Metabolomic Profile of Weaned Pigs Challenged with E. coli and Supplemented with Carbadox or Bacillus subtilis
Abstract
1. Introduction
2. Results
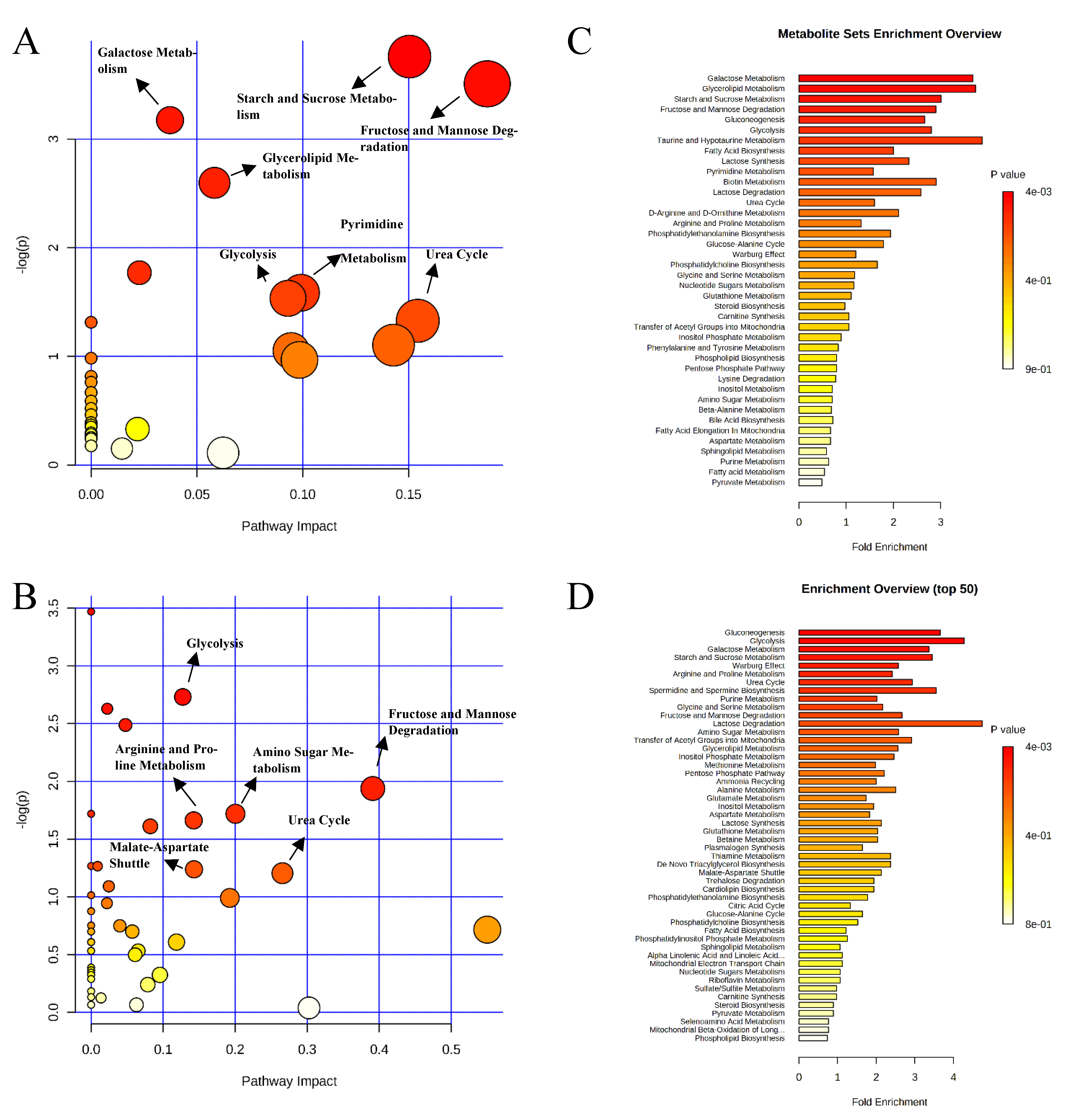
2.1. Metabolite Profile in Ileal Mucosa
2.2. Metabolite Profile in Colon Digesta
3. Discussion
3.1. Metabolites Related to Host Metabolism
3.2. Metabolites Related to Microbial Metabolism
4. Material and Methods
4.1. Ethical Statement
4.2. Animals, Housing and Experimental Design
4.3. Sample Collection
4.4. Untargeted Metabolomics Analysis
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health. Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, É. Chapter 52 Colibacillosis. In Diseases of Swine; Zimmerman, J.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 807–834. [Google Scholar]
- National Animal Health Monitoring System (NAHMS). Swine 2012 Part II: Reference of Swine Health and Health Management in the United States; USDA: Washington, DC, USA, 2012.
