Features of the Metabolic Profile of Saliva in Lung Cancer and COPD: The Effect of Smoking Status
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
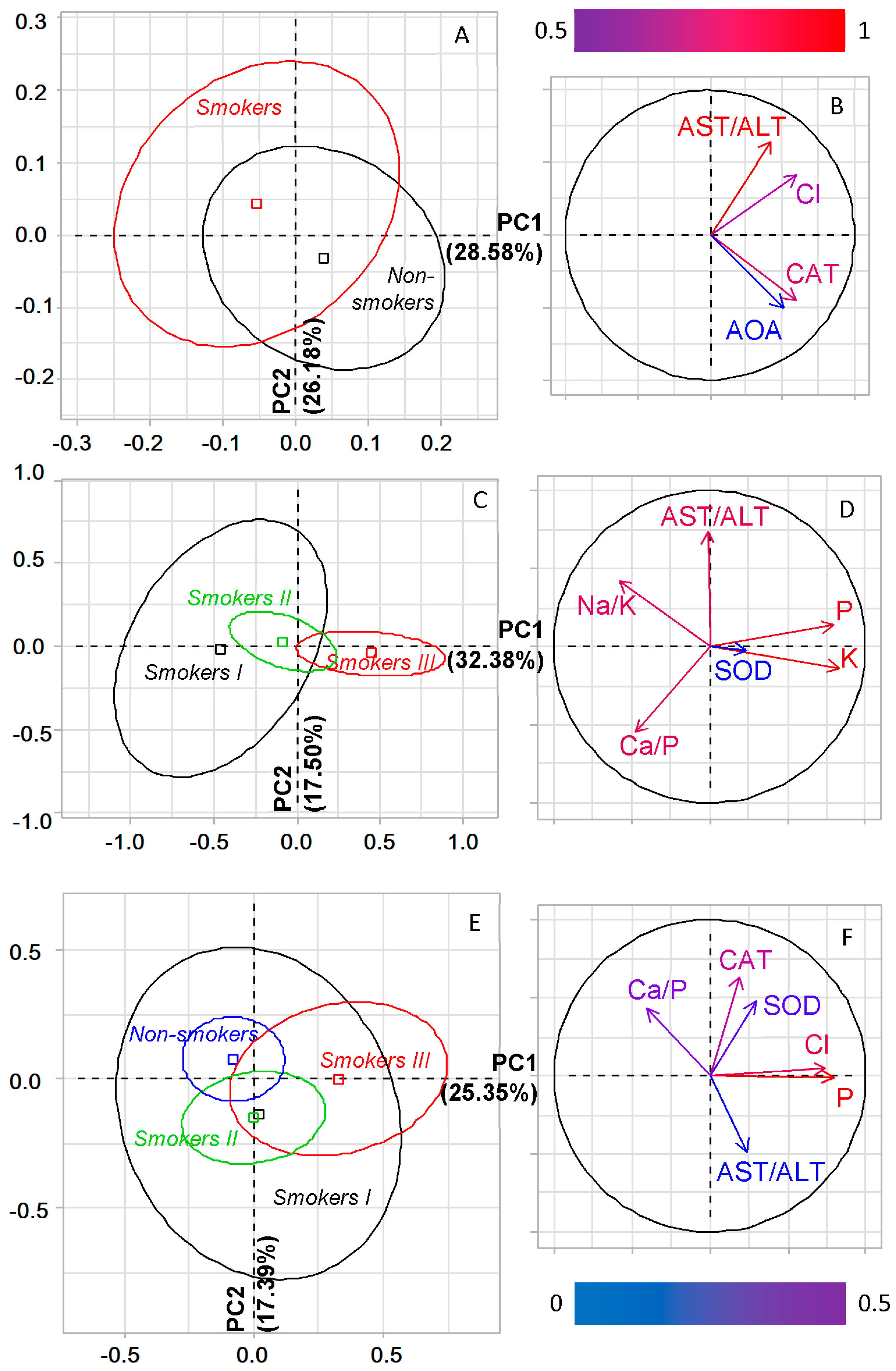
2.2. The Salivary Biochemical Composition of Lung Cancer Patients Depending on Smoking
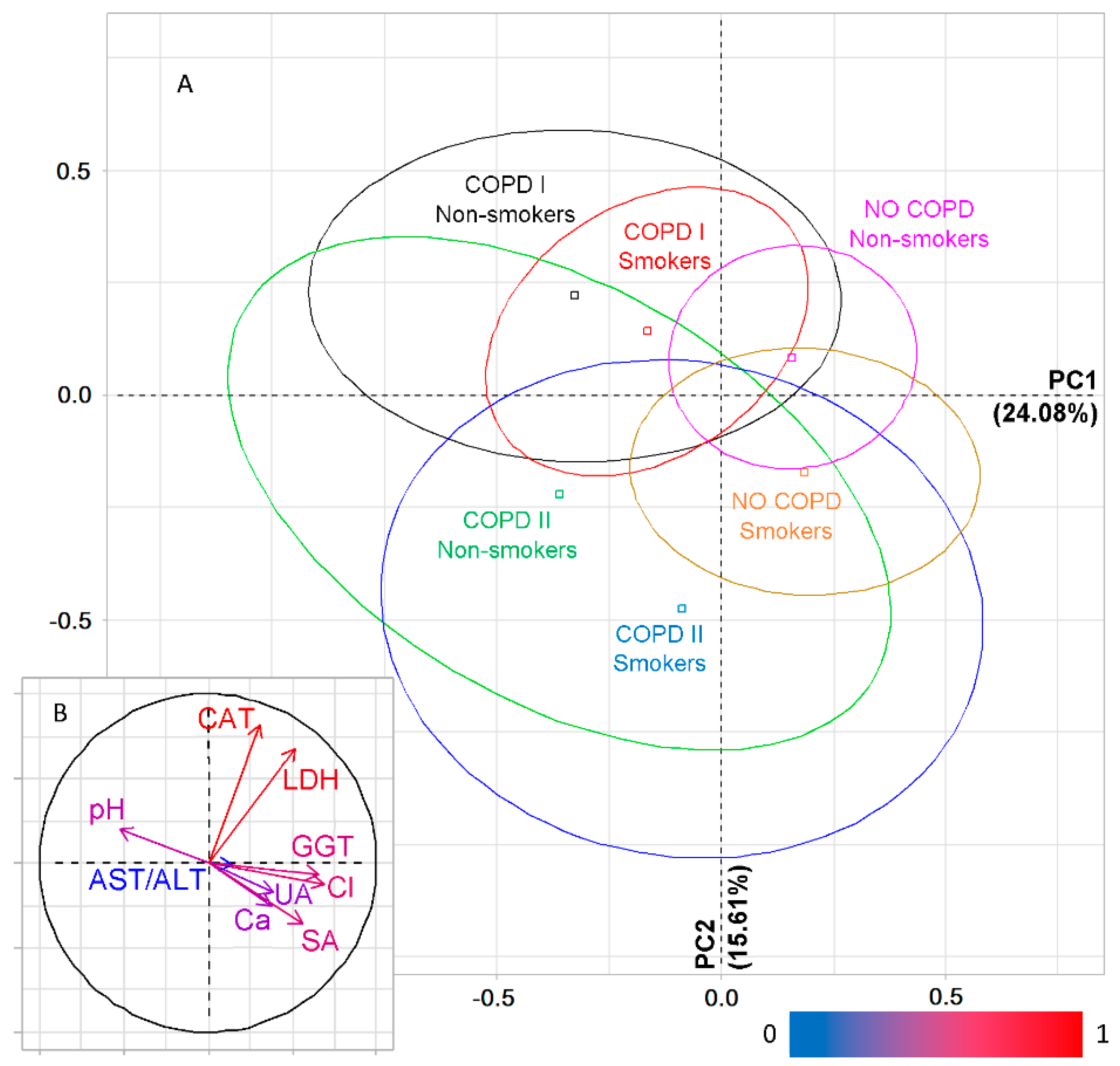
2.3. The Salivary Biochemical Composition in Lung Cancer Patients with COPD
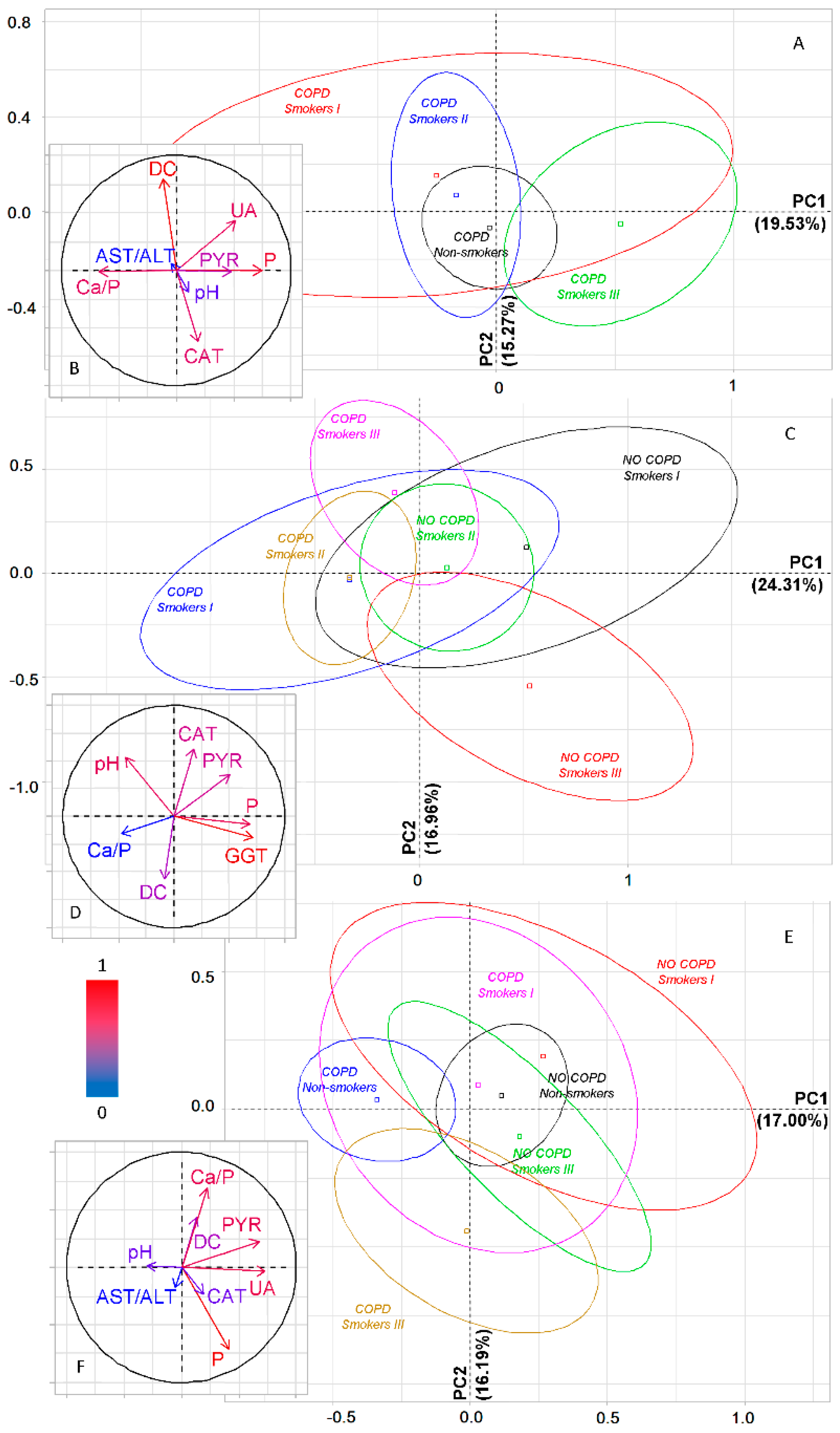
2.4. Features of the Salivary Biochemical Composition in Lung Cancer Patients with COPD Depending on Smoking
2.5. Metabolic Features of Salivary Composition in Lung Cancer and COPD, Depending on Smoking Experience
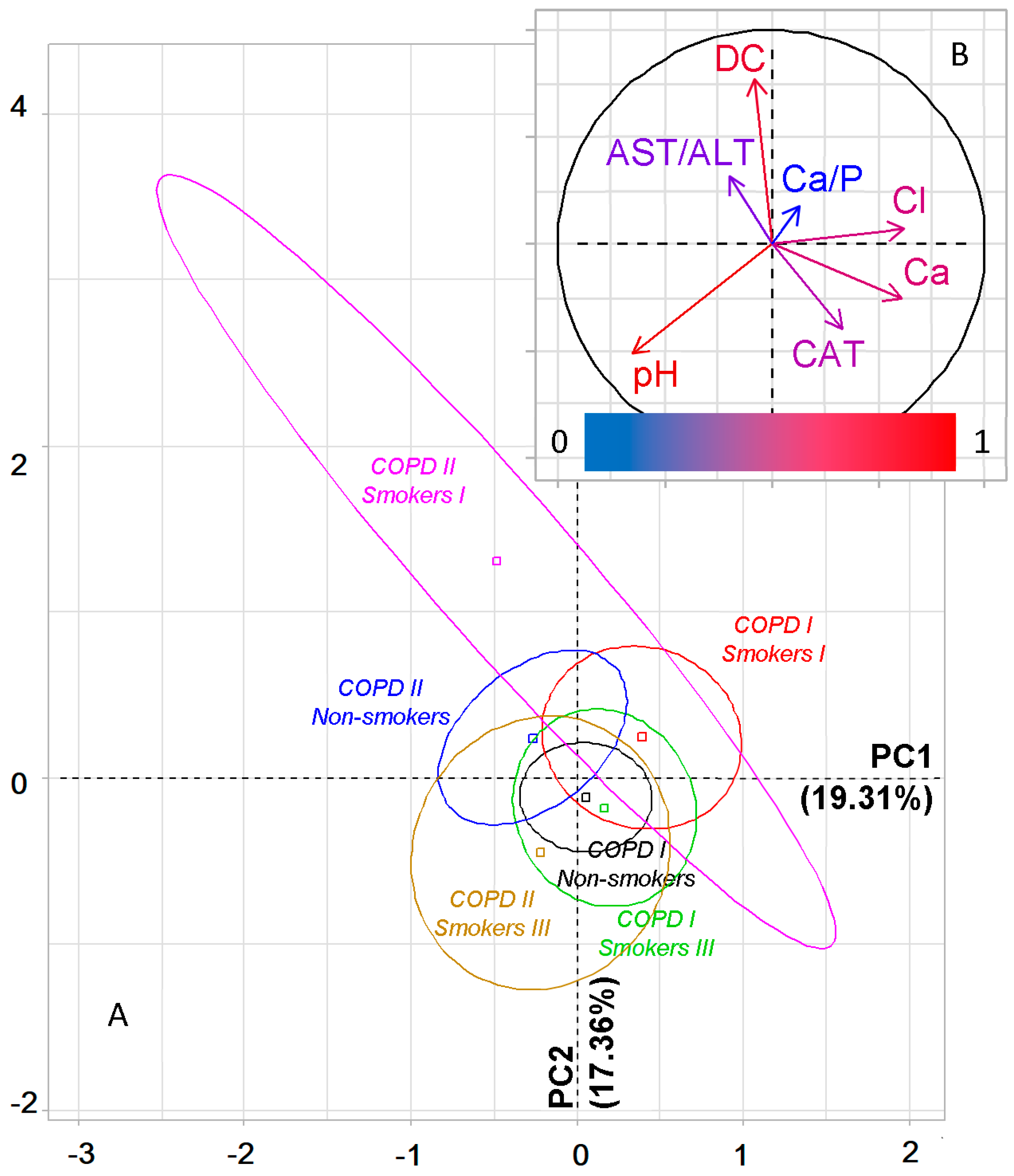
2.6. Metabolic Features of Saliva Taking into Account the Type of COPD and Smoking History
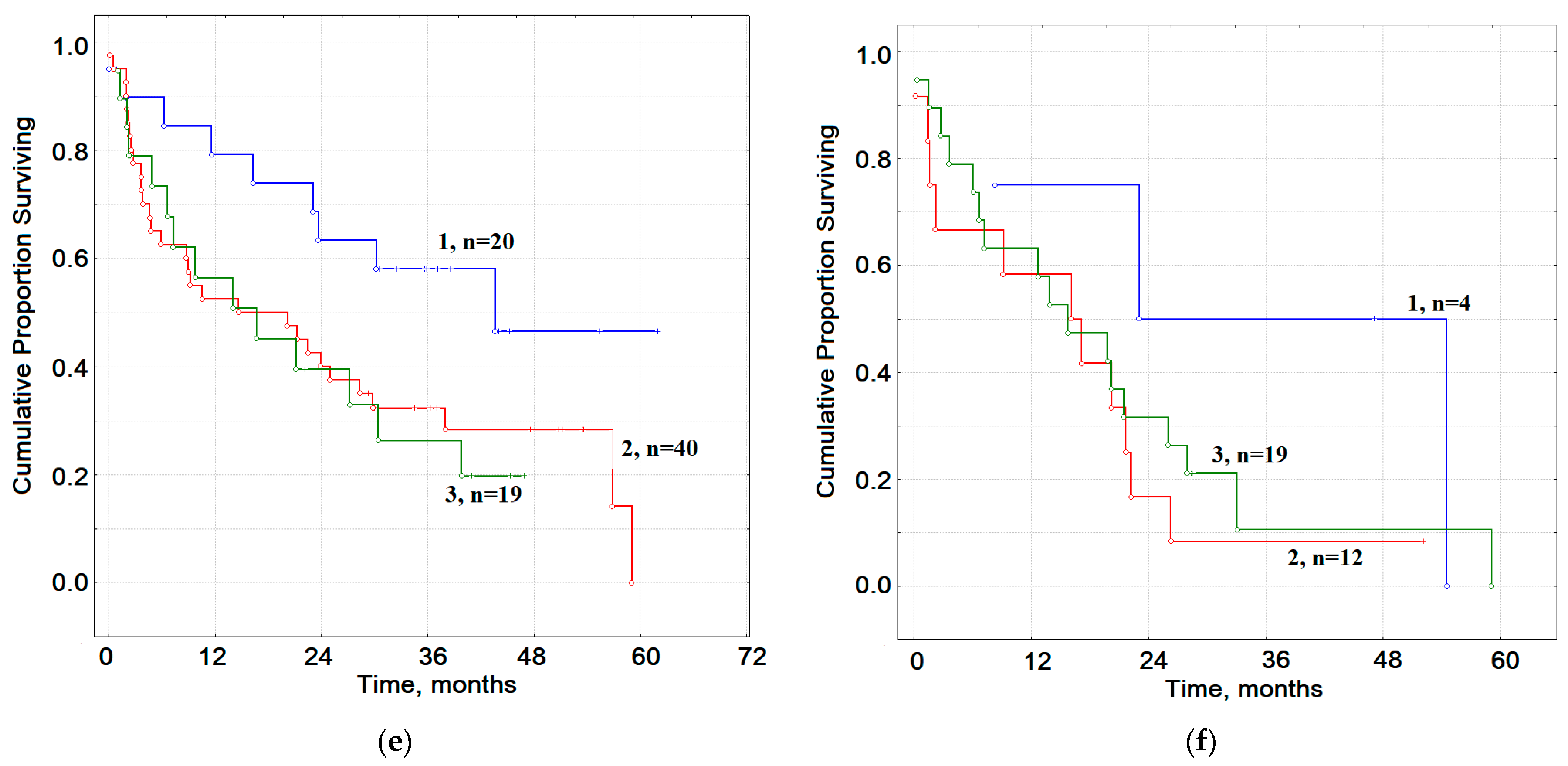
2.7. The Prognostic Value of Smoking in Groups of Patients with Lung Cancer and COPD of Varying Severity
3. Discussion
4. Materials and Methods
4.1. Study Design and Group Description
4.2. Collection, Processing and Storage of Saliva Samples
4.3. Biochemical Analysis of Saliva
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Abal Arca, J.; Parente Lamelas, I.; Almazán Ortega, R.; Blanco Pérez, J.; Toubes Navarro, M.E.; Marcos Velázquez, P. Lung cancer and COPD: A common combination. Arch. Bronconeumol. 2009, 45, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, R.S.; Stover, D.E.; Shi, W.; Venkatraman, E. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest 2006, 129, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Henschke, C.I.; Yip, R.; Boffetta, P.; Markowitz, S.; Miller, A.; Hanaoka, T.; Wu, N.; Zulueta, J.J.; Yankelevitz, D.F. CT screening for lung cancer: Importance of emphysema for never smokers and smokers. Lung Cancer 2015, 88, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Mouronte-Roibás, C.; Leiro-Fernández, V.; Fernández-Villar, A.; Botana-Rial, M.; Ramos-Hernández, C.; Ruano-Ravina, A. COPD, emphysema and the onset of lung cancer. A systematic review. Cancer Lett. 2016, 382, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Young, R.P.; Hopkins, R.J.; Christmas, T.; Black, P.N.; Metcalf, P.; Gamble, G.D. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir. J. 2009, 34, 380–386. [Google Scholar] [CrossRef] [PubMed]
- De-Torres, J.P.; Wilson, D.O.; Sanchez-Salcedo, P.; Weissfeld, J.L.; Berto, J.; Campo, A.; Alcaide, A.B.; García-Granero, M.; Celli, B.R.; Zulueta, J.J. Lung cancer in patients with chronic obstructive pulmonary disease. Development and validation of the COPD Lung Cancer Screening Score. Am. J. Respir. Crit. Care Med. 2015, 191, 285–291. [Google Scholar] [CrossRef]
