. BMC Bioinform. 2008, 9, 507. [Google Scholar] [CrossRef]
- Tardivel, P.J.C.; Canlet, C.; Lefort, G.; Tremblay-Franco, M.; Debrauwer, L.; Concordet, D.; Servien, R. ASICS: An automatic method for identification and quantification of metabolites in complex 1D 1H NMR spectra. Metabolomics 2017, 13, 109. [Google Scholar] [CrossRef]
- Draper, J.; Enot, D.P.; Parker, D.; Beckmann, M.; Snowdon, S.; Lin, W.; Zubair, H. Metabolite signal identification in accurate mass metabolomics data with MZedDB, an interactive m/z annotation tool utilising predicted ionisation behaviour ’rules’. BMC Bioinform. 2009, 10, 227. [Google Scholar] [CrossRef]
- Klein, M.S.; Oefner, P.J.; Gronwald, W. MetaboQuant: A tool combining individual peak calibration and outlier detection for accurate metabolite quantification in 1D 1H and 1H-13C HSQC NMR spectra. Biotechniques 2013, 54, 251–256. [Google Scholar] [CrossRef]
- Röhnisch, H.E.; Eriksson, J.; Müllner, E.; Agback, P.; Sandström, C.; Moazzami, A.A. AQuA: An Automated Quantification Algorithm for High-Throughput NMR-Based Metabolomics and Its Application in Human Plasma. Anal. Chem. 2018, 90, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Purohit, P.V.; Rocke, D.M.; Viant, M.R.; Woodruff, D.L. Discrimination Models Using Variance-Stabilizing Transformation of Metabolomic NMR Data. OMICS A J. Integr. Biol. 2004, 8, 118–130. [Google Scholar] [CrossRef]
- Eriksson, L.; Antti, H.; Gottfries, J.; Holmes, E.; Johansson, E.; Lindgren, F.; Long, I.; Trygg, J.; Wold, S. Using chemometrics for navigating in the large data sets of genomics, proteomics, and metabonomics (gpm). Anal. Bioanal. Chem. 2004, 380, 419–429. [Google Scholar] [CrossRef]
- Huber, W.; Von Heydebreck, A.; Sültmann, H.; Poustka, A.; Vingron, M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 2002, 18, S96–S104. [Google Scholar] [CrossRef] [PubMed]
- Chawade, A.; Alexandersson, E.; Levander, F. Normalyzer: A Tool for Rapid Evaluation of Normalization Methods for Omics Data Sets. J. Proteome Res. 2014, 13, 3114–3120. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tang, J.; Yang, Q.; Cui, X.; Li, S.; Chen, S.; Cao, Q.; Xue, W.; Chen, N.; Zhu, F. Performance Evaluation and Online Realization of Data-driven Normalization Methods Used in LC/MS based Untargeted Metabolomics Analysis. Sci. Rep. 2016, 6, 38881. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R. Team. Development Core Team. R A Lang. Environ. Stat. Comput. 2013, 55, 275–286. [Google Scholar]
- Kuo, T.C.; Tian, T.F.; Tseng, Y.J. 3Omics: A web-based systems biology tool for analysis, integration and visualization of human transcriptomic, proteomic and metabolomic data. BMC Syst. Biol. 2013, 7, 64. [Google Scholar] [CrossRef]
- Cambiaghi, A.; Ferrario, M.; Masseroli, M. Analysis of metabolomic data: Tools, current strategies and future challenges for omics data integration. Brief. Bioinform. 2016, 18, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Ruhe, A.; Wold, H.; Dunn, I.W.J. The Collinearity Problem in Linear Regression. The Partial Least Squares (PLS) Approach to Generalized Inverses. SIAM J. Sci. Stat. Comput. 1984, 5, 735–743. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Vapnik, V.; Chervonenkis, A. Theory of Pattern Recognition. Nauka. 1974. Available online: https://www.bibsonomy.org/bibtex/936f556afc966ddda07ba175241d6924 (accessed on 15 July 2021).
