Use of Clopidogrel and Proton Pump Inhibitors Alone or in Combinations in Persons with Diabetes in Denmark; Potential for CYP2C19 Genotype-Guided Drug Therapy
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Westergaard, N.; Nielsen, R.S.; Jørgensen, S.; Vermehren, C. Drug Use in Denmark for Drugs Having Pharmacogenomics (PGx) Based Dosing Guidelines from CPIC or DPWG for CYP2D6 and CYP2C19 Drug–Gene Pairs: Perspectives for Introducing PGx Test to Polypharmacy Patients. J. Pers. Med. 2020, 10, 3. [Google Scholar] [CrossRef]
- Vermehren, C.; Søgaard Nielsen, R.; Jørgensen, S.; Drastrup, A.M.; Westergaard, N. Drug Use among Nursing Home Residents in Denmark for Drugs Having Pharmacogenomics Based (PGx) Dosing Guidelines: Potential for Preemptive PGx Testing. J. Pers. Med. 2020, 10, 78. [Google Scholar] [CrossRef]
- Schilling, U.; Dingemanse, J.; Ufer, M. Pharmacokinetics and Pharmacodynamics of Approved and Investigational P2Y12 Receptor Antagonists. Clin. Pharmacokinet. 2020, 59, 545–566. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.S.; Hlatky, M.A.; Antman, E.M.; Bhatt, D.L.; Bjorkman, D.J.; Clark, C.B.; Furberg, C.D.; Johnson, D.A.; Kahi, C.J.; Laine, L.; et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: A focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID. J. Am. Coll. Cardiol. 2010, 56, 2051–2066. [Google Scholar] [CrossRef] [PubMed]
- Rivas Rios, J.R.; Franchi, F.; Rollini, F.; Angiolillo, D.J. Diabetes and antiplatelet therapy: From bench to bedside. Cardiovasc. Diagn. Ther. 2018, 8, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Carreras, E.T.; Hochholzer, W.; Frelinger, A.L.; Nordio, F.; O’Donoghue, M.L.; Wiviott, S.D.; Angiolillo, D.J.; Michelson, A.D.; Sabatine, M.S.; Mega, J.L. Diabetes mellitus, CYP2C19 genotype, and response to escalating doses of clopidogrel: Insights from the ELEVATE-TIMI 56 trial. Thromb. Haemost. 2016, 116, 69–77. [Google Scholar] [CrossRef]
- Bøtker, H.E.; Gustafsson, I.; Egstrup, K.; Jensen, M.T.; Krarup, N.T.; Rosing, P.; Knudsen, S.T. Diabetes og Hjertesygdom. Available online: https://nbv.cardio.dk/diabetes#264-behandling--modifikation-af-risikofaktorer (accessed on 28 December 2020).
- Plavix. Product Monograph. Available online: http://products.sanofi.ca/en/plavix.pdf (accessed on 28 December 2020).
- Gower, M.N.; Ratner, L.R.; Williams, A.K.; Rossi, J.S.; Stouffer, G.A.; Lee, C.R. Clinical utility of cyp2c19 genotype-guided antiplatelet therapy in patients at risk of adverse cardiovascular and cerebrovascular events: A review of emerging evidence. Pharmgenomics. Pers. Med. 2020, 13, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Sangkuhl, K.; Stein, C.M.; Hulot, J.S.; Mega, J.L.; Roden, D.M.; Klein, T.E.; Sabatine, M.S.; Johnson, J.A.; Shuldiner, A.R. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin. Pharmacol. Ther. 2013, 94, 317–323. [Google Scholar] [CrossRef]
- Barbarino, J.M.; Whirl-Carrillo, M.; Altman, R.B.; Klein, T.E. PharmGKB: A worldwide resource for pharmacogenomic information. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1417. [Google Scholar] [CrossRef]
- Flockhart, D.A. Drug Interactions: Cytochrome P450 Drug Interaction Table; Indiana University School of Medicine: Indianapolis, IN, USA, 2007; Available online: https://drug-interactions.medicine.iu.edu/home.aspx. (accessed on 28 December 2020).