- De-Torres, J.P.; Marín, J.M.; Casanova, C.; Cote, C.; Carrizo, S.; Cordoba-Lanus, E.; Baz-Dávila, R.; Zulueta, J.J.; Aguirre-Jaime, A.; Saetta, M.; et al. Lung cancer in patients with chronic obstructive pulmonary disease–incidence and predicting factors. Am. J. Respir. Crit. Care Med. 2011, 184, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Mannino, D.M.; Aguayo, S.M.; Petty, T.L.; Redd, S.C. Low lung function and incident lung cancer in the United States: Data From the First National Health and Nutrition Examination Survey follow-up. Arch. Intern. Med. 2003, 163, 1475–1480. [Google Scholar] [CrossRef]
- Yao, H.; Rahman, I. Current concepts on the role of inflammation in COPD and lung cancer. Curr. Opin. Pharmacol. 2009, 9, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Reiman, J.M.; Kmieciak, M.; Manjili, M.H.; Knutson, K.L. Tumor immunoediting and immunosculpting pathways to cancer progression. Semin. Cancer Biol. 2007, 17, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.J.; Li, H.; Cho, S.N.; Dishop, M.K.; Wistuba, I.I.; Ji, L.; Kurie, J.M.; Dickey, B.F.; Demayo, F.J. Promotion of lung carcinogenesis by COPD-like airway inflammation in a K-ras induced mouse model. Am. J. Respir. Cell Mol. Biol. 2009, 40, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Parimon, T.; Chien, J.W.; Bryson, C.L.; McDonell, M.B.; Udris, E.M.; Au, D.H. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 175, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Solimini, N.L.; Elledge, S.J. Principles of cancer therapy: Oncogene and non-oncogene addiction. Cell 2009, 136, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Punturieri, A.; Szabo, E.; Croxton, T.L.; Shapiro, S.D.; Dubinett, S.M. Lung cancer and chronic obstructive pulmonary disease: Needs and opportunities for integrated research. J. Natl. Cancer Inst. 2009, 101, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Sarf, E.A.; Kosenok, V.K.; Gundyrev, I.A. Biochemical Markers of Saliva in Lung Cancer: Diagnostic and Prognostic Perspectives. Diagnostics 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Kisluk, J.; Ciborowski, M.; Niemira, M.; Kretowski, A.; Niklinski, J. Proteomics biomarkers for non-small cell lung cancer. J. Pharm. Biomed. Anal. 2014, 101, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qian, L.; Ji, L.; Liu, S.; Wahid, A.; Jiang, X.; Sohail, A.; Ji, Y.; Zhang, Y.; Wang, P.; et al. Affinity chromatography assisted comprehensive phosphoproteomics analysis of human saliva for lung cancer. Anal. Chim. Acta 2020, 1111, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Grassl, N.; Kulak, N.A.; Pichler, G.; Geyer, P.E.; Jung, J.; Schubert, S.; Sinitcyn, P.; Cox, J.; Mann, M. Ultra-deep and quantitative saliva proteome reveals dynamics of the oral microbiome. Genome Med. 2016, 8, 44. