Galantamine Quantity and Alkaloid Profile in the Bulbs of Narcissus tazetta and daffodil cultivars (Amaryllidaceae) Grown in Israel
Abstract
1. Introduction
2. Results
2.1. Extraction and Quantification of Galantamine from Narcissus Bulbs—Method Optimization and Validation
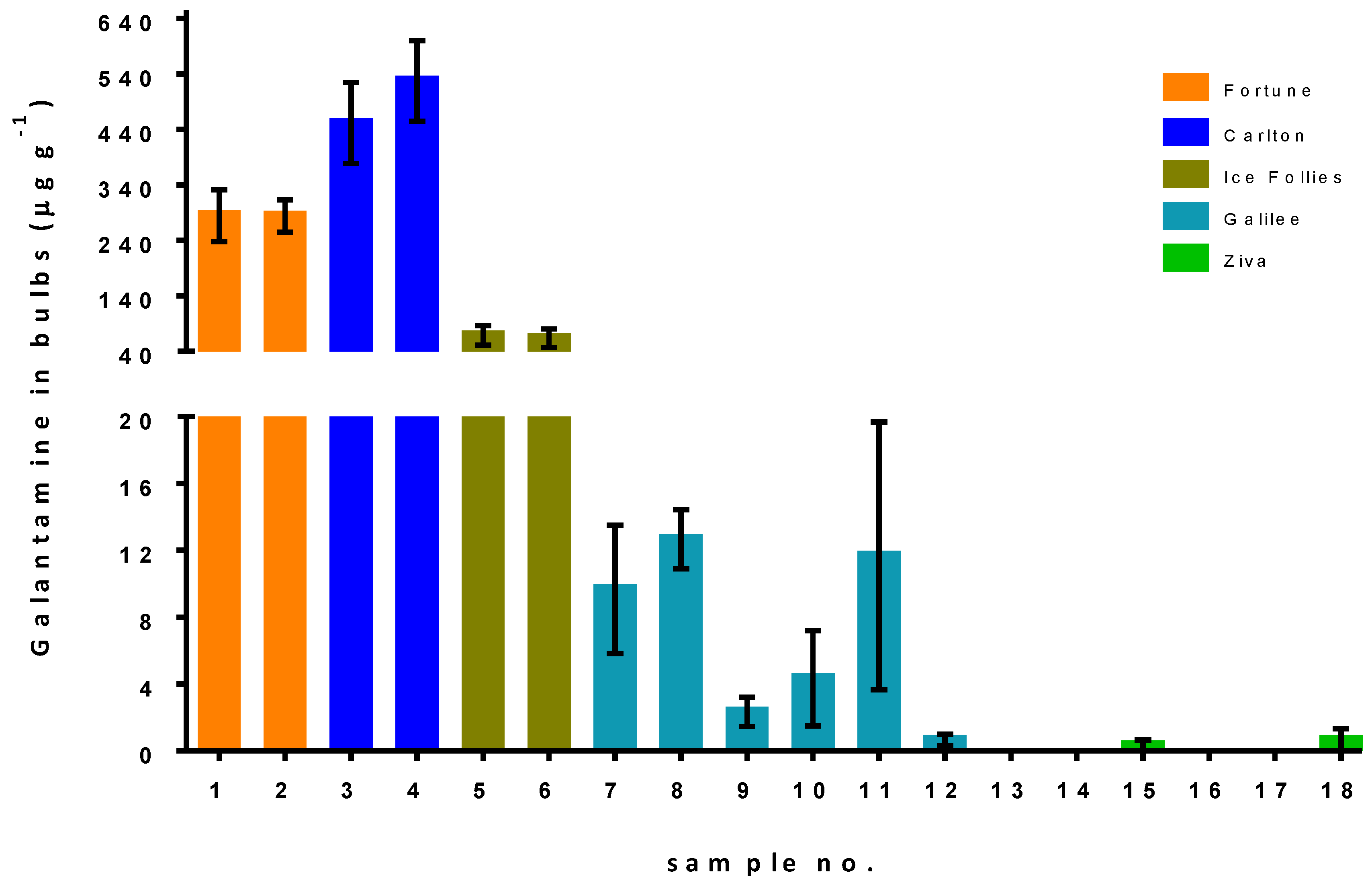
2.2. Galantamine Quantification in Different Narcissus Cultivars Grown under Different Conditions
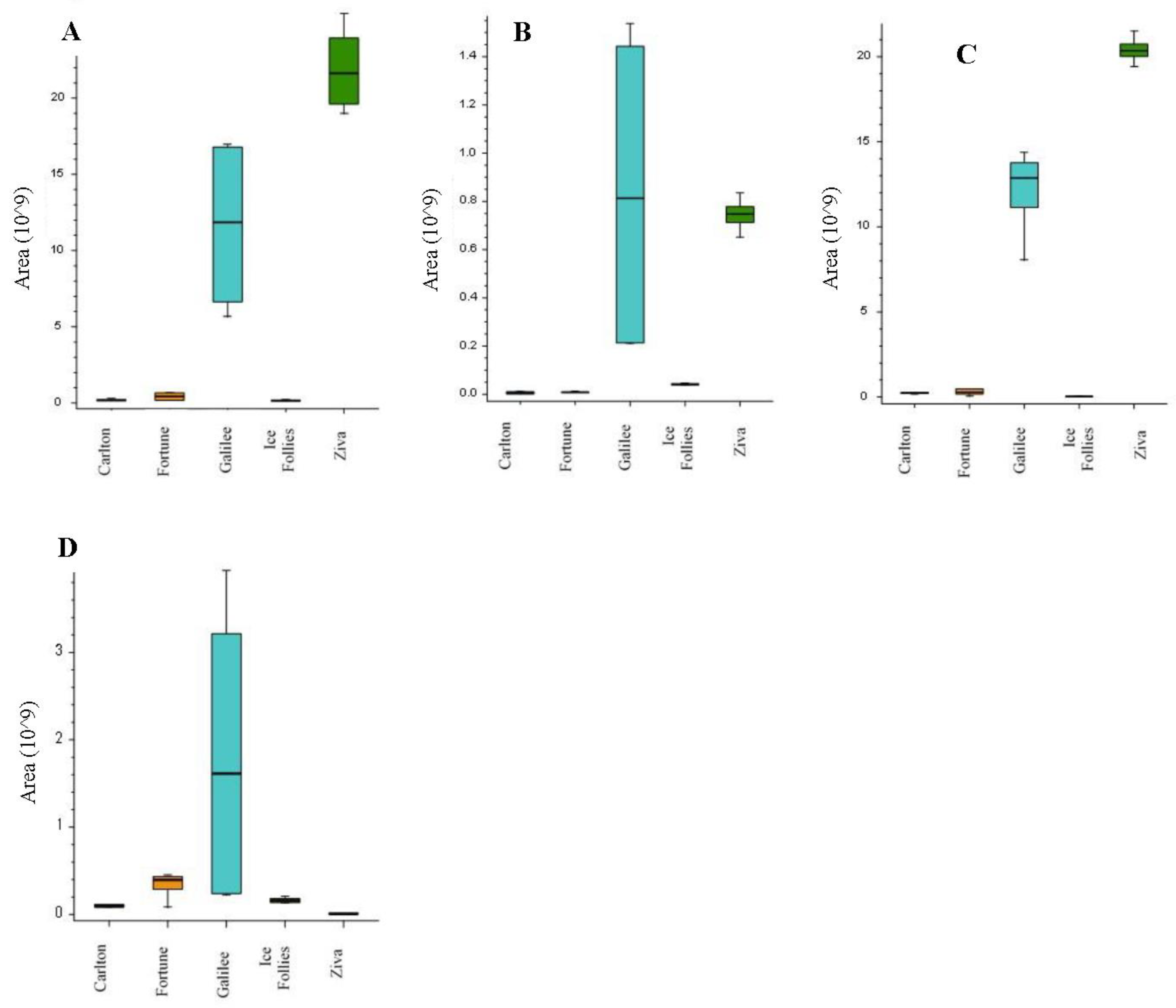
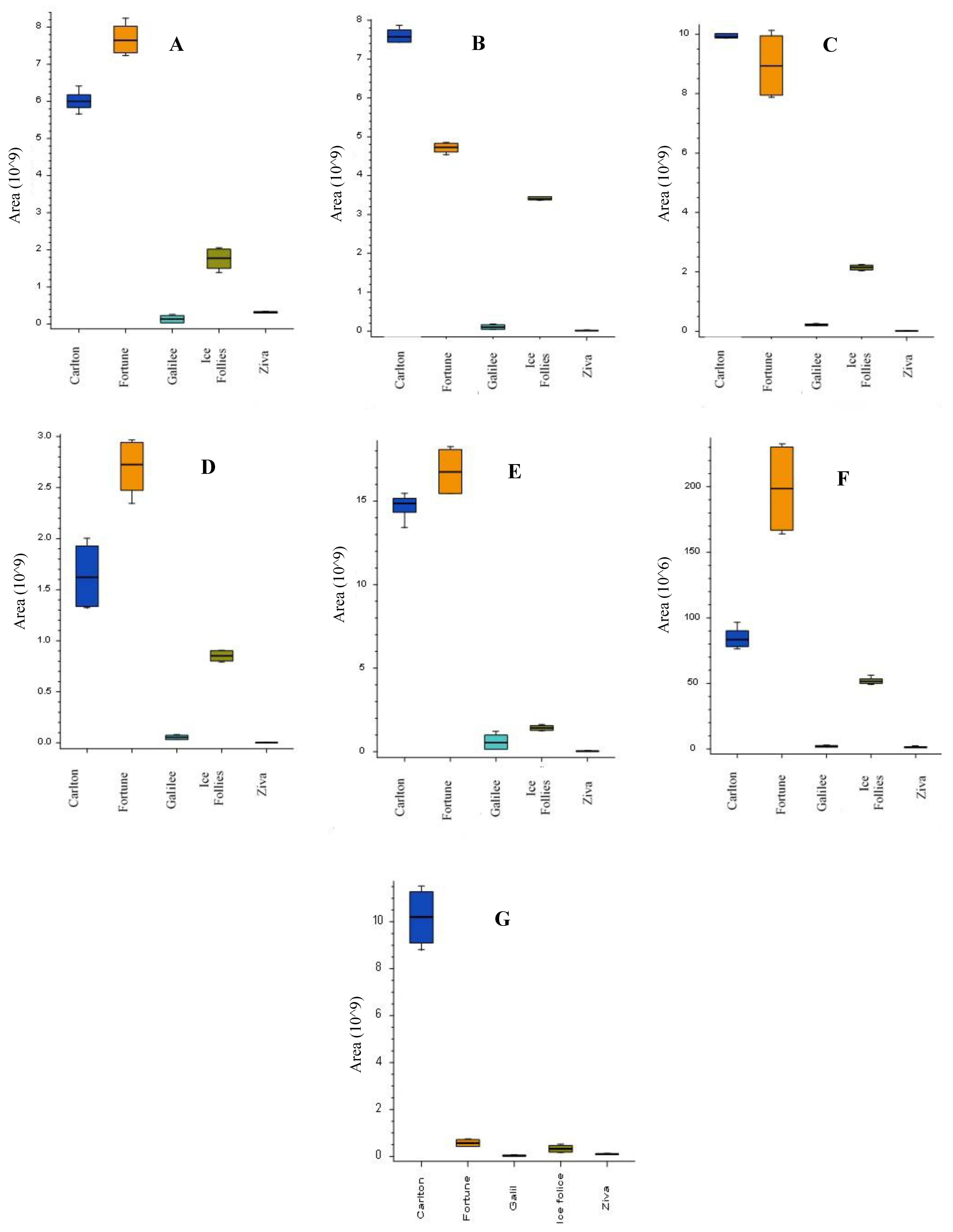
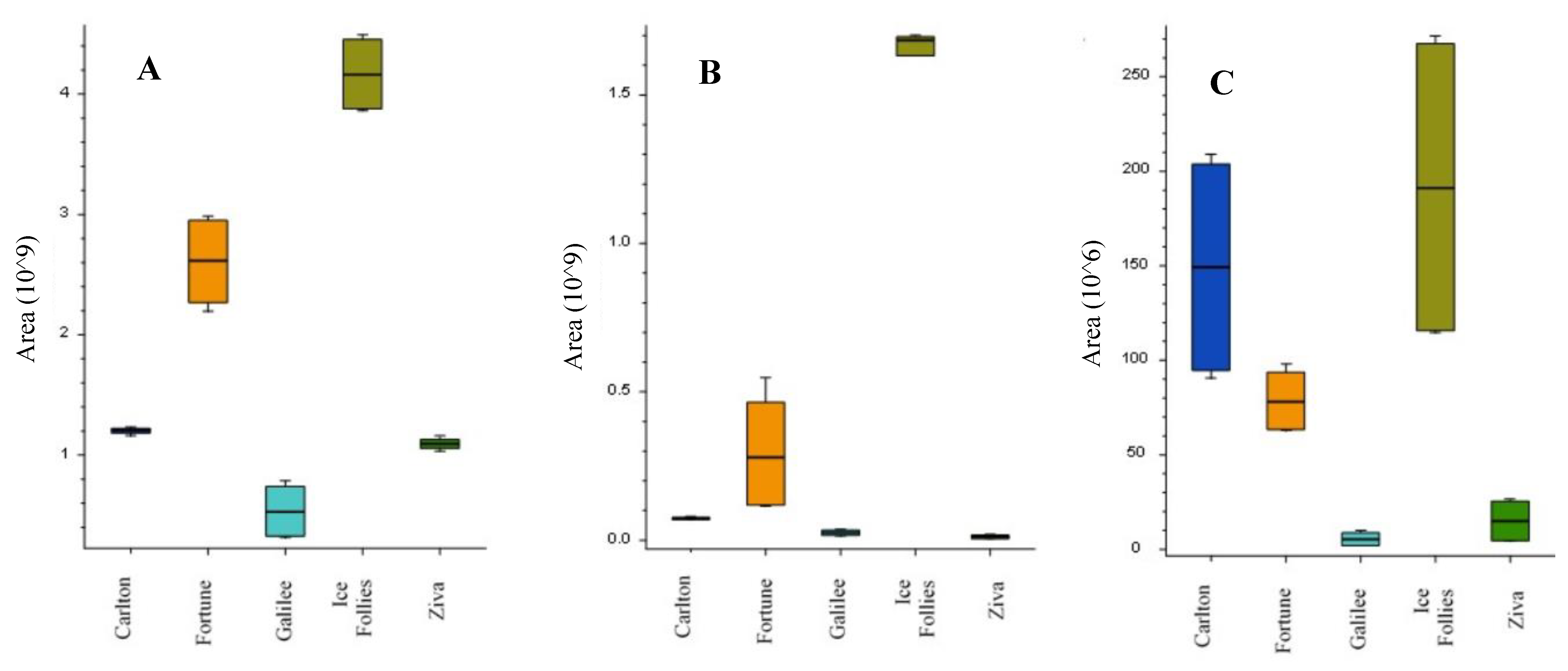
2.3. Alkaloid Profiles in Narcissus Cultivars Using LC-MS/MS Analysis
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Narcissus Cultivars, Growth Conditions and Bulb Collection
4.3. Extraction of Alkaloids from Narcissus Bulbs
4.4. Galantamine Extraction and Quantification Methods
4.5. Galantamine Quantification by UHPLC–HRMS
4.6. Galantamine Linearity and Sensitivity
4.7. Validation of Galantamine Extraction Method
4.8. Repeatability
4.9. Alkaloids Profile Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Takos, A.M.; Rook, F. Towards a Molecular Understanding of the Biosynthesis of Amaryllidaceae Alkaloids in Support of Their Expanding Medical Use. Int. J. Mol. Sci. 2013, 14, 11713–11741. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Okubo, H. (Eds.) Ornamental Geophytes: From Basic Science to Sustainable Production; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Rees, A.R. Ornamental Bulbs, Corms and Tubers; CABI Publishing: Wallingford, UK, 1992. [Google Scholar]