- Tibshirani, R. The lasso method for variable selection in the cox model. Stat. Med. 1997, 16, 385–395. [Google Scholar] [CrossRef]
- Hoerl, A.E.; Kennard, R.W. Ridge Regression: Biased Estimation for Nonorthogonal Problems. Technometrics 1970, 12, 55. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Dimitriadou, E.; Hornik, K.; Weingessel, A.; Leisch, F.; Chang, C.C.; Lin, C.C.; e1071: Misc Functions of the Department of Statistics (e1071), TU Wien. R Package Version 1. 6–3. Available online: https://rdrr.io/rforge/e1071/ (accessed on 15 July 2021).
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Hochrein, J.; Klein, M.S.; Zacharias, H.U.; Li, J.; Wijffels, G.; Schirra, H.J.; Spang, R.; Oefner, P.J.; Gronwald, W. Performance Evaluation of Algorithms for the Classification of Metabolic 1H NMR Fingerprints. J. Proteome Res. 2012, 11, 6242–6251. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.F.R.S. LIII. On lines and planes of closest fit to systems of points in space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1901, 2, 559–572. [Google Scholar] [CrossRef]
- Kohonen, T. Self-Organizing Maps; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Wehrens, R.; Buydens, L.M.C. Self- and Super-organizing Maps in R: The kohonen Package. J. Stat. Software 2007, 1. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: New York, NY, USA, 2013; Volume 103. [Google Scholar]
- Bictash, M.; Ebbels, T.; Chan, Q.; Loo, R.L.; Yap, I.K.; Brown, I.J.; de Iorio, M.; Daviglus, M.L.; Holmes, E.; Stamler, J.; et al. Opening up the "Black Box": Metabolic phenotyping and metabolome-wide association studies in epidemiology. J. Clin. Epidemiol. 2010, 63, 970–979. [Google Scholar] [CrossRef]
- Horvath, S. Weighted Network Analysis: Applications in Genomics and Systems Biology; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Lauritzen, S.L. Graphical Models; Clarendon Press: Oxford, UK, 1996. [Google Scholar]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, R.; Opgen-Rhein, K. Strimmer, GeneNet: Modeling and Inferring Gene Networks, (2015) R Package Version 1.2.13. Available online: https://cran.microsoft.com/snapshot/2014-09-09/web/packages/GeneNet/index.html (accessed on 15 July 2021).
- Haslbeck, J.M.B.; Waldorp, L.J. Mgm: Estimating Time-Varying Mixed Graphical Models in High-Dimensional Data. J. Stat. Softw. 2020, 1. [Google Scholar] [CrossRef]
- Altenbuchinger, M.; Weihs, A.; Quackenbush, J.; Grabe, H.J.; Zacharias, H.U. Gaussian and Mixed Graphical Models as (multi-)omics data analysis tools. Biochim. Biophys. Acta BBA Bioenerg. 2020, 1863, 194418. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Tenori, L.; Cascante, M.; Carulla, P.R.D.A.; Dos Santos, V.A.P.M.; Saccenti, E. From correlation to causation: Analysis of metabolomics data using systems biology approaches. Metabolomics 2018, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Schwarzer, G. Meta: An R package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- Gurevitch, J.; Koricheva, J.; Nakagawa, S.; Stewart, G. Meta-analysis and the science of research synthesis. Nat. Cell Biol. 2018, 555, 175–182. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Ishwaran, H.; Kogalur, U.B.; Blackstone, E.H.; Lauer, M. Random survival forests. Ann. Appl. Stat. 2008, 2, 841–860. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M. The Cox Model. In Statistics for Biology and Health; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2000; pp. 39–77. [Google Scholar]
- Jager, K.J.; Van Dijk, P.C.; Zoccali, C.; Dekker, F.W. The analysis of survival data: The Kaplan—Meier method. Kidney Int. 2008, 74, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Stel, V.S.; Dekker, F.; Tripepi, G.; Zoccali, C.; Jager, K.J. Survival Analysis II: Cox Regression. Nephron Clin. Pr. 2011, 119, c255–c260. [Google Scholar] [CrossRef] [PubMed]