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, L.; Zhou, H.; Lee, J.M.; Garon, E.B.; Wong, D.T. Proteomic analysis of human saliva from lung cancer patients using two-dimensional difference gel electrophoresis and mass spectrometry. Mol. Cell. Proteomics 2012, 11, M111-012112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, H.; Zhou, H.; Santiago, S.; Lee, J.M.; Garon, E.B.; Yang, J.; Brinkmann, O.; Yan, X.; Akin, D.; et al. Development of transcriptomic biomarker signature in human saliva to detect lung cancer. Cell. Mol. Life Sci. 2012, 69, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Lin, C.C.; Joon, A.; Feng, Z.; Troche, G.; Lira, M.E.; Chia, D.; Mao, M.; Ho, C.L.; Su, W.C.; et al. Noninvasive saliva-based EGFR gene mutation detection in patients with lung cancer. Am. J. Respir. Crit. Care Med. 2014, 190, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Urbanowicz, K.E.; Wei, F.; Rao, S.L.; Kim, J.; Shin, H.; Cheng, J.; Tu, M.; Wong, D.T.W.; Kim, Y. Clinical validity of saliva and novel technology for cancer detection. BBA Rev. Cancer 2019, 1872, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, F.; Schafer, C.; Wong, D.T. Detection of tumor cell-specific mRNA and protein in exosome-like microvesicles from blood and saliva. PLoS ONE 2014, 9, e110641. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huo, C.; Qiao, Z.; Shang, Z.; Uzzaman, A.; Liu, S.; Jiang, X.; Fan, L.Y.; Ji, L.; Guan, X.; et al. Comparative proteomic analysis of exosomes and microvesicles in human saliva for lung cancer. J. Proteome Res. 2018, 17, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, S.; Qiao, Z.; Shang, Z.; Xia, Z.; Niu, X.; Qian, L.; Zhang, Y.; Fan, L.; Cao, C.X.; et al. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Anal. Chim. Acta 2017, 982, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mu, X.; Wang, Y.; Zhu, D.; Zhang, J.; Liang, C.; Chen, B.; Wang, J.; Zhao, C.; Zuo, Z.; et al. Dysbiosis of the Salivary Microbiome Is Associated With Non-smoking Female Lung Cancer and Correlated With Immunocytochemistry Markers. Front. Oncol. 2018, 8, 520. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Sarf, E.A.; Solomatin, D.V.; Kosenok, V.K. Salivary Metabolic Profile of Patients with Lung Cancer, Chronic Obstructive Pulmonary Disease of Varying Severity and Their Comorbidity: A Preliminary Study. Diagnostics 2020, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Marulli, G.; Nannini, N.; Pasello, G.; Lunardi, F.; Balestro, E.; Perissinotto, E.; Rebusso, A.; Saetta, M.; Rea, F.; et al. COPD-related adenocarcinoma presents low aggressiveness morphological and molecular features compared to smoker tumours. Lung Cancer 2014, 86, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Gemine, R.E.; Ghosal, R.; Collier, G.; Parry, D.; Campbell, I.; Davies, G.; Davies, K.; Lewis, K.E. Longitudinal study to assess impact of smoking at diagnosis and quitting on 1-year survival for people with non-small cell lung cancer. Lung Cancer 2019, 129, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Casal-Mouriño, A.; Valdés, L.; Barros-Dios, J.M.; Ruano-Ravina, A. Lung