- Lubbe, A.; Pomahačová, B.; Choi, Y.H.; Verpoorte, R. Analysis of metabolic variation and galanthamine content in Narcissus bulbs by 1H NMR. Phytochem. Anal. 2010, 21, 66–72. [Google Scholar] [CrossRef]
- Santos, A.; Fidalgo, F.; Santos, I.; Salema, R. In vitro bulb formation of Narcissus asturiensis, a threatened species of the Amaryl-lidaceae. J. Hortic. Sci. Biotechnol. 2002, 77, 149–152. [Google Scholar] [CrossRef]
- Abbott, A. Dementia: A problem for our age. Nat. Cell Biol. 2011, 475, S2–S4. [Google Scholar] [CrossRef] [PubMed]
- Klosi, R.; Mersinllari, M.; Gavani, E. Galantamine content in Leucojum aestivum popula- tions grown in northwest Albania. Albanian J. Pharm. Sci. 2016, 3, 3–5. [Google Scholar]
- Zhang, X.; Huang, H.; Liang, X.; Huang, H.; Dai, W.; Shen, Y.; Yan, S.; Zhang, W. Analysis of Amaryllidaceae alkaloids from Crinum by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2903–2916. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.-H.; Jang, I.H.; Arasu, M.V.; Al-Dhabi, N.A.; Duraipandiyan, V.; Lee, N.-H.; Lee, S.; Kim, S.-J. Isolation and identification of alkaloids and anthocyanins from flower and bulb of Lycoris radiata using HPLC and LC-ESI-MS. J. Agric. Chem. Environ. 2013, 2, 22–26. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, C.; Guo, M. Comparative Analysis of Amaryllidaceae Alkaloids from Three Lycoris Species. Molecules 2015, 20, 21854–21869. [Google Scholar] [CrossRef]
- Chen, G.L.; Tian, Y.Q.; Wu, J.L.; Li, N.; Guo, M.Q. Antiproliferative activities of Amaryllidaceae alkaloids from Lycoris radiata tar-geting DNA topoisomerase I. Sci. Rep. 2016, 6, 38284. [Google Scholar] [CrossRef]
