Impact on Bile Acid Concentrations by Alveolar Echinococcosis and Treatment with Albendazole in Mice
Abstract
1. Introduction
2. Results
2.1. Murine Model of Alveolar Echinococcosis
2.2. Bile Acid Profiles
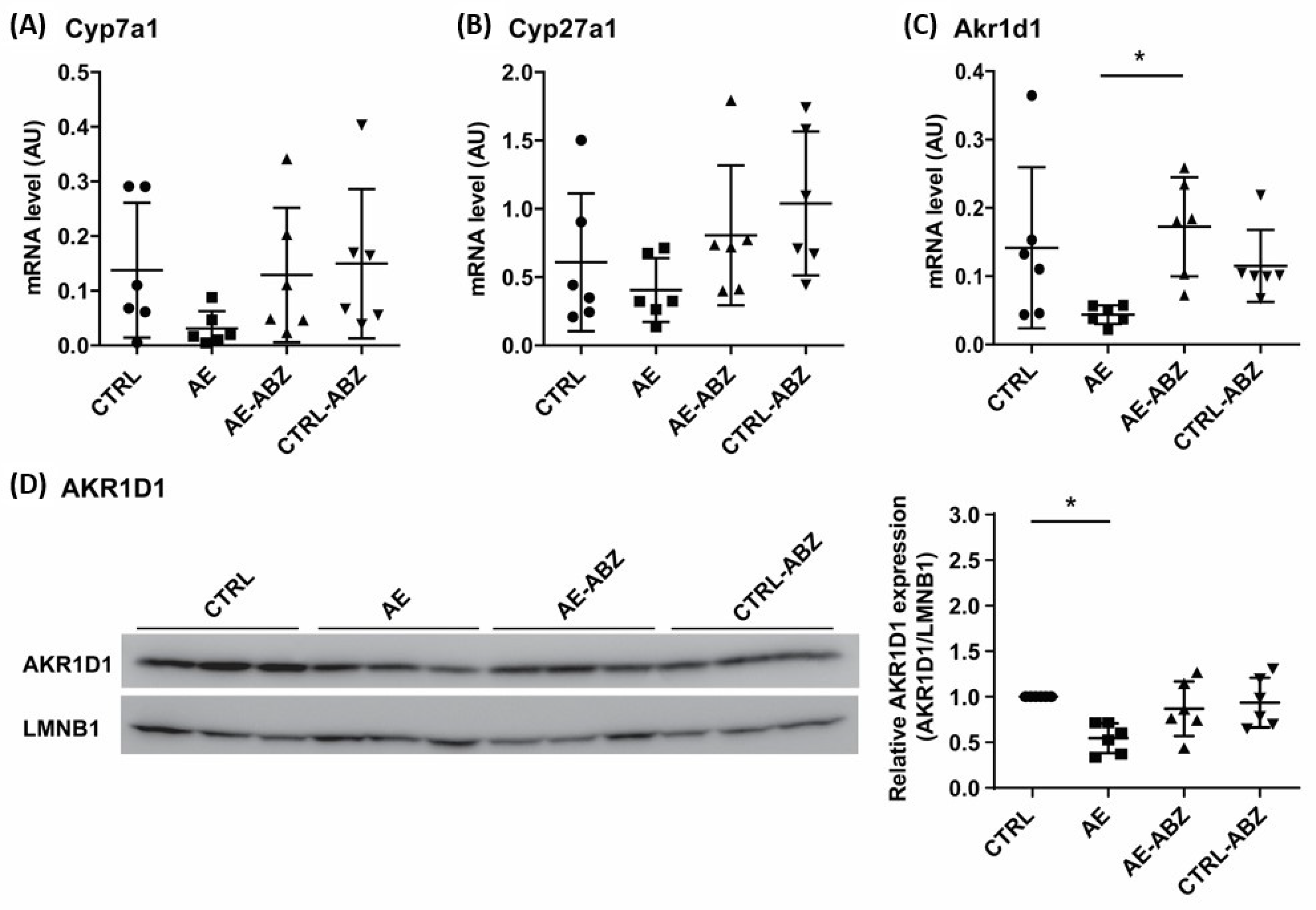
2.3. Effect of AE and ABZ Treatment on Enzymes Involved in Bile Acid Synthesis
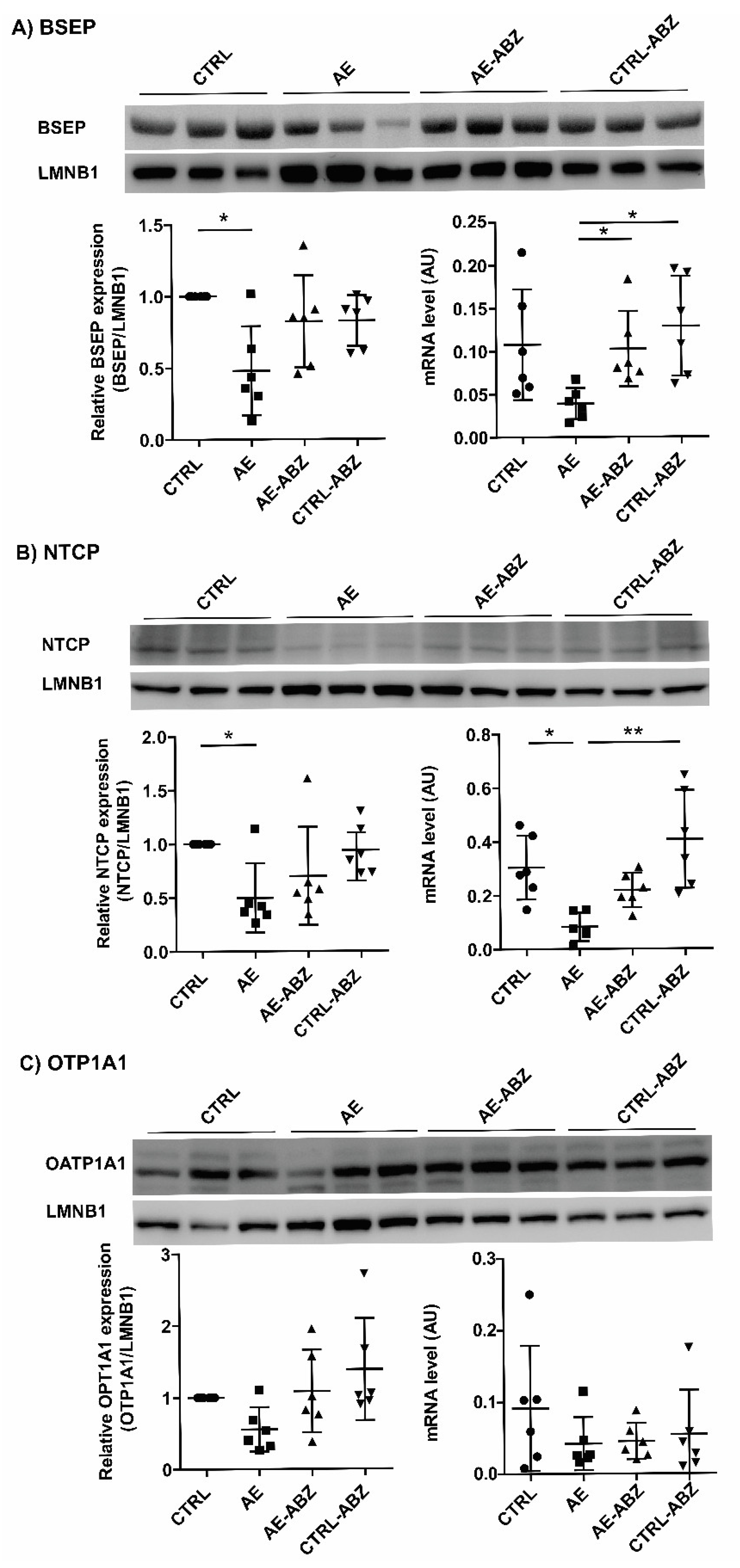
2.4. Influence of AE and ABZ Treatment on the Expression of Bile Acid Transporters
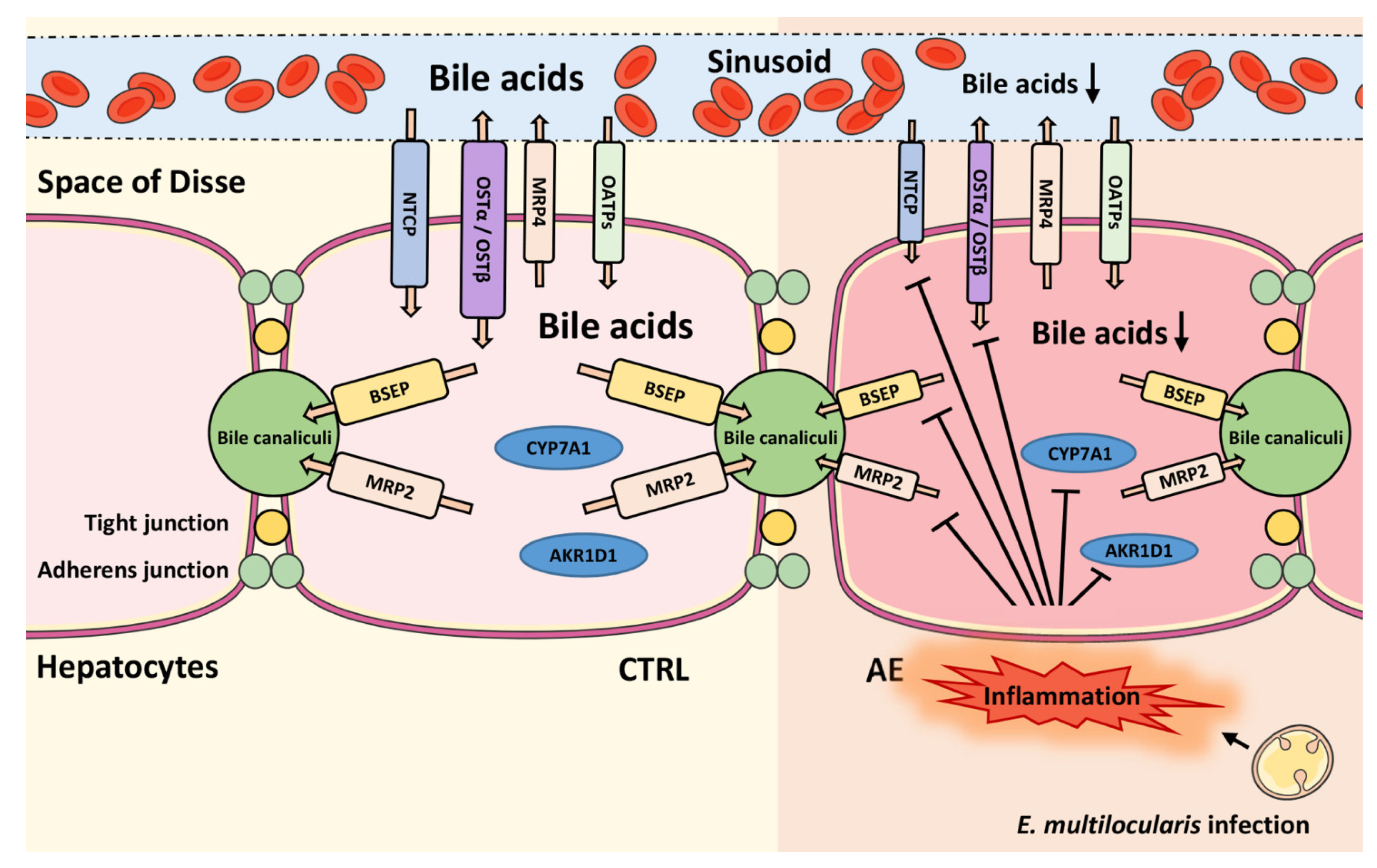
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Mice
4.3. Quantification of Bile Acids in Serum
4.4. Quantification of Bile Acids in Liver Tissue
4.5. Total RNA Extraction and qPCR
4.6. Protein Expression/Western Blot
4.7. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, P. Echinococcus multilocularis. Curr. Opin. Infect. Dis. 2003, 16, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, A.; Stadelmann, B.; Rufener, R.; Spiliotis, M.; Boubaker, G.; Müller, J.; Müller, N.; Gorgas, D.; Gottstein, B. Treatment of echinococcosis: Albendazole and mebendazole-what else? Parasite 2014, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Rinaldi, L.; Alvarez Rojas, C.A.; Torgerson, P.R.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.M.; Lahmar, S.; Cringoli, G.; et al. Global Distribution of Alveolar and Cystic Echinococcosis; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 95, pp. 315–493. [Google Scholar]
- Kato, N.; Nonaka, N.; Oku, Y.; Kamiya, M. Modified cellular immune responses in dogs infected with Echinococcus multilocularis. Parasitol. Res. 2005, 95, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, B.; Hemphill, A. Echinococcus multilocularis: The parasite-host interplay. Exp. Parasitol. 2008, 119, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Graeter, T.; Ehing, F.; Oeztuerk, S.; Mason, R.A.; Haenle, M.M.; Kratzer, W.; Seufferlein, T.; Gruener, B. Hepatobiliary complications of alveolar echinococcosis: A long-term follow-up study. World J. Gastroenterol. 2015, 21, 4925–4932. [Google Scholar] [CrossRef] [PubMed]