- Kim, K.; He, Y.; Xiong, X.; Ehrlich, A.; Li, X.; Raybould, H.; Atwill, E.R.; Maga, E.A.; Jørgensen, J.; Liu, Y. Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J. Anim. Sci. Biotechnol. 2019, 10, 52. [Google Scholar] [CrossRef]
- He, Y.; Jinno, C.; Kim, K.; Wu, Z.; Tan, B.; Li, X.; Whelan, R.; Liu, Y. Dietary Bacillus spp. enhanced growth and disease resistance of weaned pigs by modulating intestinal microbiota and systemic immunity. J. Anim. Sci. Biotechnol. 2020, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Rietschel, E.T. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Devriendt, B.; Stuyven, E.; Verdonck, F.; Goddeeris, B.M.; Cox, E. Enterotoxigenic Escherichia coli (K88) induce proinflammatory responses in porcine intestinal epithelial cells. Dev. Comp. Immunol. 2010, 34, 1175–1182. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Z.; Jiang, J.; Yu, Y.; Zhang, Q. Differential gene expression profiling of porcine epithelial cells infected with three enterotoxigenic Escherichia coli strains. BMC Genom. 2012, 13, 330. [Google Scholar] [CrossRef]
- Ren, W.; Yin, J.; Gao, W.; Chen, S.; Duan, J.; Liu, G.; Li, T.; Li, N.; Peng, Y.; Yin, Y. Metabolomics study of metabolic variations in enterotoxigenic Escherichia coli-infected piglets. RSC Adv. 2015, 5, 59550–59555. [Google Scholar] [CrossRef]
- Wu, H.; Liu, J.; Chen, S.; Zhao, Y.; Zeng, S.; Bin, P.; Zhang, D.; Tang, Z.; Zhu, G. Jejunal metabolic responses to Escherichia coli infection in piglets. Front. Microbiol. 2018, 9, 2465. [Google Scholar] [CrossRef]
- Luise, D.; Bertocchi, M.; Motta, V.; Salvarani, C.; Bosi, P.; Luppi, A.; Fanelli, F.; Mazzoni, M.; Archetti, I.; Maiorano, G.; et al. Bacillus sp. probiotic supplementation diminish the Escherichia coli F4ac infection in susceptible weaned pigs by influencing the intestinal immune response, intestinal microbiota and blood metabolomics. J. Anim. Sci. Biotechnol. 2019, 10, 74. [Google Scholar] [CrossRef]
- Becker, S.L.; Li, Q.; Burrough, E.R.; Kenne, D.; Sahin, O.; Gould, S.A.; Patience, J.F. Effects of an F18 enterotoxigenic Escherichia coli challenge on growth performance, immunological status, and gastrointestinal structure of weaned pigs and the potential protective effect of direct-fed microbial blends. J. Anim. Sci. 2020, 98, skaa113. [Google Scholar] [CrossRef]
- Li, Q.; Peng, X.; Burrough, E.R.; Sahin, O.; Gould, S.A.; Gabler, N.K.; Loving, C.L.; Dorman, K.S.; Patience, J.F. Dietary soluble and insoluble fiber with or without enzymes altered the intestinal microbiota in weaned pigs challenged with enterotoxigenic E. coli f18. Front. Microbiol. 2020, 11, 1110. [Google Scholar] [CrossRef] [PubMed]
- Loos, M.; Geens, M.; Schauvliege, S.; Gasthuys, F.; van der Meulen, J.; Dubreuil, J.D.; Goddeeris, B.M.; Niewold, T.; Cox, E. Role of heat-stable enterotoxins in the induction of early immune responses in piglets after infection with Enterotoxigenic Escherichia coli. PLoS ONE 2012, 7, e41041. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, P.F.; Ma, X.K.; Shang, Q.H.; Xu, Y.T.; Long, S.F.; Wu, Y.; Yuan, F.M.; Piao, X.S. Probiotic supplementation protects weaned pigs against enterotoxigenic Escherichia coli K88 challenge and improves performance similar to antibiotics. J. Anim. Sci. 2017, 95, 2627–2639. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, M.; Che, T.M.; Almeida, J.A.S.; Lee, J.J.; Bravo, D.; Maddox, C.W.; Pettigrew, J.E. Dietary plant extracts alleviate diarrhea and alter immune responses of weaned pigs experimentally infected with a pathogenic Escherichia coli. J. Anim. Sci. 2013, 91, 5294–5306. [Google Scholar] [CrossRef] [PubMed]
- Suter, W.; Rosselet, A.; Knusel, F. Mode of action of Quindoxin and substituted Quinoxaline-di-N-Oxides on Escherichia coli. Antimicrob. Agents Chemother. 1978, 13, 770–783. [Google Scholar] [CrossRef]