cancer survival among never smokers. Cancer Lett. 2019, 451, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Paris, C.; Clement-Duchene, C.; Vignaud, J.M.; Gislard, A.; Stoufflet, A.; Bertrand, O.; Thiberville, L.; Grosdidier, G.; Martinet, Y.; Benichou, J.; et al. Relationships between lung adenocarcinoma and gender, age, smoking and occupational risk factors: A case–case study. Lung Cancer 2010, 68, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.N.; Forey, B.A.; Coombs, K.J. Systematic review with meta-analysis of the epidemiological evidence in the 1900 relating smoking to lung cancer. BMC Cancer 2012, 12, 1–90. [Google Scholar] [CrossRef] [PubMed]
- Zaridze, D.G.; Maksimovich, D.M. Prevention of malignant neoplasms. Adv. Mol. Oncol. 2017, 4, 8–25. [Google Scholar] [CrossRef]
- Martín-Sánchez, J.C.; Bilal, U.; Clèries, R.; Lidón-Moyano, C.; Fu, M.; González-de Paz, L.; Franco, M.; Fernandez, E.; Martínez-Sánchez, J.M. Modelling lung cancer mortality rates from smoking prevalence: Fill in the gap. Cancer Epidemiol. 2017, 49, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Altynbaeva, E.I.; Teplova, S.N.; Kochengina, S.A. Quantitative evaluation of immunocytes and interleukin-17 levels in saliva from smokers at the early stages of chronic obstructive pulmonary disease. Med. Immunol. 2014, 12, 399–404. [Google Scholar] [CrossRef][Green Version]
- Callejón-Leblic, B.; Pereira-Vega, A.; Vazquez-Gandullo, E.; Sánchez-Ramos, J.L.; Gómez-Ariza, J.L.; García-Barrera, T. Study of the metabolomic relationship between lung cancer and chronic obstructive pulmonary disease based on direct infusion mass spectrometry. Biochimie 2019, 157, 111–122. [Google Scholar] [CrossRef]
- Watson, J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol. 2013, 3, 120144. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Jofré, M.; Rueda, J.R.; Asenjo-Lobos, C.; Madrid, E.; Cosp, X.B. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst. Rev. 2020, 3, CD002141. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agusti, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Munther, S. The effects of cigarette smoking and exercise on total salivary antioxidant activity. Saudi Dent. J. 2019, 31, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]


| Smoking Status | Subgroups | Number of Patients | ||
|---|---|---|---|---|
| NO COPD | COPD I | COPD II | ||
| Non-smokers | n (%) | 147 (65.9) | 49 (43.0) | 23 (45.1) |
| Age, years | 60.0 [55.0; 64.5] | 57.0 [53.0; 61.0] | 63.0 [58.0; 65.0] | |
| Smokers I | n (%) | 14 (6.3) | 16 (14.0) | 4 (7.8) |
| Age, years | 57,5 [48.0; 60.0] | 54.0 [52.0; 59.0] | 59.5 [54.5; 65.5] | |
| Smokers II | n (%) | 39 (17.5) | 35 (30.7) | 10 (19.6) |
| Age, years | 62.0 [55.0; 63.0] | 59.0 [54.0; 64.0] | 60.5 [55.5; 63.5] | |
| Smokers III | n (%) | 23 (10.3) | 14 (12.3) | 14 (27.5) |