- Lundström-Stadelmann, B.; Rufener, R.; Ritler, D.; Zurbriggen, R.; Hemphill, A. The importance of being parasiticidal… an update on drug development for the treatment of alveolar echinococcosis. Food Waterborne Parasitol. 2019, 15, e00040. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st century. Clin. Microbiol. Rev. 2019, 32, 1–39. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.L. Bile acids: Regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef]
- Russell, D.W.; Setchell, K.D.R. Bile Acid Biosynthesis. Biochemistry 1992, 31, 4737–4749. [Google Scholar] [CrossRef]
- Thakare, R.; Alamoudi, J.A.; Gautam, N.; Rodrigues, A.D.; Alnouti, Y. Species differences in bile acids I. Plasma and urine bile acid composition. J. Appl. Toxicol. 2018, 38, 1323–1335. [Google Scholar] [CrossRef]
- Esteller, A. Physiology of bile secretion. World J. Gastroenterol. 2008, 14, 5641–5649. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.L. Bile formation and secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xie, G.; Jia, W. Bile acid–microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Lan, T.; Rao, A. Bile acid transporters. J. Lipid Res. 2009, 50, 2340–2357. [Google Scholar] [CrossRef] [PubMed]
- Stieger, B. The Role of the Sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile Formation. In Drug Transporters; Springer: Berlin/Heidelberg, Germany, 2011; Volume 201, ISBN 9783642145407. [Google Scholar]
- Cheng, X.; Buckley, D.; Klaassen, C.D. Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem. Pharmacol. 2007, 74, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Alrefai, W.A.; Gill, R.K. Bile Acid Transporters: Structure, Function, Regulation and Pathophysiological Implications. Pharm. Res. 2007, 24, 1803–1823. [Google Scholar] [CrossRef]