- Nueda, M.J.; Conesa, A.; Westerhuis, J.A.; Hoefsloot, H.C.J.; Smilde, A.K.; Talón, M.; Ferrer, A. Discovering gene expression patterns in time course microarray experiments by ANOVA–SCA. Bioinformatics 2007, 23, 1792–1800. [Google Scholar] [CrossRef]
- Jansen, J.J.; Hoefsloot, H.C.J.; Van Der Greef, J.; Timmerman, M.E.; Westerhuis, J.A.; Smilde, A.K. ASCA: Analysis of multivariate data obtained from an experimental design. J. Chemom. 2005, 19, 469–481. [Google Scholar] [CrossRef]
- Zwanenburg, G.; Hoefsloot, H.C.; Westerhuis, J.A.; Jansen, J.J.; Smilde, A.K. ANOVA-principal component analysis and ANOVA-simultaneous component analysis: A comparison. J. Chemom. 2011, 25, 561–567. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Smilde, A.K.; Westerhuis, J.A.; Hoefsloot, H.C.J.; Bijlsma, S.; Rubingh, C.M.; Vis, D.J.; Jellema, R.; Pijl, H.; Roelfsema, F.; van der Greef, J. Dynamic metabolomic data analysis: A tutorial review. Metabolomics 2010, 6, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. MSEA: A web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res. 2010, 38, W71–W77. [Google Scholar] [CrossRef] [PubMed]
- Goeman, J.J.; van de Geer, S.A.; de Kort, F.; van Houwelingen, H.C. A global test for groups of genes: Testing association with a clinical outcome. Bioinformatics 2004, 20, 93–99. [Google Scholar] [CrossRef]
- Picart-Armada, S.; Fernández-Albert, F.; Vinaixa, M.; Yanes, O.; Perera-Lluna, A. FELLA: An R package to enrich metabolomics data. BMC Bioinform. 2018, 19, 538. [Google Scholar] [CrossRef] [PubMed]
- Al-Akwaa, F.M.; Yunits, B.; Huang, S.; Alhajaji, H.; Garmire, L.X. Lilikoi: An R package for personalized pathway-based classification modeling using metabolomics data. GigaScience 2018, 7. [Google Scholar] [CrossRef]
- Nguyen, T.-M.; Shafi, A.; Nguyen, T.; Draghici, S. Identifying significantly impacted pathways: A comprehensive review and assessment. Genome Biol. 2019, 20, 203. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.; Simon, R. Bias in error estimation when using cross-validation for model selection. BMC Bioinform. 2006, 7, 91. [Google Scholar] [CrossRef]
- Becker, G.J.; Hewitson, T. Animal models of chronic kidney disease: Useful but not perfect. Nephrol. Dial. Transplant. 2013, 28, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.; Weljie, A.M.; Turner, R.J. Computational tools for the secondary analysis of metabolomics experiments. Comput. Struct. Biotechnol. J. 2013, 4, e201301003. [Google Scholar] [CrossRef] [PubMed]
- Abbiss, H.; Maker, G.L.; Trengove, R.D. Metabolomics Approaches for the Diagnosis and Understanding of Kidney Diseases. Metabolites 2019, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chen, D.-Q.; Chen, L.; Liu, J.-R.; Vaziri, N.D.; Guo, Y.; Zhao, Y.-Y. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J. Transl. Med. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Klein, J.; Jupp, S.; Moulos, P.; Fernandez, M.; Buffin-Meyer, B.; Casemayou, A.; Chaaya, R.; Charonis, A.; Bascands, J.-L.; Stevens, R.; et al. The KUPKB: A novel Web application to access multiomics data on kidney disease. FASEB J. 2012, 26, 2145–2153. [Google Scholar] [CrossRef]
- Fernandes, M.; Husi, H. Establishment of a integrative multi-omics expression database CKDdb in the context of chronic kidney disease (CKD). Sci. Rep. 2017, 7, 40367. [Google Scholar] [CrossRef]
- Papadopoulos, T.; Krochmal, M.; Cisek, K.; Fernandes, M.; Husi, H.; Stevens, R.; Bascands, J.-L.; Schanstra, J.P.; Klein, J. Omics databases on kidney disease: Where they can be found and how to benefit from them. Clin. Kidney J. 2016, 9, 343–352. [Google Scholar] [CrossRef]