- Tarakemeh, A.; Azizi, M.; Rowshan, V.; Salehi, H.; Spina, R.; Dupire, F.; Arouie, H.; Laurain-Mattar, D. Screening of Amaryllidaceae alkaloids in bulbs and tissue cultures of Narcissus papyraceus and four varieties of N. tazetta. J. Pharm. Biomed. Anal. 2019, 172, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Ao, M.; Zhang, P.; Yu, L. Extracts of Lycoris aurea Induce Apoptosis in Murine Sarcoma S180 Cells. Molecules 2012, 17, 3723–3735. [Google Scholar] [CrossRef]
- Katoch, D.; Kumar, S.; Kumar, N.; Singh, B. Simultaneous quantification of Amaryllidaceae alkaloids from Zephyranthes grandiflora by UPLC–DAD/ESI-MS/MS. J. Pharm. Biomed. Anal. 2012, 71, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Lamoral-Theys, D.; Decaestecker, C.; Mathieu, V.; Dubois, J.; Kornienko, A.; Kiss, R.; Evidente, A.; Pottier, L. Lycorine and its Derivatives for Anti-cancer Drug Design. Mini-Rev. Med. Chem. 2010, 10, 41–50. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Galanthamine Pattern in Narcissus confuses Plants. Planta Medica 2003, 69, 1166–1168. [Google Scholar] [CrossRef]
- Ndagijimana, A.; Wang, X.; Pan, G.; Zhang, F.; Feng, H.; Olaleye, O. A review on indole alkaloids isolated from Uncaria rhyncho-phylla and their pharmacological studies. Fitoterapia 2013, 86, 35–47. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (Part I). TrAC-Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Daffodils as potential crops of galanthamine. Assessment of more than 100 ornamental varieties for their alkaloid content and acetylcholinesterase inhibitory activity. Ind. Crop. Prod. 2013, 43, 237–244. [Google Scholar] [CrossRef]