- Slijepcevic, D.; Van De Graaf, S.F.J. Bile Acid Uptake Transporters as Targets for Therapy. Dig. Dis. 2017, 35, 251–258. [Google Scholar] [CrossRef]
- Halilbasic, E.; Claudel, T.; Trauner, M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J. Hepatol. 2013, 58, 155–168. [Google Scholar] [CrossRef]
- Mennone, A.; Soroka, C.J.; Cai, S.-Y.; Harry, K.; Adachi, M.; Hagey, L.; Schuetz, J.D.; Boyer, J.L. Mrp4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology 2006, 43, 1013–1021. [Google Scholar] [CrossRef]
- Sugita, T.; Amano, K.; Nakano, M.; Masubuchi, N.; Sugihara, M.; Matsuura, T. Analysis of the serum bile acid composition for differential diagnosis in patients with liver disease. Gastroenterol. Res. Pract. 2015, 2015, 717431. [Google Scholar] [CrossRef]
- Luo, L.; Aubrecht, J.; Li, D.; Warner, R.L.; Johnson, K.J.; Kenny, J.; Colangelo, J.L. Assessment of serum bile acid profiles as biomarkers of liver injury and liver disease in humans. PLoS ONE 2018, 13, e0193824. [Google Scholar] [CrossRef]
- Manzotti, C.; Casazza, G.; Stimac, T.; Nikolova, D.; Gluud, C. Total serum bile acids or serum bile acid profile, or both, for the diagnosis of intrahepatic cholestasis of pregnancy. Cochrane Database Syst. Rev. 2019, 2019, CD012546. [Google Scholar] [CrossRef] [PubMed]
- Cepa, S.; Potter, D.; Wong, L.; Schutt, L.; Tarrant, J.; Pang, J.; Zhang, X.; Andaya, R.; Salphati, L.; Ran, Y.; et al. Individual serum bile acid profiling in rats aids in human risk assessment of drug-induced liver injury due to BSEP inhibition. Toxicol. Appl. Pharmacol. 2018, 338, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Makino, I.; Nakagawa, S.; Mashimo, K. Conjugated and Unconjugated Serum Bile Acid Levels in Patients with Hepatobiliary Diseases. Gastroenterology 1969, 56, 1033–1039. [Google Scholar] [CrossRef]
- Jebbawi, F.; Bellanger, A.; Lunström-Stadelmann, B.; Rufener, R.; Dosch, M.; Goepfert, C.; Gottstein, B.; Millon, L.; Grandgirard, D.; Leib, S.L.; et al. Innate and adaptive immune responses following PD-L1 blockade in treating chronic murine alveolar echinococcosis. Parasite Immunol. 2021, e12834. [Google Scholar] [CrossRef]