- Breit, M.; Weinberger, K.M. Metabolic biomarkers for chronic kidney disease. Arch. Biochem. Biophys. 2016, 589, 62–80. [Google Scholar] [CrossRef]
- Davies, R. The metabolomic quest for a biomarker in chronic kidney disease. Clin. Kidney J. 2018, 11, 694–703. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Hollywood, K.A.; Goodacre, R. Metabolomics for the masses: The future of metabolomics in a personalized world. Eur. J. Mol. Clin. Med. 2017, 3, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Goldansaz, S.A.; Jaine, J. Translational Metabolomics: Current Challenges and Future Opportunities. Metabolites 2019, 9, 108. [Google Scholar] [CrossRef] [PubMed]
| Study Design/Type | Study Question | Study Population and Investigated Biofluids | References | Detected Metabolites/Metabolic Biomarkers/Pathways |
|---|---|---|---|---|
| case–control study | AKI prediction | patients undergoing cardiac surgery, urine specimens collected before and after surgery | [18] | carnitine (elevated in AKI-free patients), tranexamic acid (elevated in AKI patients) and others |
| case–control study | AKI prediction | patients undergoing cardiac surgery, plasma specimens collected 24h after surgery | [19] | glucuronide conjugate of propofol, Mg2+, lactate and others |
| case–control study | indicators of AKI | hospitalized, newly diagnosed AKI patients, serum specimens | [20] | increases in acylcarnitines and amino acids and reduction of arginine and lysophosphatidyl cholines in AKI patients |
| case–control study | distinct metabolic profile of ADPKD | 54 patients with ADPKD, several control groups, urine specimens | [21] | on average 51 out of 701 NMR features could reliably discriminate ADPKD patients from other kidney disease patients and healthy controls |
| case–control study | non-invasive diagnosis of TCMR in pediatric kidney transplant recipients | pediatric kidney replacement recipients, urine specimens | [22] | proline, kynurenine, phosphatidylcholines, diacylglycerols elevated in TCMR patients |
| case–control study | identify metabolic pathways altered in CKD stage 3–4 non-diabetics | CKD patients from the Paricalcitol study; healthy controls: employees of study centers, urine and plasma specimens | [23] | 27 urine and 33 plasma metabolites differed between CKD vs. controls; pathway analysis: citric acid cycle significantly affected: reduction of urinary excretion of citrate, cis-aconitate, isocitrate, 2-oxoglutarate, succinate; expression of genes regulating these metabolites were reduced |
| 2 independent nested case–control studies (=analysis vs. replication cohort) | metabolites predicting CVD mortality in incident KRT patients | ArMORR study, plasma specimens | [24] | oleoylcarnitine, linoleoylcarnitine, palmitoylcarnitine, stearoylcarnitine, strongest association with CVD mortality: oleoylcarnitine |
| cross-sectional CKD study | plasma metabolite profile differences in CKD stages 2, 3, and 4 | 30 participants with differing CKD stages, plasma specimens | [25] | CKD stages 3 vs. 2: 62 differing metabolites (39 higher and 23 lower in CKD stage 3); CKD stages 4 vs. 2: 111 differing metabolites (66 higher and 45 lower in CKD stage 4); CKD stages 4 vs. 3: 11 differing metabolites (7 higher and 4 lower in CKD stage 4); major differences for higher CKD stages: altered arginine metabolism, elevated coagulation/inflammation, impaired carboxylate anion transport, decreased adrenal steroid hormone production |
| cross-sectional study (proof-of-concept study) | identfication of serum metabolites to provide a more accurate GFR estimate | AASK study, MESA study: participants with mGFR, serum specimens | [26] | (1) serum metabolites from untargeted quantification: AASK—283 and MESA—387 significantly associated metabolites with mGFR; (2) targeted metabolites: 15 metabolites used for GFR estimation |