| Age, years | 63.5 [61.0; 68.0] *,** | 62.0 [59.0; 66.0] ** | 65.0 [61.0; 69.0] ** | |
| Indicators | Smoking | LC+SM, n = 76 | COPDI+SM, n = 65 | COPDII+SM, n = 28 | Kruskal–Wallis Test (H, p) |
|---|---|---|---|---|---|
| LC+NO SM, n = 147 | COPDI+NO SM, n = 49 | COPDII+NO SM, n = 23 | |||
| Electrolytes | |||||
| pH | YES | 6.44 [6.22; 6.64] | 6.60 [6.35; 6.82] | 6.60 [6.29; 6.91] | 11.55, 0.0415 * |
| NO | 6.47 [6.20; 6.71] | 6.52 [6.22; 6.88] | 6.68 [6.21; 7.06] | ||
| Calcium, mmol/L | YES | 1.50 [1.03; 2.13] | 1.28 [0.86; 1.80] | 1.35 [0.99; 1.76] | 11.17, 0.0481 * |
| NO | 1.44 [1.07; 1.80] | 1.48 [1.03; 1.82] | 1.21 [0.81; 1.47] | ||
| Phosphorus, mmol/L | YES | 4.50 [3.28; 6.26] | 4.07 [3.10; 5.43] | 4.90 [3.51; 6.69] | 5.405, 0.3685 |
| NO | 4.62 [3.53; 5.72] | 4.61 [3.25; 5.54] | 4.26 [3.17; 4.98] | ||
| Ca/P ratio, c.u. | YES | 0.32 [0.22; 0.48] | 0.30 [0.18; 0.50] | 0.28 [0.17; 0.38] | 4.765, 0.4452 |
| NO | 0.32 [0.23; 0.47] | 0.35 [0.23; 0.48] | 0.28 [0.20; 0.44] | ||
| Potassium, mmol/L | YES | 13.2 [8.9; 16.3] | 13.1 [9.9; 14.9] | 14.3 [10.8; 19.9] | 4.469, 0.4841 |
| NO | 12.5 [9.9; 15.7] | 12.4 [8.3; 16.9] | 12.5 [8.5; 15.8] | ||
| Na/K ratio, c.u. | YES | 0.72 [0.45; 1.50] | 0.89 [0.48; 1.52] | 0.57 [0.48; 1.01] | 3.871, 0.5682 |
| NO | 0.75 [0.47; 1.13] | 0.70 [0.44; 0.94] | 0.84 [0.46; 1.39] | ||
| Chlorides, mmol/L | YES | 28.9 [22.3; 37.3] | 29.5 [24.0; 38.0] | 31.6 [21.0; 36.9] | 9.752, 0.0826 ** |
| NO | 28.0 [22.3; 33.7] | 24.7 [18.0; 31.0] | 28.0 [20.0; 34.6] | ||
| Magnesium, mmol/L | YES | 0.311 [0.231; 0.383] | 0.291 [0.228; 0.339] | 0.302 [0.200; 0.372] | 6.395, 0.2697 |
| NO | 0.311 [0.246; 0.390] | 0.286 [0.219; 0.379] | 0.277 [0.231; 0.301] | ||
| Protein Metabolism | |||||
| Uric acid, μmol/L | YES | 67.4 [35.3; 166.4] | 79.1 [37.1; 159.1] | 113.9 [34.5; 196.5] | 10.83, 0.0550 ** |
| NO | 97.6 [46.3; 180.8] | 53.5 [22.3; 121.3] | 94.0 [34.6; 180.8] | ||
| Sialic acids, mmol/L | YES | 0.201 [0.119; 0.287] | 0.146 [0.085; 0.229] | 0.183 [0.095; 0.317] | 9.977, 0.0759 ** |
| NO | 0.177 [0.104; 0.287] | 0.114 [0.061; 0.269] | 0.189 [0.092; 0.317] | ||
| Pyruvic acid, μmol/L | YES | 16.91 [10.54; 25.00] | 13.48 [9.31; 18.63] | 12.62 [8.82; 16.91] | 6.512, 0.2595 |
| NO | 15.20 [10.29; 21.81] | 14.22 [8.33; 18.87] | 13.97 [8.82; 16.67] | ||
| Enzymes | |||||
| AST/ALT ratio, c.u. | YES | 1.19 [0.88; 1.54] | 1.29 [1.02; 1.75] | 1.02 [0.76; 1.56] | 10.02, 0.0746 ** |
| NO | 1.38 [1.04; 1.73] | 1.33 [0.97; 1.66] | 1.13 [1.01; 1.34] | ||
| LDH, U/L | YES | 1144.0 [615.9; 1619.0] | 1248.5 [625.3; 1715.5] | 691.6 [462.3; 1254.1] | 9.959, 0.0764 ** |
| NO | 1265.0 [524.2; 2022.0] | 1110.0 [605.2; 1761.7] | 728.6 [469.3; 1277.5] | ||
| GGT, U/L | YES | 23.7 [19.4; 26.8] | 21.6 [17.8; 25.1] | 20.0 [18.1; 22.6] | 9.427, 0.0932 ** |
| NO | 22.0 [18.2; 26.0] | 20.2 [16.0; 26.0] | 22.9 [19.0; 25.4] | ||