- Rahimi Khonakdari, M.; Mirjalili, M.H.; Gholipour, A.; Rezadoost, H.; Moridi Farimani, M. Quantification of galantamine in Nar-cissus tazetta and Galanthus nivalis (Amaryllidaceae) populations growing wild in Iran. Plant Genet Resour. Characterisa-tion Util. 2018, 16, 188–192. [Google Scholar] [CrossRef]
- Ka, S.; Koirala, M.; Mérindol, N.; Desgagné-Penix, I. Biosynthesis and Biological Activities of Newly Discovered Amaryllida-ceae Alkaloids. Molecules 2020, 25, 4901. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Desgagné-Penix, I. Transcriptome and metabolome profiling of Narcissus pseudonarcissus ‘King Alfred’ reveal components of Amaryllidaceae alkaloid metabolism. Sci. Rep. 2017, 7, 17356. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Y.; Xu, Y.; Ma, C.; Qin, C.; Zhang, L. Lycorine reduces mortality of human enterovirus 71-infected mice by inhibiting virus replication. Virol. J. 2011, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-N.; Zhang, Q.-Y.; Li, X.-D.; Xiong, J.; Xiao, S.-Q.; Wang, Z.; Zhang, Z.-R.; Deng, C.-L.; Yang, X.-L.; Wei, H.-P.; et al. Gemcitabine, lycorine and oxysophoridine inhibit novel coronavirus (SARS-CoV-2) in cell culture. Emerg. Microbes Infect. 2020, 9, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Berkov, S.; Georgiev, M.; Burrus, M.; Codina, C.; Bastida, J.; Ilieva, M.; Pavlov, A. Optimized nutrient medium for galanthamine pro-duction in Leucojum aestivum L. in vitro shoot system. Z. Nat. C J. Biosci. 2009, 64, 219–224. [Google Scholar]