| 2 cross-sectional observational studies of the general population | association of serum metabolites and their ratios with eGFR | KORA F4 study, TwinsUK registry, serum specimens | [15] | association with eGFR: 22 metabolites and 516 metabolite ratios; acylcarnitines were associated inversely, ratio with the lowest p-value: serine to glutarylcarnitine |
| differing study design per cohort | metabolites correlating with clinical markers of kidney disease | 4 cohorts: training cohort, validation cohort, prospective cohort, drug treatment cohort | [27] | 5 metabolites, e.g., 5-metohydroxytryptophan, correlate with markers of kidney function |
| nested case–control study | CKD progression | CRIC study, serum specimens | [14] | 10 nominally associated metabolites; 6 higher in cases (uric acid, glucuronate, 4-hydroxy-mandelate, 3-methyladipate/pimelate, cytosine, homo-gentisate) and 4 lower in cases (threonine, methionine, phenylalanine, arginine) |
| prospective CKD cohort | risk of progression to KRT | GCKD study, plasma specimens | [17] | 24 NMR features—highest weights: creatinine, high-density lipoprotein, valine, acetyl groups of glycoproteins, Ca2+-EDTA |
| prospective CKD cohort | urinary 6-bromotryptophan and incident ESKD | GCKD study, urine specimens | [28] | higher 6-bromotryptophan levels were associated with lower risk of ESKD |
| prospective CKD cohort | urine metabolites associated with adverse kidney outcomes and mortality | GCKD study, urine specimens | [29] | 55 metabolites significantly associated with kidney failure, kidney failure + AKI or death; significant enrichment for phosphatidylcholine pathway |
| prospective CKD cohort | adverse cardiac events in CKD stage 3 patients | GCKD study, plasma specimens | [30] | association of trimethylamine N-oxide (TMAO) with cardiac arrhythmia and myocardial infarction |
| prospective CKD cohort, prospective population-based cohort | genetic studies of urinary metabolites | GCKD study, UK Biobank, urine specimens | [31] | 240 unique metabolite-locus associations highlighting novel candidate substrates for transport proteins; genes identified are enriched in absorption, distribution, metabolism, and excretion (ADME) relevant tissues, potentially novel candidates for biotransformation and detoxification reactions |
| prospective diabetic cohort study | multimetabolite models of disease process from type 1 diabetic patients w/o CKD | Finnish Diabetic Nephropathy Study Group, serum specimens | [32] | cross-sectionally: patients w/o DKD complications: low lipids, less inflammation, better glycemic control vs. patients with advanced CKD: high sphingomyelin, cystatin-C; shared features: low unsaturated fatty acids (UFA), phospholipids; prospectively: progressive albuminuria: high UFAs, phospholipids, IDL, LDL; accelerated DKD progression: high saturated fatty acids, low HDL |
| prospective observational transplant recipient study | prediction of allograft status via urine metabolites | kidney graft recipients of the CTOT-04 study, urine specimens | [33] | best discrimination between acute cellular rejection vs. no rejection: ratio of urinary 3-sialyllactose to xanthosine |
| prospective population-based study | metabolite associations with eGFR; incident CKD | ARIC study, serum specimens | [16] | eGFR associations: 34 metabolites detected—strongest positive = creatinine, strongest negative = 3-indoxyl sulfate; lower risk of incident CKD: 5-oxoproline, 1,5-anhydroglucitol |
| prospective population-based study | kidney function decline, incident CKD | KORA S4/F4 study, serum specimens | [34] | kidney function decline: spermidine, phosphatidylcholine diacyl C42:5-to-phosphatidyl acyl-alkyl C36:0 ratio; incident CKD: kynerunine-to-tryptophan ratio |