| Catalase, ncat/mL | YES | 2.53 [1.98; 3.92] | 2.69 [2.02; 3.85] | 2.40 [1.80; 2.95] | 10.16, 0.0708 ** |
| NO | 2.82 [2.14; 4.56] | 2.80 [2.32; 4.17] | 2.56 [1.72; 2.82] | ||
| SOD, c.u. | YES | 52.6 [18.4; 100.0] | 63.2 [26.3; 110.5] | 73.7 [42.1; 86.8] | 3.927, 0.5600 |
| NO | 68.4 [28.9; 136.8] | 68.4 [50.0; 113.2] | 63.2 [28.9; 139.5] | ||
| AOA, mmol/L | YES | 1.92 [1.38; 2.14] | 1.83 [1.41; 2.14] | 1.70 [1.60; 1.86] | 5.722, 0.3342 |
| NO | 1.67 [1.49; 1.85] | 1.78 [1.49; 1.87] | 1.40 [1.14; 1.90] | ||
| Lipoperoxidation Products | |||||
| DC, c.u. | YES | 4.06 [3.88; 4.22] | 3.92 [3.78; 4.18] | 3.92 [3.75; 4.09] | 8.655, 0.1236 |
| NO | 3.99 [3.78; 4.16] | 3.93 [3.68; 4.11] | 3.99 [3.85; 4.23] | ||
| SB, c.u. | YES | 0.564 [0.501; 0.670] | 0.538 [0.480; 0.656] | 0.574 [0.501; 0.661] | 5.069, 0.4075 |
| NO | 0.561 [0.488; 0.676] | 0.531 [0.463; 0.654] | 0.571 [0.534; 0.665] | ||
| MDA, μmol/L | YES | 7.01 [5.90; 9.32] | 6.32 [5.56; 8.89] | 8.12 [6.07; 10.85] | 8.473, 0.1320 |
| NO | 7.09 [5.73; 9.06] | 6.92 [5.64; 9.40] | 8.50 [5.90; 10.13] | ||
| Indicator | COPD Type | Smokers I n = 14/16/4 | Smokers II n = 39/35/10 | Smokers III n = 23/14/14 | Kruskal–Wallis Test (H, p) |
|---|---|---|---|---|---|
| Electrolytes | |||||
| pH | NO COPD | 6.41 [6.18; 6.55] | 6.48 [6.24; 6.69] | 6.36 [6.02; 6.64] | 15.35, 0.1670 |
| COPD I | 6.66 [6.32; 6.78] | 6.56 [6.38; 6.82] | 6.61 [6.35; 7.07] | ||
| COPD II | 6.54 [6.19; 6.75] | 6.57 [6.23; 6.80] | 6.62 [6.35; 7.17] | ||
| Calcium, mmol/L | NO COPD | 1.49 [0.91; 2.24] | 1.56 [1.17; 2.17] | 1.28 [0.97; 2.08] | 15.75, 0.1507 |
| COPD I | 1.53 [0.85; 1.90] | 1.26 [0.75; 1.79] | 1.28 [1.11; 1.68] | ||
| COPD II | 1.31 [0.68; 2.24] | 1.46 [1.10; 1.99] | 1.21 [0.90; 1.52] | ||
| Phosphorus, mmol/L | NO COPD | 4.32 [2.42; 4.79] | 4.68 [3.60; 6.04] | 5.01 [2.94; 8.84] | 13.75, 0.2473 |
| COPD I | 4.35 [3.16; 5.56] | 3.82 [2.93; 5.09] | 4.79 [3.58; 5.91] | ||
| COPD II | 4.70 [1.76; 7.37] | 3.92 [3.29; 5.67] | 5.68 [4.33; 7.76] | ||
| Ca/P ratio, c.u. | NO COPD | 0.35 [0.30; 0.49] | 0.33 [0.26; 0.51] | 0.24 [0.15; 0.42] | 15.39, 0.1653 |
| COPD I | 0.35 [0.16; 0.54] | 0.29 [0.21; 0.51] | 0.30 [0.18; 0.41] | ||
| COPD II | 0.30 [0.20; 6.57] | 0.34 [0.28; 0.47] | 0.24 [0.13; 0.30] | ||
| Potassium, mmol/L | NO COPD | 8.9 [6.7; 15.0] | 13.1 [9.2; 15.9] | 13.5 [8.9; 19.2] | 9.458, 0.5797 |
| COPD I | 12.5 [7.3; 14.1] | 14.1 [10.6; 15.9] | 13.0 [8.4; 14.9] | ||
| COPD II | 12.1 [8.5; 13.8] | 12.7 [10.3; 14.7] | 17.5 [12.3; 21.1] | ||
| Na/K ratio, c.u. | NO COPD | 1.70 [0.45; 2.00] | 0.67 [0.40; 1.50] | 0.72 [0.50; 1.33] | 12.47, 0.3293 |
| COPD I | 1.19 [0.64; 1.55] | 0.60 [0.39; 1.30] | 1.08 [0.59; 2.29] | ||
| COPD II | 1.01 [0.65; 1.29] | 0.65 [0.50; 1.03] | 0.49 [0.36; 0.82] | ||
| Chlorides, mmol/L | NO COPD | 28.9 [22.9; 41.7] | 29.2 [22.8; 37.6] | 25.1 [21.2; 35.6] | 15.65, 0.1546 |