- Gómez, C.; Stücheli, S.; Kratschmar, D.V.; Bouitbir, J.; Odermatt, A. Development and Validation of a Highly Sensitive LC-MS/MS Method for the Analysis of Bile Acids in Serum, Plasma, and Liver Tissue Samples. Metabolites 2020, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Wang, X.G.; Chen, Y.T. Computerized tomography of liver in alveolar echinococcosis treated with albendazole. Zhonghua Nei Ke Za Zhi 1993, 32, 733–735. [Google Scholar] [PubMed]
- Rosenfeld, G.; Nimmo, M.; Hague, C.; Buczkowski, A.; Yoshida, E.M. Echinococcus presenting as painless jaundice. Can. J. Gastroenterol. 2012, 26, 684–685. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jenniskens, M.; Langouche, L.; Vanwijngaerden, Y.M.; Mesotten, D.; Van den Berghe, G. Cholestatic liver (dys)function during sepsis and other critical illnesses. Intensive Care Med. 2016, 42, 16–27. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Vale, N.; Gouveia, M.J.; Botelho, M.; Sripa, B.; Suttiprapa, S.; Rinaldi, G.; Gomes, P.; Brindley, P.J.; Correia da Costa, J.M. Carcinogenic liver fluke Opisthorchis viverrini oxysterols detected by LC-MS/MS survey of soluble fraction parasite extract. Parasitol. Int. 2013, 62, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, W.; Zhang, L.; Zhang, Z.; Li, J.; Lu, G.; Zhu, Y.; Wang, Y.; Huang, Y.; Liu, J.; et al. The genome of the hydatid tapeworm Echinococcus granulosus. Nat. Genet. 2013, 45, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.B.; Zou, Y.; Elsheikha, H.M.; Liu, G.H.; Hu, M.H.; Wang, S.L.; Zhu, X.Q. Serum metabolomic alterations in Beagle dogs experimentally infected with Toxocara canis. Parasites Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Corbin, I.; Blackburn, B.J.; Wolowiec, T.; Novak, M. Metabolic profile of the liver of mice infected with cysticerci of Taenia crassiceps. Parasitol. Int. 