- Cheng, G.; Li, B.; Wang, C.; Zhang, H.; Liang, G.; Weng, Z.; Hao, H.; Wang, X.; Liu, Z.; Dai, M.; et al. Systematic and molecular basis of the antibacterial action of Quinoxaline 1,4-Di-N-Oxides against Escherichia coli. PLoS ONE 2015, 10, e0136450. [Google Scholar] [CrossRef]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions: Bacillus subtilis antibiotics. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef]
- Hu, Y.; Dun, Y.; Li, S.; Zhao, S.; Peng, N.; Liang, Y. Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and fecal bacterial flora of weaned piglets. Asian Australas. J. Anim. Sci 2014, 27, 1131–1140. [Google Scholar] [CrossRef]
- Lee, S.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lokhande, A.; Kim, E.K.; Kwon, I.K.; Kim, Y.H.; Chae, B.J. Effects of dietary supplementation with Bacillus subtilis LS 1–2 fermentation biomass on growth performance, nutrient digestibility, cecal microbiota and intestinal morphology of weanling pig. Anim. Feed Sci. Technol. 2014, 188, 102–110. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Escherichia coli STb toxin and colibacillosis: Knowing is half the battle. FEMS Microbiol. Lett. 2008, 278, 137–145. [Google Scholar] [CrossRef]
- Johnson, A.M.; Kaushik, R.S.; Rotella, N.J.; Hardwidge, P.R. Enterotoxigenic Escherichia coli modulates host intestinal cell membrane asymmetry and metabolic activity. IAI 2009, 77, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, C.; Li, C.; Li, P.; Fu, E.; Xie, Y.; Jin, F. Heat-Labile enterotoxin-induced PERK-CHOP pathway activation causes intestinal epithelial cell apoptosis. Front. Cell. Infect. Microbiol. 2017, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Mammen, J.M.V.; Matthews, J.B. Mucosal repair in the gastrointestinal tract. Crit. Care Med. 2003, 31, S532–S537. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Cocchi, S.; Suzzi, G. Polyamines and gut microbiota. Front. Nutr. 2019, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining mysteries of molecular biology: The role of polyamines in the cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Wang, J.; Golovina, V.A.; Li, L.; Platoshyn, O.; Yuan, J.X.-J. Role of K + channel expression in polyamine-dependent intestinal epithelial cell migration. Am. J. Physiol. Cell Physiol. 2000, 278, C303–C314. [Google Scholar] [CrossRef]
- Wang, J.Y.; Johnson, L.R. Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am. J. Physiol. Gastrointest. Liver Physiol. 1990, 259, G584–G592. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Lane, A.N.; Fan, T.W.-M. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef]
- Sanderson, I.R.; He, Y. Nucleotide uptake and metabolism by intestinal epithelial cells. J. Nutr. 1994, 124, 131S–137S. [Google Scholar] [CrossRef]
- McCauley, R.; Kong, S.-E.; Hall, J. Review: Glutamine and nucleotide metabolism within enterocytes. JPEN J Parenter Enteral Nutr. 1998, 22, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Gil, A. Modulation of the immune response mediated by dietary nucleotides. Eur. J. Clin. Nutr. 2002, 56, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Paulick, M.G.; Bertozzi, C.R. The glycosylphosphatidylinositol anchor: A complex membrane-anchoring structure for proteins. Biochemistry 2008, 47, 6991–7000. [Google Scholar] [CrossRef] [PubMed]
- Aebi, M. N-linked protein glycosylation in the ER. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Scott, D.A.; Losfeld, M.-E.; Freeze, H.H. The Metabolic origins of mannose in glycoproteins. J. Biol. Chem. 2014, 289, 6751–6761. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bhardwaj, R.; Thukral, A.K.; Handa, N.; Kaur, R.; Kumar, V. Osmolyte Dynamics. In Emerging Technologies and Management of Crop Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 405–430. [Google Scholar]
- Alton, G. Direct utilization of mannose for mammalian glycoprotein biosynthesis. Glycobiology 1998, 8, 285–295. [Google Scholar] [CrossRef]