- Simon, T.; Verstuyft, C.; Mary-Krause, M.; Quteineh, L.; Drouet, E.; Méneveau, N.; Steg, P.G.; Ferrières, J.; Danchin, N.; Becquemont, L. Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 2009, 360, 363–375. [Google Scholar] [CrossRef]
- Mega, J.L.; Close, S.L.; Wiviott, S.D.; Shen, L.; Hockett, R.D.; Brandt, J.T.; Walker, J.R.; Antman, E.M.; Macias, W.; Braunwald, E.; et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 2009, 360, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Swen, J.J.; Nijenhuis, M.; de Boer, A.; Grandia, L.; Maitland-van der Zee, A.H.; Mulder, H.; Rongen, G.A.P.J.M.; van Schaik, R.H.N.; Schalekamp, T.; Touw, D.J.; et al. Pharmacogenetics: From Bench to Byte—An Update of Guidelines. Clin. Pharmacol. Ther. 2011, 89, 662–673. [Google Scholar] [CrossRef]
- Lamoureux, F.; Duflot, T. Pharmacogenetics in cardiovascular diseases: State of the art and implementation-recommendations of the French National Network of Pharmacogenetics (RNPGx). Therapie 2017, 72, 257–267. [Google Scholar] [CrossRef]
- FDA. Table of Pharmacogenomic Biomarkers in Drug Labels. Available online: https://www.fda.gov/drugs/science-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling (accessed on 28 December 2020).
- Kernan, W.N.; Ovbiagele, B.; Black, H.R.; Bravata, D.M.; Chimowitz, M.I.; Ezekowitz, M.D.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2160–2236. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Dehmer, G.J.; Kaul, S.; Leifer, D.; O’Gara, P.T.; Stein, C.M. ACCF/AHA clopidogrel clinical alert: Approaches to the FDA “boxed warning”: A report of the American college of cardiology foundation task force on clinical expert consensus documents and the American heart association. J. Am. Coll. Cardiol. 2010, 56, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Wu, Q.; Zhang, Z.; Zheng, T.; Xiang, Q.; Zhang, P.; Liu, X.; Zhang, C.; Tan, H.; Huang, J.; et al. Efficacy and safety of clopidogrel only vs. clopidogrel added proton pump inhibitors in the treatment of patients with coronary heart disease after percutaneous coronary intervention: A systematic review and meta-analysis. IJC Hear. Vasc. 2019, 23, 100317. [Google Scholar] [CrossRef] [PubMed]
- Farhat, N.; Haddad, N.; Crispo, J.; Birkett, N.; McNair, D.; Momoli, F.; Wen, S.W.; Mattison, D.R.; Krewski, D. Trends in concomitant clopidogrel and proton pump inhibitor treatment among ACS inpatients, 2000–2016. Eur. J. Clin. Pharmacol. 2019, 75, 227–235. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Chen, M.; Zhu, L.L.; Yu, L.S.; Zeng, S.; Xiang, M.X.; Zhou, Q. Pharmacokinetic drug interactions with clopidogrel: Updated review and risk management in combination therapy. Ther. Clin. Risk Manag. 2015, 11, 449–467. [Google Scholar]
- Lima, J.J.; Thomas, C.D.; Barbarino, J.; Desta, Z.; Van Driest, S.L.; El Rouby, N.; Johnson, J.A.; Cavallari, L.H.; Shakhnovich, V.; Thacker, D.L.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C19 and Proton Pump Inhibitor Dosing. Clin. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef]
- Würtz, M.; Grove, E.L. Proton pump inhibitors in cardiovascular disease: Drug interactions with antiplatelet drugs. Adv. Exp. Med. Biol. 2017, 906, 325–350. [Google Scholar] [CrossRef] [PubMed]
- Guerin, A.; Mody, R.; Carter, V.; Ayas, C.; Patel, H.; Lasch, K.; Wu, E. Changes in practice patterns of clopidogrel in combination with proton pump inhibitors after an fda safety communication. PLoS ONE 2016, 11, e0145504. [Google Scholar] [CrossRef] [PubMed]