| prospective population-based study; prospective twin cohort | metabolite association with eGFR, incident CKD | KORA F4 study, replication in TwinsUK registry, serum specimens | [35] | 54 metabolites replicated and significantly associated with eGFR; 6 with pair-wise correlation with established kidney function measures (C-mannosyltryptophan, pseudouridine, N-acetylalanine, erythronate, myo-inositol, N-acetylcarnosine); incident CKD: C-mannosyltryptophan, pseudouridine, O-sulfo-l-tyrosine |
| prospective small patient sample | metabolic changes after kidney allograft transplantation | 19 allograft recipients, serum specimens | [36] | hippurate, mannitol, and alanine associate with changes in transplant allograft function over time; hippurate/histine are more sensitive to short-term changes in kidney activity than creatinine |
| two clinical trials | cross-sectional association of UACR with 637 known, non-drug, blood metabolites | AASK, MDRD study, serum specimens | [37] | 58 metabolites associated with proteinuria; metabolites with lowest p-value: 4-hydroxychlorthalonil and 1,5-anhydroglucitol with all 6 metabolites of the phosphatidylethanolamine pathway being significant |
| review | DKD associated metabolites | multiple studies | [38] | early stages of DKD: association with tricarboxylic acid cycle, glucose metabolites; uremic toxins in DKD progression: phenyl sulfate and tryptophan derivatives |
| review | differential metabolites in MGN, FSGS, IgAN | multiple studies | [39] | amongst others—MGN: 13 urinary metabolites as most important (dopamine, fumarate, carnosine, nicotinamide d-ribonucleotide, pyridoxal, deoxyguanosine triphosphate, adenosine monophosphate, l-citrulline, nicotinamide, deoxyuridine, phenylalanine, tryptamine, succinate); FSGS: 10 prognostic urine metabolites (citrulline, proline, dimethylamine, acetoacetate, valine, alphaketoisovaleric acid, isobutyrate, histidine, d-palmitylcarnitine, N-methylnicotinamide); IgAN vs. controls: higher serum metabolite levels (phenylalanine, lactate, myo-Inositol, L6 lipids L5 lipids, L3 lipids) and lower serum metabolite levels (alpha-, beta-glucose, valine, phosphocholine, tyrosine, lysine, isoleucine, glycine, glycerolphosphocholine, glutamate, glutamine, alanine, acetate, 1-methylhistidine, 3-hydroxybutyrate) |
| perspectives, no study design | metabolomics in CKD research: metabolites and future risk of mortality | AASK study, serum specimens | [13] | number of associated metabolites reduced after adjustment for eGFR—metabolite classes detected: amino acid, carbohydrate, cofactors/vitamins, energy, lipid, nucleotide, peptide, xenobiotic, unkown |
| Preprocessing Step | Goal | Available Methods | Commercially Available Software | Freely Available Software | ||||
|---|---|---|---|---|---|---|---|---|
| NMR Spectroscopy | Hyphenated MS | NMR Spectroscopy | Hyphenated MS | NMR Spectroscopy | Hyphenated MS | NMR Spectroscopy | Hyphenated MS | |
| spectral preprocessing | transform spectral data from time to frequency domain, correct baseline and phase distortions | reproducible identification and quantification of peak features across multiple MS spectra | Fourier transformation, zero filling, apodization, phase correction, baseline correction, spectral alignment, removal of unwanted regions | deisotoping, retention time alignment, baseline and noise filtering, recalibration | TopSpin (BrukerBioSpin GmbH, Rheinstetten, Germany), AMIX (BrukerBioSpin GmbH, Rheinstetten, Germany), ACD (ACD labs) | ACD (ACD labs), AMIX (BrukerBioSpin GmbH, Rheinstetten, Germany), vendor-specific software, Mnova | Automics (Softpedia), NMRFx, NMRPipe [88], BAYESIL [89], R-package AlpsNMR [90], R-package speaq [91] | ChromA [92], Chromaligner [93], MetAlign [94], MZmine [95,96], MZmine 2 [97], OpenMS [98], XCMS [99], XCMS2 [100], MAVEN [101], eRah [102] |