- Sharma, V.; Freeze, H.H. Mannose efflux from the cells: A potential source of mannose in blood. J. Biol. Chem. 2011, 286, 10193–10200. [Google Scholar] [CrossRef]
- Park, J.; Kotani, T.; Konno, T.; Setiawan, J.; Kitamura, Y.; Imada, S.; Usui, Y.; Hatano, N.; Shinohara, M.; Saito, Y.; et al. Promotion of intestinal epithelial cell turnover by commensal bacteria: Role of short-chain fatty acids. PLoS ONE 2016, 11, e0156334. [Google Scholar] [CrossRef]
- Link-Lenczowski, P.; Jastrzębska, M.; Chwalenia, K.; Pierzchalska, M.; Leja-Szpak, A.; Bonior, J.; Pierzchalski, P.; Jaworek, J. A switch of N-glycosylation of proteome and secretome during differentiation of intestinal epithelial cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 118555. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Braun, A.; Treede, I.; Gotthardt, D.; Tietje, A.; Zahn, A.; Ruhwald, R.; Schoenfeld, U.; Welsch, T.; Kienle, P.; Erben, G.; et al. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: A clue to pathogenesis. Inflamm. Bowel Dis. 2009, 15, 1705–1720. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A. Very long-chain fatty acids: Elongation, physiology and related disorders. J. Biochem. 2012, 152, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Hengstermann, S.; Valentini, L.; Schaper, L.; Buning, C.; Koernicke, T.; Maritschnegg, M.; Buhner, S.; Tillinger, W.; Regano, N.; Guglielmi, F.; et al. Altered status of antioxidant vitamins and fatty acids in patients with inactive inflammatory bowel disease. Clin. Nutr. 2008, 27, 571–578. [Google Scholar] [CrossRef]
- Uchiyama, K.; Odahara, S.; Nakamura, M.; Koido, S.; Katahira, K.; Shiraishi, H.; Ohkusa, T.; Fujise, K.; Tajiri, H. The fatty acid profile of the erythrocyte membrane in initial-onset inflammatory bowel disease patients. Dig. Dis. Sci. 2013, 58, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.; Hutchings, M.R.; Hutchings, K.E.K.; Gally, D.L.; Houdijk, J.G.M. Changes in the ileal, but not fecal, microbiome in response to increased dietary protein level and enterotoxigenic Escherichia coli Exposure in pigs. Appl. Environ. Microbiol. 2019, 85, e01252-19. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.R.; Dickinson, D.B. Studies of sugars and sorbitol in developing corn kernels. Plant Physiol. 1984, 75, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Kelker, N.E.; Anderson, R.L. Sorbitol metabolism in Aerobacter aerogenes. J. Bacteriol. 1971, 105, 160–164. [Google Scholar] [CrossRef]
- Aldridge, P.; Metzger, M.; Geider, K. Genetics of sorbitol metabolism in Erwinia amylovora and its influence on bacterial virulence. Mol. Gen. Genet. 1997, 256, 611–619. [Google Scholar] [CrossRef]
- Beaugerie, L.; Flourié, B.; Pernet, P.; Achour, L.; Franchisseur, C.; Rambaud, J.C. Glucose does not facilitate the absorption of sorbitol perfused in situ in the human small intestine. J. Nutr. 1997, 127, 341–344. [Google Scholar] [CrossRef][Green Version]
- Yebra, M.A.J.; Pérez-Martínez, G. Cross-talk between the L-sorbose and D-sorbitol (D-glucitol) metabolic pathways in Lactobacillus casei. Microbiology 2002, 148, 2351–2359. [Google Scholar] [CrossRef]
- Alcántara, C.; Sarmiento-Rubiano, L.A.; Monedero, V.; Deutscher, J.; Pérez-Martínez, G.; Yebra, M.J. Regulation of Lactobacillus casei sorbitol utilization genes requires DNA-binding transcriptional activator gutr and the conserved protein gutm. Appl. Environ. Microbiol. 2008, 74, 5731–5740. [Google Scholar] [CrossRef] [PubMed]
- Anastassiadis, S.; Morgunov, I.G. Gluconic acid production. Recent Pat. Biotechnol. 2007, 1, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- Asano, T.; Yuasa, K.; Kunugita, K.; Teraji, T.; Mitsuoka, T. Effects of gluconic acid on human faecal bacteria. Microb. Ecol. Health Dis. 1994, 7, 247–256. [Google Scholar] [CrossRef]