- Kruik-Kollöffel, W.J.; van der Palen, J.; Kruik, H.J.; van Herk-Sukel, M.P.P.; Movig, K.L.L. Prescription behavior for gastroprotective drugs in new users as a result of communications regarding clopidogrel—Proton pump inhibitor interaction. Pharmacol. Res. Perspect. 2016, 4. [Google Scholar] [CrossRef]
- Berger, P.B. Should proton pump inhibitors be withheld from patients taking clopidogrel? The issue that has been giving me heartburn! Circ. Cardiovasc. Qual. Outcomes 2015, 8, 6–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Melloni, C.; Washam, J.B.; Jones, W.S.; Halim, S.A.; Hasselblad, V.; Mayer, S.B.; Heidenfelder, B.L.; Dolor, R.J. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: Systematic review. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Serbin, M.A.; Guzauskas, G.F.; Veenstra, D.L. Clopidogrel-proton pump inhibitor drug-drug interaction and risk of adverse clinical outcomes among PCI-treated ACS patients: A meta-analysis. J. Manag. Care Spec. Pharm. 2016, 22, 939–947. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Teeluck, A.R.; Bhurtu, A.; Huang, W.Q. Is the concomitant use of clopidogrel and Proton Pump Inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: A systematic review and meta-analysis of recently published studies (2012—2016). BMC Cardiovasc. Disord. 2017, 17. [Google Scholar] [CrossRef]
- Shah, R.R.; Smith, R.L. Addressing phenoconversion: The Achilles’ heel of personalized medicine. Br. J. Clin. Pharmacol. 2015, 79, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.A.; Setiawan, D.; Hak, E.; Wilffert, B. Pharmacogenetics of drug–drug interaction and drug–drug–gene interaction: A systematic review on CYP2C9, CYP2C19 and CYP2D6. Pharmacogenomics 2017, 18, 701–739. [Google Scholar] [CrossRef] [PubMed]
- Ellithi, M.; Baye, J.; Wilke, R.A. CYP2C19 genotype-guided antiplatelet therapy: Promises and pitfalls. Pharmacogenomics 2020, 21, 889–897. [Google Scholar] [CrossRef]
- Depta, J.P.; Lenzini, P.A.; Lanfear, D.E.; Wang, T.Y.; Spertus, J.A.; Bach, R.G.; Cresci, S. Clinical outcomes associated with proton pump inhibitor use among clopidogrel-treated patients within CYP2C19 genotype groups following acute myocardial infarction. Pharm. J. 2015, 15, 20–25. [Google Scholar] [CrossRef][Green Version]
- Furuta, T.; Iwaki, T.; Umemura, K. Influences of different proton pump inhibitors on the anti-platelet function of clopidogrel in relation to CYP2C19 genotypes. Br. J. Clin. Pharmacol. 2010, 70, 383–392. [Google Scholar] [CrossRef]
- Danish Medicines Agency. Interaktionsdatabasen. Available online: http://www.interaktionsdatabasen.dk/Default.aspx (accessed on 30 April 2020).
- Felleskatalogen Interaksjonsanalyse. Felleskatalogen. Available online: https://www.felleskatalogen.no/medisin/interaksjon/ (accessed on 8 October 2020).
- Medscape Drug Interactions Checker. Medscape Drug Reference Database. Available online: https://reference.medscape.com/drug-interactionchecker (accessed on 7 October 2020).
- Drug Interactions Drug Interactions Checker. For Drugs, Food & Alcohol. Available online: https://www.drugs.com/drug_interactions.html (accessed on 8 October 2020).
- Schmidt, M.; Hallas, J.; Laursen, M.; Friis, S. Data Resource Profile: Danish online drug use statistics (MEDSTAT). Int. J. Epidemiol. 2016, 45, 1401–1402. [Google Scholar] [CrossRef] [PubMed]
- Sundhed. Cerebralt infarkt og TCI. Available online: https://www.sundhed.dk/sundhedsfaglig/information-til-praksis/hovedstaden/almen-praksis/laegemidler/basislisten-hovedstaden/cerebralt-infarkt-og-tci/ (accessed on 28 December 2020).