| Purification Method | Recovery (%) | RSD (%) |
|---|---|---|
| LLE | 75 ± 3 | 7 |
| strata X-C cartridges | 64 ± 1 | 2 |
| mcx cartridges | 82 ± 2 | 4 |
| Sample No. | Cultivar | Normal Irrigation | Reduced Irrigation | No Pruning | High Pruning | Low Pruning |
|---|---|---|---|---|---|---|
| 1 | Fortune | V | ||||
| 2 | Fortune | V | ||||
| 3 | Carlton | V | ||||
| 4 | Carlton | V | ||||
| 5 | Ice Follies | V | ||||
| 6 | Ice Follies | V | ||||
| 7 | Galilee | V | V | |||
| 8 | Galilee | V | V | |||
| 9 | Galilee | V | V | |||
| 10 | Galilee | V | V | |||
| 11 | Galilee | V | V | |||
| 12 | Galilee | V | V | |||
| 13 | Ziva | V | V | |||
| 14 | Ziva | V | V | |||
| 15 | Ziva | V | V | |||
| 16 | Ziva | V | V | |||
| 17 | Ziva | V | V | |||
| 18 | Ziva | V | V |
| Peak Number | Name | Calculated Formula | Molecular Weight | RT [min] | [M+H]+ | MS/MS | References |
|---|---|---|---|---|---|---|---|
| 1 | Latifaliumin C | C16H19NO3 | 273.1362 | 1.751 | 274.144 | 256.133; 225.091; 217.085; 209.096; 199.075; 184.051 | [8,10] |
| 2 | Lycorine | C16H17NO4 | 287.1153 | 2.31 | 288.1227 | 270.1126; 252.102; 240.1057; 177.054; 147.044; 119.049. | [10,14] |
| 3 | Carinatine | C17H21NO4 | 303.1466 | 2.44 | 304.1539 | 286.1434; 268.13297; 193.086; 162.097; 147.044; 119.0495; 95.049; 91.055; 65.039 | FISh scoring coverage |