1996, 82, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Kalia, N.; Hardcastle, J.; Keating, C.; Pelegrin, P.; Grundy, D.; Grasa, L.; Bardhan, K.D. Intestinal secretory and absorptive function in Trichinella spiralis mouse model of postinfective gut dysfunction: Role of bile acids. Gut 2008, 57, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gan, Y.; Lv, J.W.; Qin, M.Q.; Hu, W.R.; Liu, Z.B.; Ma, L.; Song, B.D.; Li, J.; Jiang, W.Y.; et al. The protective effect of obeticholic acid on lipopolysaccharide-induced disorder of maternal bile acid metabolism in pregnant mice. Int. Immunopharmacol. 2020, 83, 106442. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Li, H.; Li, J.R.; Lv, X.Q.; Jiang, J.D.; Peng, Z.G. Farnesoid X receptor agonist GW4064 indirectly inhibits HCV entry into cells via down-regulating scavenger receptor class B type I. Eur. J. Pharmacol. 2019, 853, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Mouzannar, K.; Fusil, F.; Lacombe, B.; Ollivier, A.; Ménard, C.; Lotteau, V.; Cosset, F.L.; Ramière, C.; André, P. Farnesoid X receptor-α is a proviral host factor for hepatitis B virus that is inhibited by ligands in vitro and in vivo. FASEB J. 2019, 33, 2472–2483. [Google Scholar] [CrossRef]
- Stadelmann, B.; Rufener, R.; Aeschbacher, D.; Spiliotis, M.; Gottstein, B.; Hemphill, A. Screening of the Open Source Malaria Box Reveals an Early Lead Compound for the Treatment of Alveolar Echinococcosis. PLoS Negl. Trop. Dis. 2016, 10, e0004535. [Google Scholar] [CrossRef]
- Gerloff, T.; Stieger, B.; Hagenbuch, B.; Madon, J.; Landmann, L.; Roth, J.; Hofmann, A.F.; Meier, P.J. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J. Biol. Chem. 1998, 273, 10046–10050. [Google Scholar] [CrossRef]