- Farrell, B.; Pottie, K.; Thompson, W.; Boghossian, T.; Pizzola, L.; Rojas-Fernandez, R.J.; Walsh, K.; Welch, V.; Moayyedi, P. Proton Pump Inhibitor (PPI) Deprescribing Algorithm. Can. Fam. Physician 2017, 63, 354–364. Available online: https://deprescribing.org/resources/deprescribing-guidelines-algorithms/ (accessed on 15 December 2020). [PubMed]
- Carstensen, B.; Rønn, P.F.; Jørgensen, M.E. Prevalence, incidence and mortality of type 1 and type 2 diabetes in Denmark 1996–2016. BMJ Open Diabetes Res. Care 2020, 8. [Google Scholar] [CrossRef]
- Peng, Y.L.; Leu, H.B.; Luo, J.C.; Huang, C.C.; Hou, M.C.; Lin, H.C.; Lee, F.Y. Diabetes is an independent risk factor for peptic ulcer bleeding: A nationwide population-based cohort study. J. Gastroenterol. Hepatol. 2013, 28, 1295–1299. [Google Scholar] [CrossRef]
- Christensen, L.D.; Reilev, M.; Juul-Larsen, H.G.; Jørgensen, L.M.; Kaae, S.; Andersen, O.; Pottegård, A.; Petersen, J. Use of prescription drugs in the older adult population-a nationwide pharmacoepidemiological study. Eur. J. Clin. Pharmacol. 2019, 75, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Prami, T.; Khanfir, H.; Hasvold, P.; Reissell, E.; Airaksinen, J.; Kytö, V. Concomitant use of drugs known to cause interactions with oral antiplatelets—polypharmacy in acute coronary syndrome outpatients in Finland. Eur. J. Clin. Pharmacol. 2020, 76, 257–265. [Google Scholar] [CrossRef]
- Klein, M.E.; Parvez, M.M.; Shin, J.G. Clinical Implementation of Pharmacogenomics for Personalized Precision Medicine: Barriers and Solutions. J. Pharm. Sci. 2017, 106, 2368–2379. [Google Scholar] [CrossRef]
- Jürgens, G.; Jacobsen, C.B.; Rasmussen, H.B.; Werge, T.; Nordentoft, M.; Andersen, S.E. Utility and adoption of CYP2D6 and CYP2C19 genotyping and its translation into psychiatric clinical practice. Acta Psychiatr. Scand. 2012, 125, 228–237. [Google Scholar] [CrossRef]
- Samwald, M.; Xu, H.; Blagec, K.; Empey, P.E.; Malone, D.C.; Ahmed, S.M.; Ryan, P.; Hofer, S.; Boyce, R.D. Incidence of Exposure of Patients in the United States to Multiple Drugs for Which Pharmacogenomic Guidelines Are Available. PLoS ONE 2016, 11, e0164972. [Google Scholar] [CrossRef]
- Alwhaibi, M.; Balkhi, B.; Alhawassi, T.M.; Alkofide, H.; Alduhaim, N.; Alabdulali, R.; Drweesh, H.; Sambamoorthi, U. Polypharmacy among patients with diabetes: A cross-sectional retrospective study in a tertiary hospital in Saudi Arabia. BMJ Open 2018, 8, e020852. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Magness, J.W.; Nelson, R.; Baron, V.; Brixner, D.I. Clinical utility of pharmacogenetic testing and a clinical decision support tool to enhance the identification of drug therapy problems through medication therapy management in polypharmacy patients. J. Manag. Care Spec. Pharm. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Swanson, K.M.; Rojas, R.L.; Wang, Z.; Sauver, J.L.S.; Visscher, S.L.; Prokop, L.J.; Bielinski, S.J.; Wang, L.; Weinshilboum, R.; et al. Systematic review of the evidence on the cost-effectiveness of pharmacogenomics-guided treatment for cardiovascular diseases. Genet. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Brixner, D.; Biltaji, E.; Bress, A.; Unni, S.; Ye, X.; Mamiya, T.; Ashcraft, K.; Biskupiak, J. The effect of pharmacogenetic profiling with a clinical decision support tool on healthcare resource utilization and estimated costs in the elderly exposed to polypharmacy. J. Med. Econ. 2016, 19, 213–228. [Google Scholar] [CrossRef]
- Yuan, J.; He, Q.; Nguyen, L.H.; Wong, M.C.S.; Huang, J.; Yu, Y.; Xia, B.; Tang, Y.; He, Y.; Zhang, C. Regular use of proton pump inhibitors and risk of type 2 diabetes: Results from three prospective cohort studies. Gut 2020. [Google Scholar] [CrossRef] [PubMed]
- Statistics Denmark. Statistics Denmark. Available online: https://www.dst.dk/en# (accessed on 28 December 2020).
- Schmidt, M.; Pedersen, L.; Sørensen, H.T. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 2014, 29, 541–549. [Google Scholar] [CrossRef]
- World Health Organization. Collaborating Centre for Drug Statistics Methodology. WHOCC—ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 7 October 2020).