- Tsukahara, T.; Koyama, H.; Okada, M.; Ushida, K. Stimulation of butyrate production by gluconic acid in batch culture of pig cecal digesta and identification of butyrate-producing bacteria. J. Nutr. 2002, 132, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Legler, G. Active site directed inhibitors and mechanism of action of glycosidases. Mol. Cell Biochem. 1973, 2, 31–38. [Google Scholar] [CrossRef]
- Pandey, S.; Sree, A.; Dash, S.S.; Sethi, D.P.; Chowdhury, L. Diversity of marine bacteria producing beta-glucosidase inhibitors. Microb. Cell Fact. 2013, 12, 35. [Google Scholar] [CrossRef]
- Singh, G.; Verma, A.K.; Kumar, V. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech 2016, 6, 3. [Google Scholar] [CrossRef]
- Balci, M. Synthesis of conduritols and related compounds. Pure Appl. Chem. 1997, 69, 97–104. [Google Scholar] [CrossRef]
- Drackley, J.K. Lipid metabolism. In Farm Animal Metabolism and Nutrition: Critical Reviews; D’Mello, J.P.F., Ed.; CAB International: Wallingford, CT, USA; Oxford, UK, 2000; pp. 97–119. [Google Scholar]
- Russell, R.W.; Gahr, S.A. Glucose availability and associated metabolism. In Farm Animal Metabolism and Nutrition: Critical Reviews; D’Mello, J.P.F., Ed.; CAB International: Wallingford, CT, USA; Oxford, UK, 2000; pp. 121–148. [Google Scholar]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Andersson, U.; Radstrom, P. beta-Glucose 1-phosphate-interconverting enzymes in maltose- and trehalose-fermenting lactic acid bacteria. Environ. Microbiol. 2002, 4, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, A.; Duncan, S.H.; Holtrop, G.; Anderson, S.E.; Lobley, G.E.; Flint, H.J. Impact of pH on lactate formation and utilization by human fecal microbial communities. AEM 2007, 73, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Zhang, S.; Yang, F.; Thacker, P.A.; Zhang, G.; Qiao, S.; Ma, X. Oral Administration of Lactobacillus fermentum i5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets. J. Agric. Food Chem. 2014, 62, 860–866. [Google Scholar] [CrossRef]
- Bhandari, S.K.; Xu, B.; Nyachoti, C.M.; Giesting, D.W.; Krause, D.O. Evaluation of alternatives to antibiotics using an Escherichia coli K88+ model of piglet diarrhea: Effects on gut microbial ecology. J. Anim. Sci. 2008, 86, 836–847. [Google Scholar] [CrossRef]
- Tang, W.; Qian, Y.; Yu, B.; Zhang, T.; Gao, J.; He, J.; Huang, Z.; Zheng, P.; Mao, X.; Luo, J.; et al. Effects of Bacillus subtilis DSM32315 supplementation and dietary crude protein level on performance, gut barrier function and microbiota profile in weaned piglets. J. Anim. Sci. 2019, 97, 2125–2138. [Google Scholar] [CrossRef]
- Kreuzer, S.; Reissmann, M.; Brockmann, G.A. New fast and cost-effective gene test to get the ETEC F18 receptor status in pigs. Vet. Microbiol. 2013, 163, 392–394. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Swine, 11th revised ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M. Setup and annotation of metabolomic experiments by integrating biological and mass spectrometric metadata. In Data Integration in the Life Sciences; Ludäscher, B., Raschid, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3615. [Google Scholar] [CrossRef]
- Sun, Y.; Su, Y.; Zhu, W. Microbiome-metabolome responses in the cecum and colon of pig to a high resistant starch diet. Front. Microbiol. 2016, 7, 779–788. [Google Scholar] [CrossRef]
- Wang, H.; Ren, E.; Xiang, X.; Su, Y.; Zhu, W. Dynamic changes in serum metabolomic profiles of growing pigs induced by intravenous infusion of sodium butyrate. Metabolites 2020, 10, 20. [Google Scholar] [CrossRef]
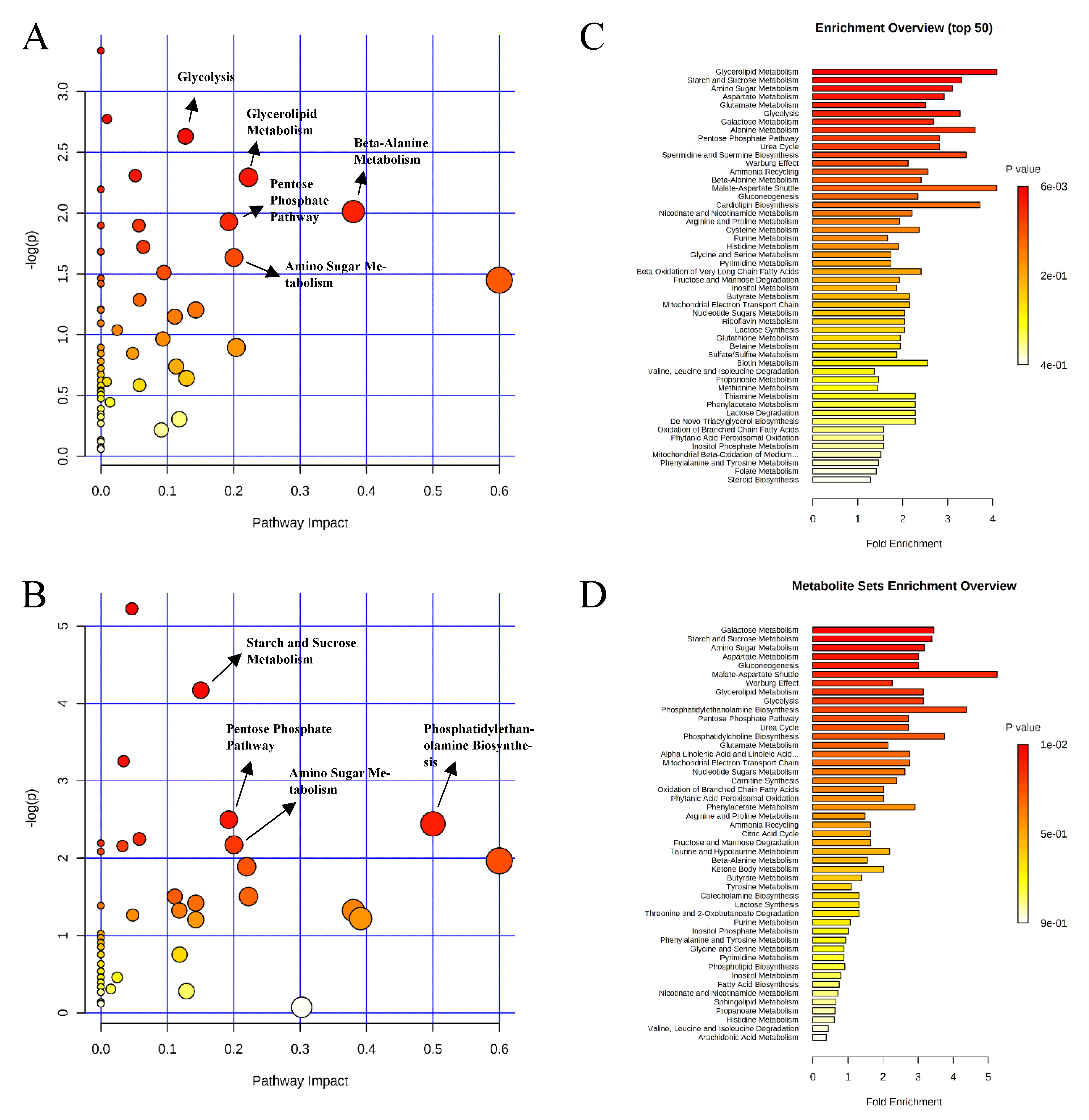
 , negative control (A),
, negative control (A),  , positive control (B),
, positive control (B),  , carbadox (C), and
, carbadox (C), and  , probiotic (D). Shaded areas in different colors represent the 95% confidence interval.
, probiotic (D). Shaded areas in different colors represent the 95% confidence interval.