- Eckhardt, U.; Schroeder, A.; Stieger, B.; Höchli, M.; Landmann, L.; Tynes, R.; Meier, P.J.; Hagenbuch, B. Polyspecific substrate uptake by the hepatic organic anion transporter Oatp1 in stably transfected CHO cells. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 276, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Stieger, B.; Hagenbuch, B.; Landmann, L.; Höchli, M.; Schroeder, A.; Meier, P.J. In situ localization of the hepatocytic na+/taurocholate cotransporting polypeptide in rat liver. Gastroenterology 1994, 107, 1781–1787. [Google Scholar] [CrossRef]
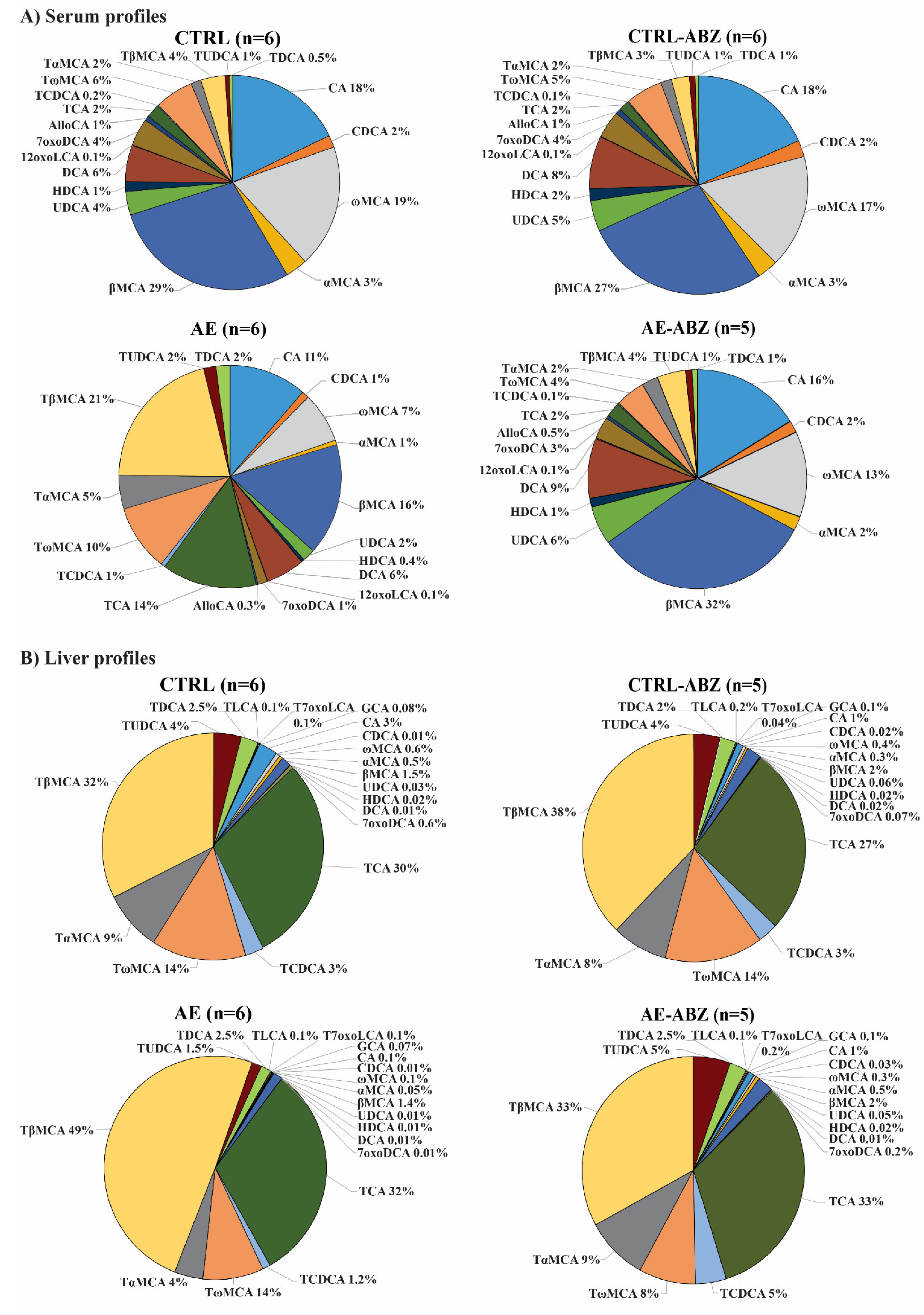
| Compound | CTRL (n = 6) (nM Mean ± SD) | AE (n = 6) (nM Mean ± SD) | AE-ABZ (n = 5) (nM Mean ± SD) | CTRL-ABZ (n = 6) (nM Mean ± SD) |
|---|---|---|---|---|
| Unconjugated | ||||
| CA | 2960 ± 1586 | 753 ± 761 *,† | 2218 ± 2861 | 2760 ± 1521 |
| CDCA | 305 ± 148 | 70 ± 34 *,† | 237 ± 200 | 365 ± 346 |
| ωMCA | 3074 ± 1654 | 489 ± 192 *,† | 1744 ± 1467 | 2522 ± 741 |
| αMCA | 554 ± 385 | 44 ± 37 *,† | 287 ± 308 | 463 ± 399 |
| βMCA | 4747 ± 3497 | 1080 ± 899 *,† | 4452 ± 4387 | 4131 ± 2559 |
| UDCA | 590 ± 358 | 122 ±81 *,† | 784 ± 811 | 686 ± 549 |
| HDCA | 245 ± 95 | 29 ± 18 *,† | 174 ± 137 | 263 ± 91 |
| DCA | 917 ± 261 | 374 ± 146 *,† | 1217 ± 939 | 1176 ± 654 |
| 12oxoLCA | 15 ± 6 | 5 ± 1 *,‡ | 17 ± 13 | 14 ± 7 |
| 7oxoDCA | 686 ± 441 | 93 ± 74 *,† | 443 ± 430 | 609 ± 305 |
| AlloCA | 122 ± 45 | 18 ± 13 *,† | 63 ± 73 | 119 ± 52 |
| Total unconjugated | 14,215 ± 7740 | 3078 ± 2082 * | 11,636 ± 11,085 | 13,107 ± 6512 |
| Taurine-conjugated | ||||
| TCA | 345 ± 173 | 920 ± 620 † | 305 ± 282 | 232 ± 110 |
| TCDCA | 27 ± 29 | 40 ± 23 | 19 ± 8 | 22 ± 19 |
| TωMCA | 970 ± 228 | 642 ± 208 | 607 ±446 | 822 ± 236 |
| TαMCA | 253 ± 104 | 325 ± 188 | 329 ± 372 | 244 ± 158 |
| TβMCA | 606 ± 285 | 1396 ± 916 † | 578 ± 596 | 398 ± 187 |
| TUDCA | 110 ± 37 | 120 ± 33 | 132 ± 80 | 125 ± 36 |
| TDCA | 81 ± 43 | 137 ± 69 | 108 ± 72 | 80 ± 39 |
| Total taurine-conjugated | 2392 ± 755 | 3578 ± 1820 | 2078 ± 1787 | 1924 ± 685 |
| Total bile acids | 16,607 ± 8135 | 6656 ± 3172 | 13,714 ± 12,722 | 15,031 ± 6963 |