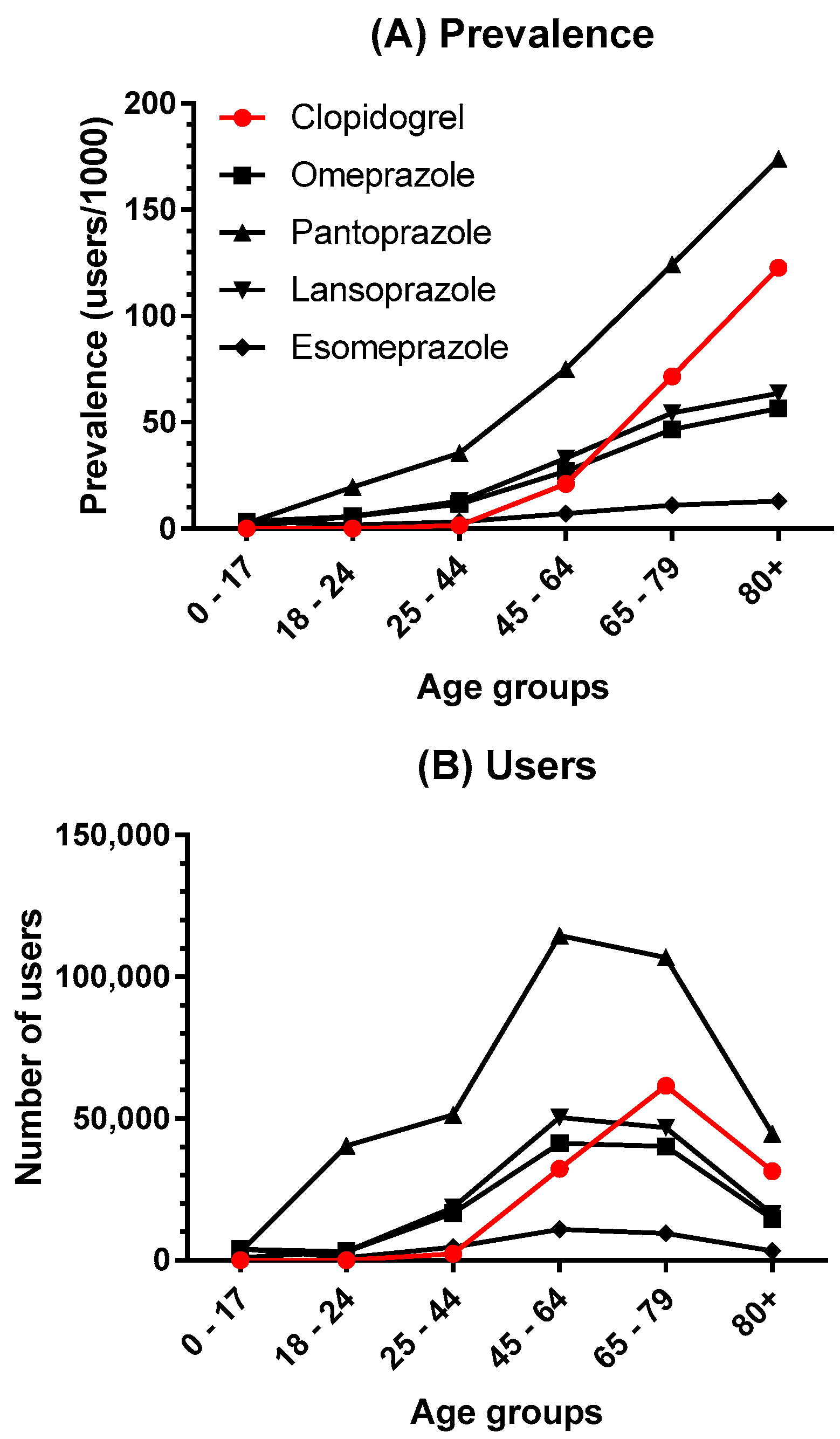
| 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|
| PPIs A02BC | 545,990 (97.0) | 570,745 (100.8) | 583,345 (102.4) | 591,195 (102.8) | 596,035 (103.1) |
| Clopidogrel B01AC04 | 87,770 (15.6) | 100,835 (17.8) | 111,315 (19.5) | 119,735 (20.8) | 127,755 (22.1) |
| Prasugrel B01AC22 | 1460 (0.3) | 1035 (0.2) | 575 (0.1) | 360 (<0.1) | 325 (<0.1) |
| Ticagrelor B01AC24 | 9345 (1.7) | 9605 (1.7) | 9790 (1.7) | 9465 (1.7) | 9500 (1.7) |
| Esomeprazole A02BC05 | Lansoprazole A02BC03 | Omeprazole A02BC01 | Pantoprazole A02BC02 | ||
|---|---|---|---|---|---|
| Users: | 32,295 | 135,980 | 119,274 | 329,222 | |
| Clopidogrel | 127,480 | 2388 [1217] | 9570 [5213] | 7188 [3900] | 25,641 [13,850] |
| Users with diabetes: | 3054 | 17,246 | 14,286 | 39,287 | |
| Clopidogrel | 21,746 | 484 [250] | 1952 [1081] | 1459 [813] | 5285 [2876] |
| Esomeprazole A02BC05 | Lansoprazole A02BC03 | Omeprazole A02BC01 | Pantoprazole A02BC02 | ||
|---|---|---|---|---|---|
| Prevalence: | 5.6 | 23.5 | 20.6 | 56.9 | |
| Clopidogrel | 22.1 | 0.4 (1.8%) [0.2 (0.9%)] | 1.7 (7.7%) [0.9 (4.1%)] | 1.2 (5.4%) [0.7 (3.2%)] | 4.4 (20.0%) [2.4 (10.9%)] |
| Prevalence diabetics: | 11.8 | 66.7 | 55.3 | 152.0 | |
| Clopidogrel | 84.1 | 1.9 (2.3%) * [1.0 (1.2%)] * | 7.6 (9.0%) * [4.2 (5.0%)] * | 5.6 (6.7%) * [3.1 (3.7%)] * | 20.4 (24.3%) * [11.1 (13.2%)] * |
| Prevalence Ratio: (Prevalence Diabetes/Prevalence General Population) | Esomeprazole | Lansoprazole | Omeprazole | Pantoprazole | ||
|---|---|---|---|---|---|---|
| 2.1 | 2.8 | 2.7 | 2.7 | |||