| COPD I | 34.7 [26.2; 38.8] | 29.1 [22.5; 37.0] | 29.5 [22.9; 38.4] | ||
| COPD II | 23.1 [16.8; 29.1] | 35.6 [31.6; 38.9] | 28.5 [20.4; 35.1] | ||
| Magnesium, mmol/L | NO COPD | 0.317 [0.188; 0.368] | 0.311 [0.257; 0.384] | 0.280 [0.221; 0.381] | 7.678, 0.7419 |
| COPD I | 0.317 [0.248; 0.339] | 0.294 [0.232; 0.358] | 0.281 [0.197; 0.336] | ||
| COPD II | 0.256 [0.201; 0.335] | 0.309 [0.228; 0.442] | 0.305 [0.164; 0.372] | ||
| Protein Metabolism | |||||
| Uric acid, μmol/L | NO COPD | 84.4 [40.5; 167.2] | 63.1 [28.3; 165.5] | 61.9 [36.3; 160.9] | 14.59, 0.2019 |
| COPD I | 94.5 [29.7; 176.3] | 69.5 [41.0; 130.0] | 93.5 [34.7; 211.5] | ||
| COPD II | 49.3 [29.5; 125.9] | 109.4 [56.6; 172.4] | 144.4 [40.8; 283.9] | ||
| Sialic acids, mmol/L | NO COPD | 0.171 [0.098; 0.201] | 0.207 [0.137; 0.281] | 0.183 [0.119; 0.317] | 12.35, 0.3381 |
| COPD I | 0.146 [0.101; 0.204] | 0.116 [0.067; 0.232] | 0.183 [0.104; 0.226] | ||
| COPD II | 0.122 [0.098; 0.317] | 0.177 [0.078; 0.397] | 0.207 [0.095; 0.323] | ||
| Pyruvic acid, μmol/L | NO COPD | 17.65 [11.27; 32.60] | 13.11 [8.82; 26.23] | 19.24 [14.46; 22.79] | 15.20, 0.1735 |
| COPD I | 14.83 [8.58; 27.08] | 11.03 [8.70; 15.81] | 16.67 [13.48; 22.79] | ||
| COPD II | 12.75 [7.23; 48.16] | 14.22 [10.05; 17.40] | 11.64 [7.84; 16.42] | ||
| Enzymes | |||||
| AST/ALT ratio, c.u. | NO COPD | 1.25 [1.04; 1.61] | 1.19 [0.87; 1.57] | 1.08 [0.77; 1.38] | 15.28, 0.1699 |
| COPD I | 1.33 [1.07; 1.77] | 1.35 [1.03; 1.75] | 1.10 [0.82; 1.35] | ||
| COPD II | 1.07 [0.84; 14.30] | 1.28 [0.75; 1.59] | 0.97 [0.76; 1.50] | ||
| LDH, U/L | NO COPD | 1177.0 [535.9; 1958.5] | 1251.0 [649.7; 1549.0] | 907.6 [604.3; 1575.0] | 12.65, 0.3170 |
| COPD I | 1494.0 [697.8; 2321.0] | 981.0 [492.4; 1586.5] | 1278.5 [882.0; 1610.0] | ||
| COPD II | 547.7 [223.0; 1006.4] | 729.3 [488.5; 1197.0] | 726.3 [484.1; 1496.0] | ||
| GGT, U/L | NO COPD | 25.0 [20.7; 31.2] | 22.3 [19.5; 26.0] | 24.4 [18.8; 27.6] | 12.85, 0.3033 |
| COPD I | 20.2 [18.0; 24.5] | 20.0 [17.4; 25.2] | 22.6 [21.1; 23.3] | ||
| COPD II | 18.8 [16.7; 24.2] | 21.5 [19.3; 22.6] | 19.7 [17.9; 22.2] | ||
| Catalase, ncat/mL | NO COPD | 3.48 [2.52; 5.29] | 2.22 [2.00; 3.86] | 2.44 [1.53; 2.97] | 20.10, 0.0440 * |
| COPD I | 3.00 [1.76; 4.28] | 2.60 [2.10; 3.85] | 2.70 [2.20; 4.12] | ||
| COPD II | 2.16 [1.75; 2.81] | 2.12 [1.39; 2.64] | 2.61 [1.98; 3.18] | ||
| SOD, c.u. | NO COPD | 36.8 [13.2; 68.4] | 57.9 [18.4; 100.0] | 61.8 [27.6; 111.8] | 11.86, 0.3742 |
| COPD I | 50.0 [26.3; 71.1] | 65.8 [34.2; 160.5] | 57.9 [18.4; 165.8] | ||
| COPD II | 43.4 [39.5; 47.4] | 52.6 [40.8; 85.5] | 84.2 [67.1; 160.5] | ||
| AOA, mmol/L | NO COPD | 1.84 [1.32; 2.26] | 1.78 [1.45; 2.07] | 2.09 [1.92; 2.23] | 10.22, 0.5110 |
| COPD I | 2.03 [1.70; 2.10] | 1.67 [1.38; 2.10] | 2.13 [1.61; 2.99] | ||
| COPD II | No data | 1.76 [1.52; 1.99] | 1.70 [1.65; 1.78] | ||