| Compound | CTRL (n = 6) (pg/mg Tissue Mean ± SD) | AE (n = 6) (pg/mg Tissue Mean ± SD) | AE-ABZ (n = 5) (pg/mg Tissue Mean ± SD) | CTRL-ABZ (n = 5) (pg/mg Tissue Mean ± SD) |
|---|---|---|---|---|
| Unconjugated | ||||
| CA | 8520 ± 9256 | 151 ± 33 † | 2464 ± 3418 | 1615 ± 631 |
| CDCA | 42 ± 31 | ND ‡ | 74 ± 63 | 43 ± 19 |
| ωMCA | 1893 ± 1839 | 182 ± 43 † | 964 ± 1010 | 768 ± 260 |
| αMCA | 1627 ± 1627 | 79 ± 33 ‡ | 1466 ± 2108 | 542 ± 122 |
| βMCA | 4583 ± 3791 | 2312 ± 1080 | 6533 ± 5483 | 3748 ± 1778 |
| UDCA | 88± 74 | ND †,‡ | 127 ± 90 | 107 ± 46 |
| HDCA | 50 ± 42 | ND †,‡ | 46 ± 9 | 43 ± 9 |
| DCA | 40 ± 22 | 14 ± 2 * | 26 ± 8 | 30 ± 11 |
| 7oxoDCA | 1887 ± 2131 | ND *,† | 519 ± 1041 | 120 ± 43 |
| Total unconjugated | 18,734 ± 18,314 | 2770 ± 1142 | 12,220 ± 13,172 | 7016 ± 1726 |
| Conjugated | ||||
| TCA | 93,465 ± 58,895 | 53,864 ± 22,985 | 91,956 ± 95,949 | 47,978 ± 8176 |
| TCDCA | 8584 ± 7344 | 1980 ± 567 | 12,736 ± 16,372 | 5275 ± 2482 |
| TωMCA | 43,450 ± 33,129 | 15,090 ± 5925 | 23,250 ± 8736 | 25,676 ± 6107 |
| TαMCA | 26,984 ± 21,698 | 7092 ± 2735 ‡ | 25,708 ± 21,439 | 14,464 ± 5161 |
| TβMCA | 102,187 ± 65,883 | 84,403 ± 38,111 | 93,075 ± 51,320 | 67,780 ± 20,851 |
| TUDCA | 12,737 ± 10,579 | 2449 ± 815 ‡ | 15,399 ± 18621 | 6981 ± 1806 |
| TDCA | 8091 ± 5150 | 2602 ± 1044 | 6989 ± 6001 | 4148 ± 1787 |
| TLCA | 319 ± 188 | 83 ± 35 | 406 ± 353 | 275 ± 116 |
| T7oxoLCA | 316 ± 377 | 256 ± 273 | 462 ± 974 | 63 ± 61 |
| GCA | 264 ± 225 | 112 ± 27 | 387 ± 427 | 157 ± 30 |
| Total conjugated | 296,395 ± 198,074 | 167,929 ± 67,355 | 270,370 ± 216,375 | 172,798 ± 39,994 |
| Total bile acids | 314,865 ± 216,097 | 170,587 ± 67,462 | 282,203 ± 229,000 | 179,657 ± 39,812 |
| Serum | CTRL (n = 6) (mean ± SD) | AE (n = 6) (mean ± SD) | AE-ABZ (n = 5) (mean ± SD) | CTRL-ABZ (n = 6) (mean ± SD) |
|---|---|---|---|---|
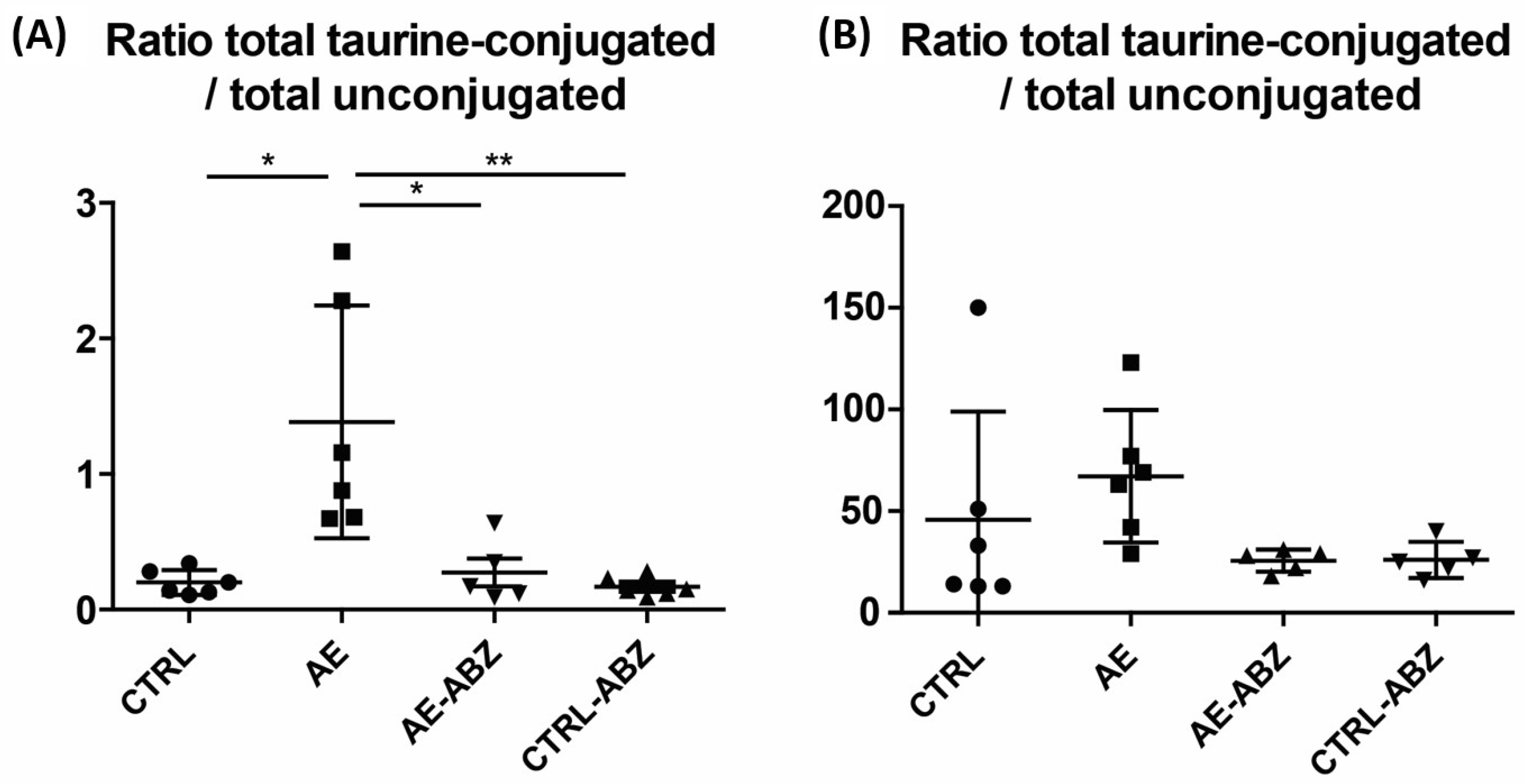
| Total taurine-conjugated/total unconjugated | 0.20 ± 0.09 | 1.38 ± 0.86 *,†,‡ | 0.17 ± 0.07 | 0.27 ± 0.23 |