| Clopidogrel | 3.8 | During 2018 | 4.8 | 4.5 | 4.7 | 4.6 |
| Same Day 2018 | 5.0 | 4.6 | 4.4 | 4.6 | ||
| Esomeprazole | Lansoprazole | Omeprazole | Pantoprazole | ||
|---|---|---|---|---|---|
| clopidogrel | (1) | ||||
| (2) | |||||
| (3) | |||||
| (4) | |||||
| Total number of users * Total number of diabetic users * | 2388 | 9570 | 7188 | 25,641 | |
| 484 | 1952 | 1459 | 5285 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westergaard, N.; Tarnow, L.; Vermehren, C. Use of Clopidogrel and Proton Pump Inhibitors Alone or in Combinations in Persons with Diabetes in Denmark; Potential for CYP2C19 Genotype-Guided Drug Therapy. Metabolites 2021, 11, 96. https://doi.org/10.3390/metabo11020096
Westergaard N, Tarnow L, Vermehren C. Use of Clopidogrel and Proton Pump Inhibitors Alone or in Combinations in Persons with Diabetes in Denmark; Potential for CYP2C19 Genotype-Guided Drug Therapy. Metabolites. 2021; 11(2):96. https://doi.org/10.3390/metabo11020096
Chicago/Turabian StyleWestergaard, Niels, Lise Tarnow, and Charlotte Vermehren. 2021. "Use of Clopidogrel and Proton Pump Inhibitors Alone or in Combinations in Persons with Diabetes in Denmark; Potential for CYP2C19 Genotype-Guided Drug Therapy" Metabolites 11, no. 2: 96. https://doi.org/10.3390/metabo11020096
APA StyleWestergaard, N., Tarnow, L., & Vermehren, C. (2021). Use of Clopidogrel and Proton Pump Inhibitors Alone or in Combinations in Persons with Diabetes in Denmark; Potential for CYP2C19 Genotype-Guided Drug Therapy. Metabolites, 11(2), 96. https://doi.org/10.3390/metabo11020096