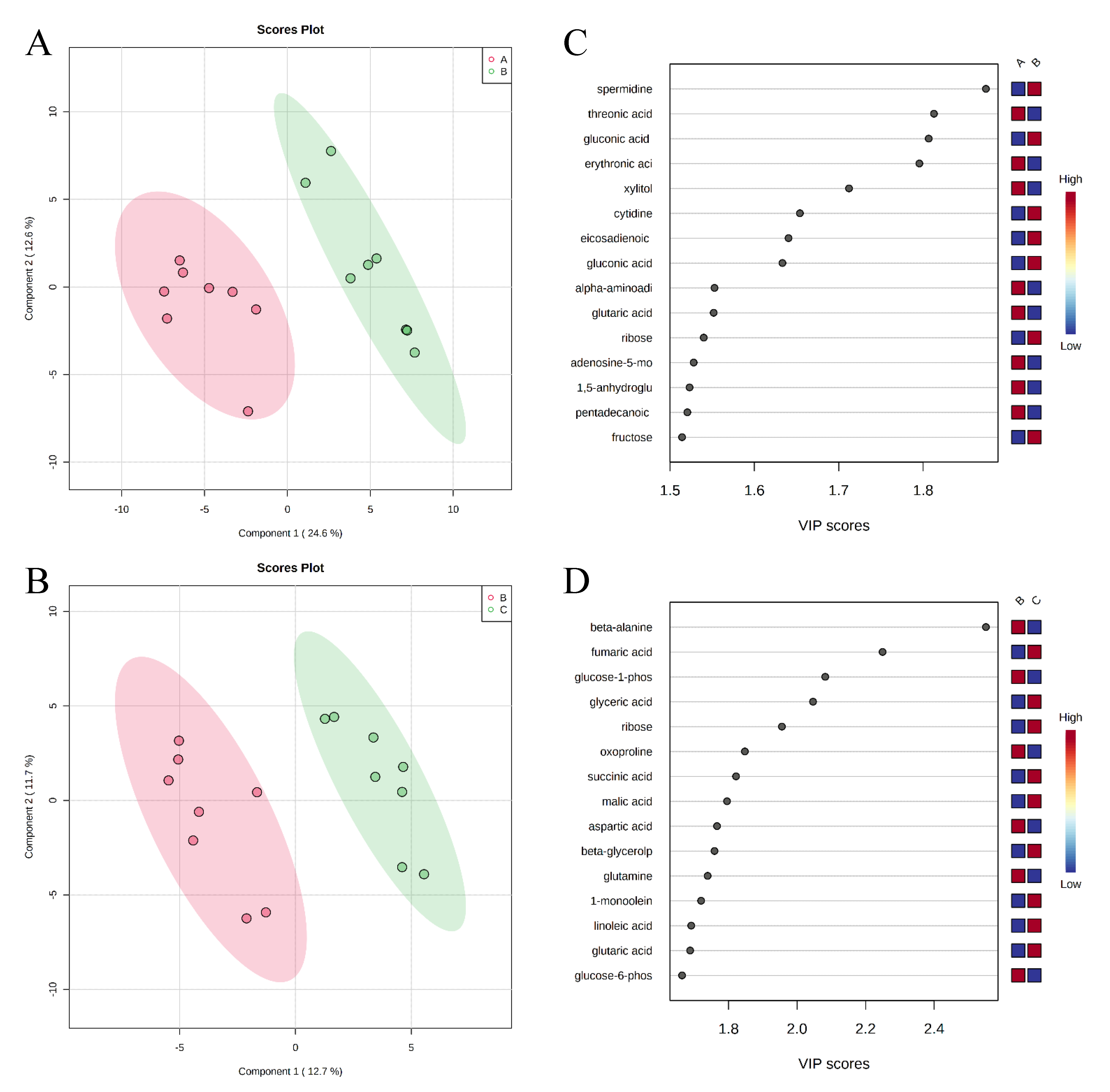
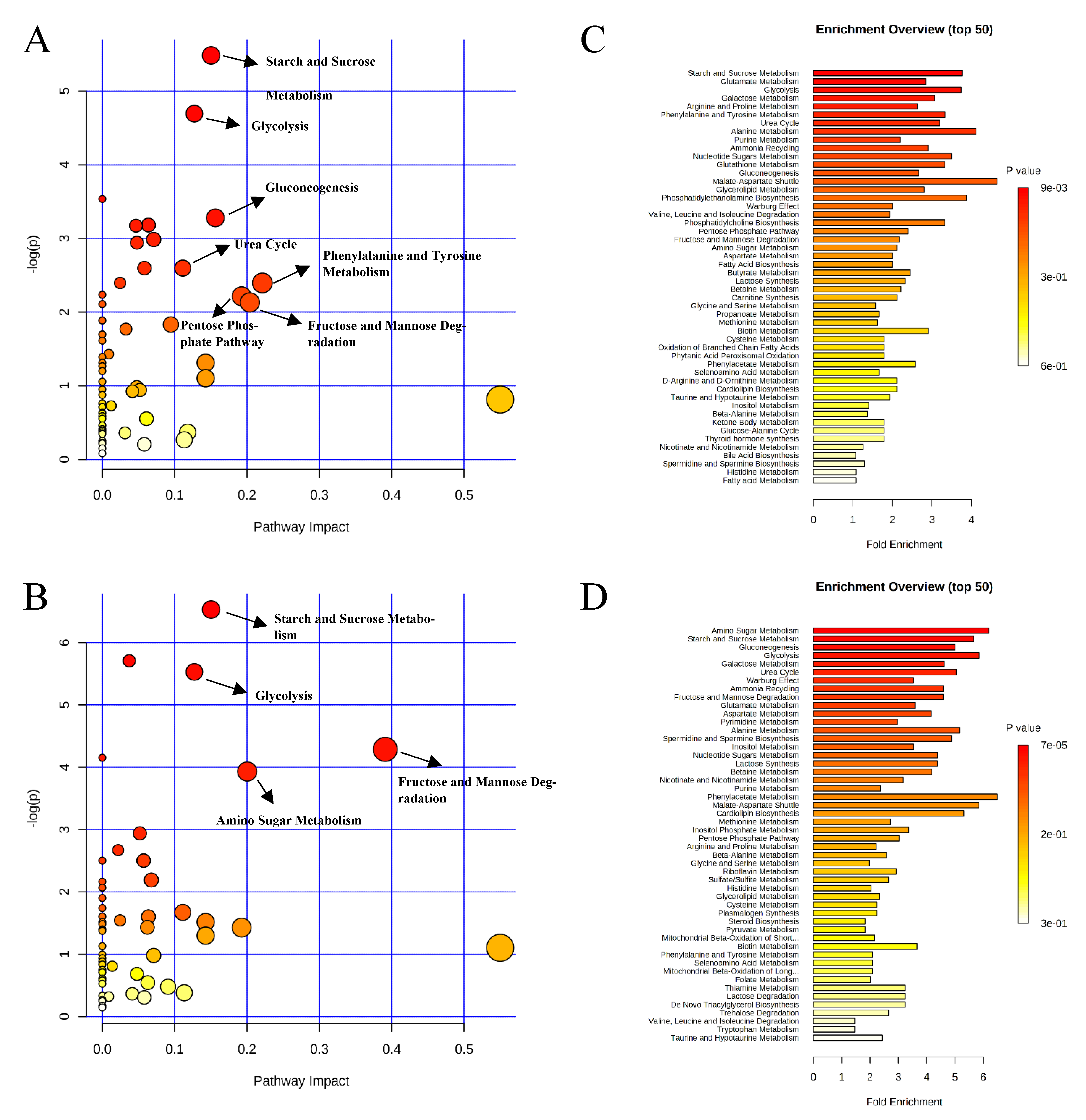
 , negative control (A),
, negative control (A),  , positive control (B),
, positive control (B),  , carbadox (C), and
, carbadox (C), and  , probiotic (D). Shaded areas in different