| TCA/CA | 0.15 ± 0.14 | 1.63 ± 1.33 *,†,‡ | 0.25 ± 0.26 | 0.09 ± 0.02 |
| TαMCA/αMCA | 0.72 ± 0.63 | 9.17 ± 5.74 *,†,‡ | 1.50 ± 1.04 | 0.58 ± 0.17 |
| TβMCA/βMCA | 0.17 ± 0.10 | 1.63 ± 1.18 *,†,‡ | 0.21 ± 0.19 | 0.12 ± 0.09 |
| Liver tissue | CTRL (n = 6) (mean ± SD) | AE (n = 6) (mean ± SD) | AE-ABZ (n = 5) (mean ± SD) | CTRL-ABZ (n = 5) (mean ± SD) |
| Total taurine-conjugated/total unconjugated | 46 ± 53 | 67 ± 33 | 26 ± 5 | 26 ± 9 |
| TCA/CA | 156 ± 312 | 359 ± 146 † | 50 ± 14 | 33 ± 13 |
| TαMCA/αMCA | 47 ± 53 | 104 ± 55 | 27 ± 9 | 29 ± 15 |
| TβMCA/βMCA | 33 ± 23 | 41 ± 22 | 16 ± 4 | 21 ± 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, C.; Jebbawi, F.; Weingartner, M.; Wang, J.; Stücheli, S.; Stieger, B.; Gottstein, B.; Beldi, G.; Lundström-Stadelmann, B.; Odermatt, A. Impact on Bile Acid Concentrations by Alveolar Echinococcosis and Treatment with Albendazole in Mice. Metabolites 2021, 11, 442. https://doi.org/10.3390/metabo11070442
Gómez C, Jebbawi F, Weingartner M, Wang J, Stücheli S, Stieger B, Gottstein B, Beldi G, Lundström-Stadelmann B, Odermatt A. Impact on Bile Acid Concentrations by Alveolar Echinococcosis and Treatment with Albendazole in Mice. Metabolites. 2021; 11(7):442. https://doi.org/10.3390/metabo11070442
Chicago/Turabian StyleGómez, Cristina, Fadi Jebbawi, Michael Weingartner, Junhua Wang, Simon Stücheli, Bruno Stieger, Bruno Gottstein, Guido Beldi, Britta Lundström-Stadelmann, and Alex Odermatt. 2021. "Impact on Bile Acid Concentrations by Alveolar Echinococcosis and Treatment with Albendazole in Mice" Metabolites 11, no. 7: 442. https://doi.org/10.3390/metabo11070442
APA StyleGómez, C., Jebbawi, F., Weingartner, M., Wang, J., Stücheli, S., Stieger, B., Gottstein, B., Beldi, G., Lundström-Stadelmann, B., & Odermatt, A. (2021). Impact on Bile Acid Concentrations by Alveolar Echinococcosis and Treatment with Albendazole in Mice. Metabolites, 11(7), 442. https://doi.org/10.3390/metabo11070442