| 4 | Norgalantamine | C16H19NO3 | 273.1361 | 2.57 | 274.1435 | 231.1015; 213.0909; 198.067; 183.044; 169.065; 155.049; 152.062; 115.0546 | [10] |
| 5 | Hamayne | C16H17NO4 | 287.1154 | 2.99 | 288.1227 | 270.112; 226.086; 224.07; 211.076; 196.075; 168.0808; 153.069; 152.062; 119.049; 115.054; 91.054; 65.039. | [14] |
| 6 | Galantamine | C17H21NO3 | 287.1517 | 3.68 | 288.1233 | 270.1486; 257.117; 231.101; 225.0908; 213.0909; 198.067; 183.044; 181.065; 169.064; 165.0699; 152.062; 115.055; 91.055. | Standard [10] |
| 7 | Lycoramine | C17H23NO3 | 289.1673 | 3.84 | 290.1747 | 272.164; 233.117; 215.107; 189.091; 145.065; 128.063; 115.055; 95.049; 91.055. | [10] |
| 8 | Latifaliumin A | C16H17NO4 | 287.1153 | 4.47 | 288.1591 | 270.149; 231.102; 225.0914; 213.091; 198.067; 183.044; 169.065; 152.062; 119.049; 115.055; 95.049; 91.055 | [8] |
| 9 | Galanthine | C18H23NO4 | 317.1623 | 4.89 | 318.1698 | 300.159; 286.143; 268.133; 193.086; 162.067; 147.044; 119.049; 91.054; 65.039 | [14] |
| 10 | Narwedine | C17H19NO3 | 285.1362 | 5.24 | 286.1070 | 257.118; 255.00 229.085; 225.091; 197.096; 181.065; 158.073; 128.062; 115.055. | [10] |
| 11 | Narcissidine | C18H23NO5 | 333.157 | 5.24 | 334.1643 | 316.155; 298.144; 284.126; 266.117; 256.132; 242.117; 165.008; 124.076; 80.05 | FISh scoring coverage |
| 12 | Vittatin | C16H17NO3 | 271.1205 | 5.28 | 272.128 | 254.117; 226.086; 224.070; 196.075; 168.08; 139.054; 136.075; 128.062. | [10,14] |
| 13 | Tazettine | C18H21NO5 | 331.1416 | 5.33 | 332.1487 | 300.12296; 282.112; 264.102; 238.086; 225.091; 169.069; 167.085; 165.065; 152.062; 141.069; 139.054; 115.055. | FISh scoring coverage |