| metabolic feature extraction | extract signal intensities in untargeted manner from spectra to perform subsequent statistical analysis, reduce dimensionality, minimize effects from peak position variations across different spectra | equidistant bucketing/binning, Gaussian binning [103], adaptive binning [104], adaptive intelligent binning [105], dynamic adaptive binning [106], SRV [107], JBA [108], peak picking, manual/automatic definition of ROIs | equidistant bucketing/binning, peak detection/picking, manual/automatic definition of ROIs | AMIX (BrukerBioSpin GmbH, Rheinstetten, Germany), Chenomx (Chenomx Inc. Edmonton, Canada) [109] | vendor-specific software | R-package mQTL [110], R-package MWASTools [111], R-package speaq [91], R-package speaq 2.0 [112], R-package AlpsNMR [90] | MetaboAnalyst [113], MZmine [95,96], MZmine 2 [97], XCMS [99], MetAlign [94], MAVEN [101], MSClust [114], ROIMCR [115] | |
| spectral deconvolution | deconvolute highly overlapping peak areas | curve fitting | MCR-ALS [116] | Chenomx (Chenomx Inc. Edmonton, Canada) [109] | vendor-specific software | BATMAN [117,118], decon1d [119], MetaboDecon1D [120], BAYESIL [89], non-linear peak fitting based on Voigt line shape model [121] | MetSign [122], DecoMetDIA [123], eRah [102] | |
| missing value imputation | – | impute missing values to obtain full data matrix | – | half minimum imputation, mean value imputation, zero imputation, median value imputation, RF [124], MICE, kNN | – | vendor-specific software | – | MZmine [95,96], MetaboAnalyst [113], eRah [102], R-package mice [125], R-package VIM [126], R-package randomForest [127] |
| metabolite identification | identify metabolites in measured spectra | compare spectral features against reference spectra of pure compounds and/or query databases | Chenomx (Chenomx Inc. Edmonton, Canada) [109], AMIX (BrukerBioSpin GmbH, Rheinstetten, Germany) with BBIOREFCODE database, Aldrich FT-NMR (Sigma-Aldrich) | vendor-specific software | COLMAR [128], KnowItAll Metabolomics (BioRad Corp.), MetaboHunter [129], MetaboMiner [130], BAYESIL [89], ASICS [131], R-package speaq 2.0 [112] | MZmine 2 [97], OpenMS [98], XCMS [99], XCMS2 [100], MZedDB [132], eRah [102] | ||
| metabolite quantification | determine absolutely quantified concentrations of identified metabolites | accurately determine area under the curve of metabolite signal and reference with respect to known concentration of internal standard | Chenomx (Chenomx Inc. Edmonton, Canada) [109], AMIX (BrukerBioSpin GmbH, Rheinstetten, Germany) | vendor-specific software | BATMAN [117], [118], MetaboQuant [133], BAYESIL [89], AQuA [134], ASICS [131] | OpenMS [98] | ||
| metabolite data transformation | scaling of data in order to reduce data heteroscedasticity | e.g., log-transformation, variance stabilization transformation [135], auto-scaling, pareto scaling [136], mean centering | R Base, R-package vsn [137], R-package speaq 2.0 [112], Normalyzer [138], MetaPre [139] | |||||
| metabolite data normalization | minimize unwanted biological and/or technical variation between samples | e.g., creatinine normalization (for urine specimens), total spectral area normalization, normalization to internal standard, probabilistic quotient normalization [140], variance stabilization normalization [137], osmolality normalization, sample-specific normalization factors (e.g., volume), alternative: normalization-invariant zero-sum regression [77,78] | AMIX (BrukerBioSpin GmbH, Rheinstetten, Germany) | vendor-specific software | MetaboAnalyst [113], R-package AlpsNMR [90], R-package speaq 2.0 [112], Normalyzer [138], MetaPre [139], R-package zeroSum [77,78] | |||