| Lipoperoxidation Products | |||||
| DC, c.u. | NO COPD | 4.08 [3.82; 4.27] | 3.99 [3.86; 4.16] | 4.09 [3.98; 4.25] | 20.94, 0.0340 * |
| COPD I | 3.94 [3.81; 4.12] | 4.01 [3.76; 4.21] | 3.86 [3.70; 4.02] | ||
| COPD II | 3.94 [3.86; 4.03] | 4.05 [3.91; 4.32] | 3.75 [3.48; 3.97] | ||
| SB, c.u. | NO COPD | 0.528 [0.494; 0.615] | 0.567 [0.500; 0.671] | 0.570 [0.505; 0.641] | 10.69, 0.4698 |
| COPD I | 0.533 [0.467; 0.642] | 0.581 [0.487; 0.686] | 0.508 [0.483; 0.548] | ||
| COPD II | 0.533 [0.491; 0.677] | 0.611 [0.561; 0.666] | 0.536 [0.457; 0.652] | ||
| MDA, μmol/L | NO COPD | 7.35 [6.45; 10.85] | 7.01 [5.90; 9.32] | 6.75 [5.85; 8.97] | 13.22, 0.2792 |
| COPD I | 6.62 [5.64; 9.57] | 6.45 [5.56; 8.89] | 5.64 [5.13; 7.01] | ||
| COPD II | 9.57 [5.98; 14.53] | 9.79 [7.09; 11.62] | 7.99 [5.60; 10.30] | ||
| Feature | Lung Cancer, n (%) | |||
|---|---|---|---|---|
| ADC, n = 189 | SCC, n = 135 | NEC, n = 68 | ||
| Age, years | 61.0 [56.0; 65.0] | 59.0 [55.0; 66.5] | 55.0 [52.0; 60.0] | |
| Gender | ||||
| Male | 129 (68.3) | 128 (94.8) | 50 (73.5) | |
| Female | 60 (31.7) | 7 (5.2) | 18 (26.5) | |
| Stage | ||||
| St IA | 16 (8.5) | 3 (2.2) | 5 (7.4) | |
| St IB | 52 (27.5) | 28 (20.7) | 10 (14.7) | |
| St IIA+B | 23 (12.2) | 19 (14.1) | 6 (8.8) | |
| St IIIA | 25 (13.2) | 34 (25.2) | 10 (14.7) | |
| St IIIB | 17 (9.0) | 24 (17.8) | 17 (25.0) | |
| St IV | 56 (29.6) | 27 (20.0) | 20 (29.4) | |
| COPD | ||||
| No | 113 (59.8) | 69 (51.1) | 41 (60.3) | |
| Yes | GOLD I | 57 (30.2) | 40 (29.6) | 17 (25.0) |
| GOLD II | 18 (9.5) | 23 (17.1) | 10 (14.7) | |
| GOLD III | 1 (0.5) | 3 (2.2) | - | |
| Smoking | ||||
| No | 112 (59.3) | 64 (47.4) | 43 (63.2) | |
| Yes | Smokers I | 14 (7.4) | 4 (3.0) | 5 (7.4) |
| Smokers II | 18 (9.5) | 26 (19.3) | 11 (16.2) | |
| Smokers III | 45 (23.8) | 41 (30.3) | 9 (13.2) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bel’skaya, L.V.; Sarf, E.A.; Solomatin, D.V.; Kosenok, V.K. Features of the Metabolic Profile of Saliva in Lung Cancer and COPD: The Effect of Smoking Status. Metabolites 2021, 11, 289. https://doi.org/10.3390/metabo11050289
Bel’skaya LV, Sarf EA, Solomatin DV, Kosenok VK. Features of the Metabolic Profile of Saliva in Lung Cancer and COPD: The Effect of Smoking Status. Metabolites. 2021; 11(5):289. https://doi.org/10.3390/metabo11050289
Chicago/Turabian StyleBel’skaya, Lyudmila V., Elena A. Sarf, Denis V. Solomatin, and Victor K. Kosenok. 2021. "Features of the Metabolic Profile of Saliva in Lung Cancer and COPD: The Effect of Smoking Status" Metabolites 11, no. 5: 289. https://doi.org/10.3390/metabo11050289
APA StyleBel’skaya, L. V., Sarf, E. A., Solomatin, D. V., & Kosenok, V. K. (2021). Features of the Metabolic Profile of Saliva in Lung Cancer and COPD: The Effect of Smoking Status. Metabolites, 11(5), 289. https://doi.org/10.3390/metabo11050289