| 14 | 4’-O-methylnorbelladine | C16H19NO3 | 273.1361 | 5.46 | 274.1435 | 137.059; 122.037; 94.042; 66.039; 65.039. | FISh scoring coverage |
| 15 | Lycorinine | C18H23NO4 | 317.1624 | 5.66 | 318.1697 | 300.159; 286.144; 268.133; 257.118; 258.148; 224.07; 175.075; 147.044. | FISh scoring coverage |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atrahimovich, D.; Harris, R.; Eitan, R.; Cohen, M.; Khatib, S. Galantamine Quantity and Alkaloid Profile in the Bulbs of Narcissus tazetta and daffodil cultivars (Amaryllidaceae) Grown in Israel. Metabolites 2021, 11, 185. https://doi.org/10.3390/metabo11030185
Atrahimovich D, Harris R, Eitan R, Cohen M, Khatib S. Galantamine Quantity and Alkaloid Profile in the Bulbs of Narcissus tazetta and daffodil cultivars (Amaryllidaceae) Grown in Israel. Metabolites. 2021; 11(3):185. https://doi.org/10.3390/metabo11030185
Chicago/Turabian StyleAtrahimovich, Dana, Raviv Harris, Ron Eitan, Menashe Cohen, and Soliman Khatib. 2021. "Galantamine Quantity and Alkaloid Profile in the Bulbs of Narcissus tazetta and daffodil cultivars (Amaryllidaceae) Grown in Israel" Metabolites 11, no. 3: 185. https://doi.org/10.3390/metabo11030185
APA StyleAtrahimovich, D., Harris, R., Eitan, R., Cohen, M., & Khatib, S. (2021). Galantamine Quantity and Alkaloid Profile in the Bulbs of Narcissus tazetta and daffodil cultivars (Amaryllidaceae) Grown in Israel. Metabolites, 11(3), 185. https://doi.org/10.3390/metabo11030185