| Research Goal | Example | LiteratureExample | Common Statistics/ Bioinformatics Method | Popular Statistics/ Bioinformatics Tools | R Software Packages | Further Reading |
|---|---|---|---|---|---|---|
| hypothesis testing | compare metabolite levels in CKD patients and healthy controls | [18] | hypothesis testing | Student’s t-test, ANOVA | >R Base: t.test, R Base: anova | [8,64] |
| multivariate bio-marker signature detection | multivariate metabolite signature to classify AKI vs. non-AKI patients | [19] | multivariate classification or linear regression | PLS-DA [148], OPLS-DA [149], support vector machine [150], Random Forest [124], LASSO regression [151], ridge regression [152], elastic net [153] | mixOmics [154], ropls [155], e1071 [156], randomForest [127], glmnet [157] | [8,64,158] |
| subgroup identification | exploratory identify CKD patient subgroups with different survival outcomes based on metabolic profiles | [32] | supervised/unsupervised machine learning | PCA [159], Hierarchical Clustering, Self-organizing maps [160] | R Base: prcomp, ropls [155], R Base: hclust, kohonen [161] | [8,64,160,162,163] |
| metabolome-wide association study | associations between all measured metabolites and eGFR, adjusted for age and sex | [35] | univariate/multivariate regression analysis (with confounder adjustment) | linear/logistic/Cox PH regression analysis | MWASTools [111] | [164] |
| statistical network analysis | exploratory identification of metabolite-metabolite associations | [30] | probabilistic graphical modeling, correlation networks | correlation network analysis, WGCNA [165], GGM [166], MGM [166] | corrr, WGCNA [167], GeneNet [168], mgm [169] | [166,170,171] |
| meta-analysis | combining p-values for creatinine and eGFR metabolite associations across multiple studies | [35] | regression model | fixed-effects model | metafor [172], meta [173] | [174] |
| time-to-event analysis | estimate the mortality of CKD patients based on a set of metabolites | [17] | survival analysis | Cox PH regression analysis [175], LASSO Cox PH regression [151], random survival forest [176] | survival [177], glmnet [157], randomForestSRC [176] | [177,178,179] |
| time-course analysis | analyze metabolite intensity changes over time under different CKD treatment conditions | [36] | time-course analysis | ASCA [180,181] | MetStaT [182], DESeq2 [183] | [184] |
| pathway (enrichment) analysis | identify set of metabolites differentiating non-CKD and CKD patients with affiliation to a specific pathway | [23] | hypergeometric test, regression model | MSEA [185], ORA, global test [186] | FELLA [187], Lilikoi [188], globaltest [186] | [171,189] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultheiss, U.T.; Kosch, R.; Kotsis, F.; Altenbuchinger, M.; Zacharias, H.U. Chronic Kidney Disease Cohort Studies: A Guide to Metabolome Analyses. Metabolites 2021, 11, 460. https://doi.org/10.3390/metabo11070460
Schultheiss UT, Kosch R, Kotsis F, Altenbuchinger M, Zacharias HU. Chronic Kidney Disease Cohort Studies: A Guide to Metabolome Analyses. Metabolites. 2021; 11(7):460. https://doi.org/10.3390/metabo11070460
Chicago/Turabian StyleSchultheiss, Ulla T., Robin Kosch, Fruzsina Kotsis, Michael Altenbuchinger, and Helena U. Zacharias. 2021. "Chronic Kidney Disease Cohort Studies: A Guide to Metabolome Analyses" Metabolites 11, no. 7: 460. https://doi.org/10.3390/metabo11070460
APA StyleSchultheiss, U. T., Kosch, R., Kotsis, F., Altenbuchinger, M., & Zacharias, H. U. (2021). Chronic Kidney Disease Cohort Studies: A Guide to Metabolome Analyses. Metabolites, 11(7), 460. https://doi.org/10.3390/metabo11070460