colors represent the 95% confidence interval.
, probiotic (D). Shaded areas in different colors represent the 95% confidence interval.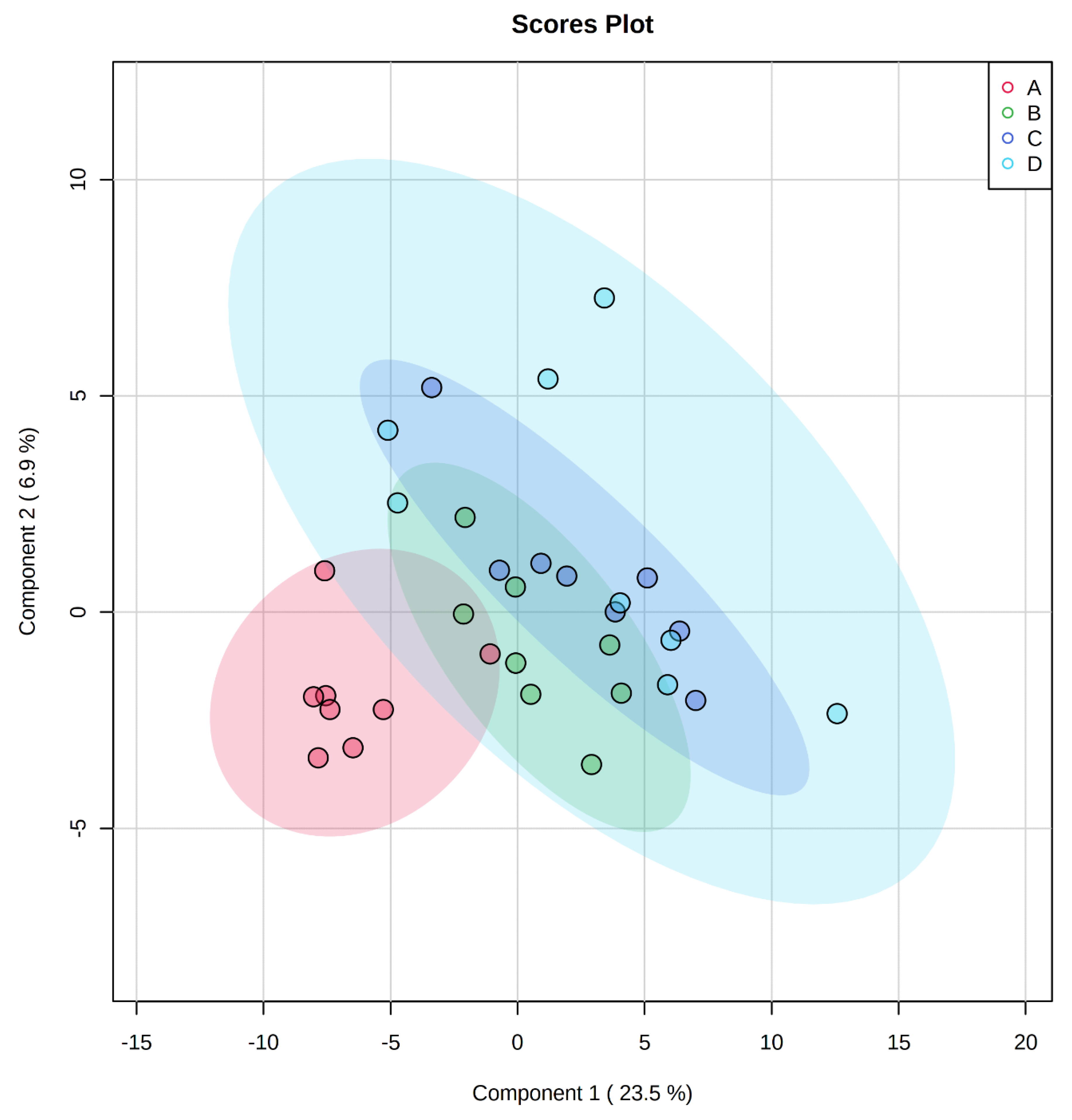
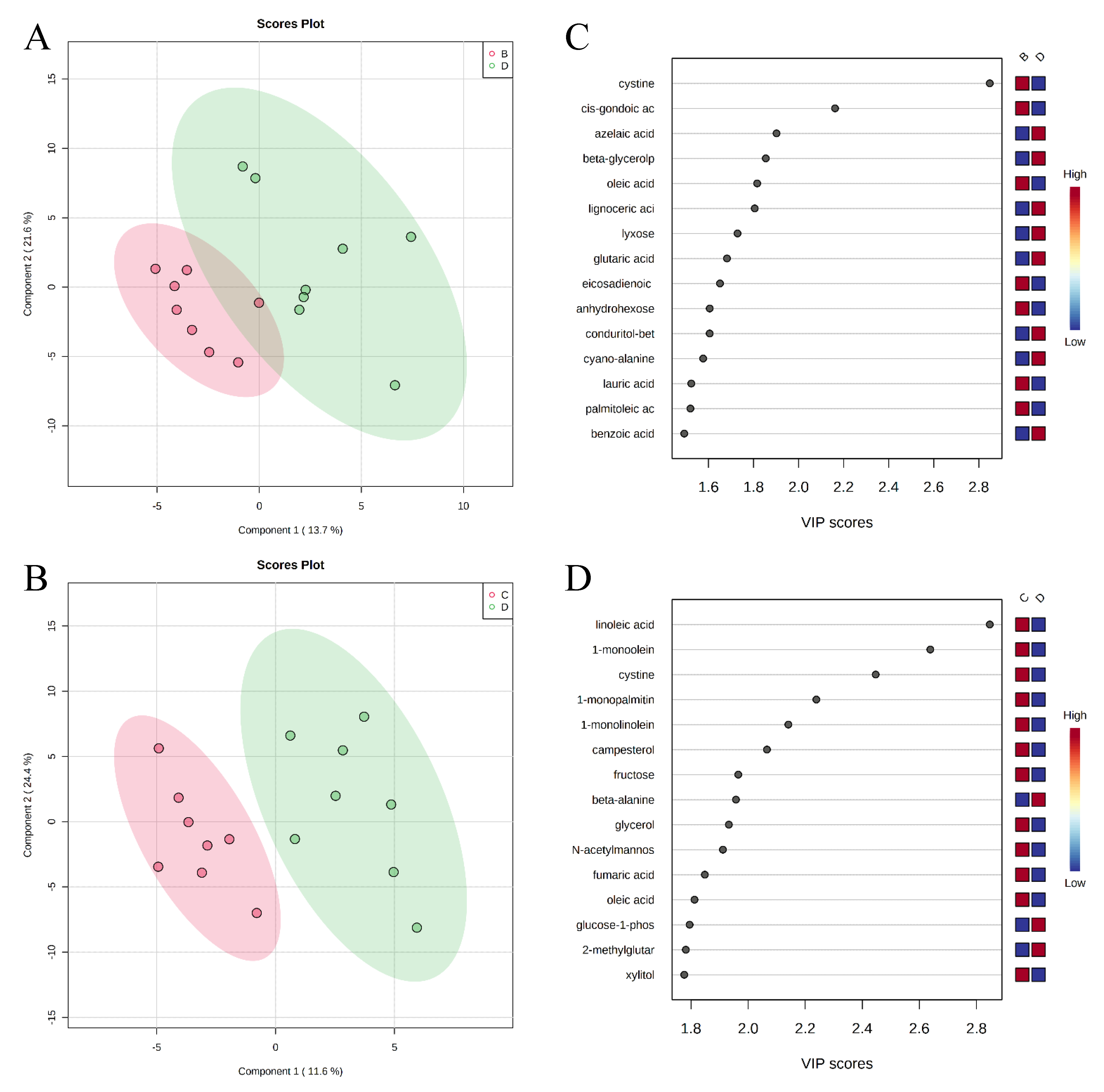
 , negative control (A),
, negative control (A),  , positive control (B),
, positive control (B),  , carbadox (C), and
, carbadox (C), and  , probiotic (D). Shaded areas in different colors represent the 95% confidence interval.
, probiotic (D). Shaded areas in different colors represent the 95% confidence interval.
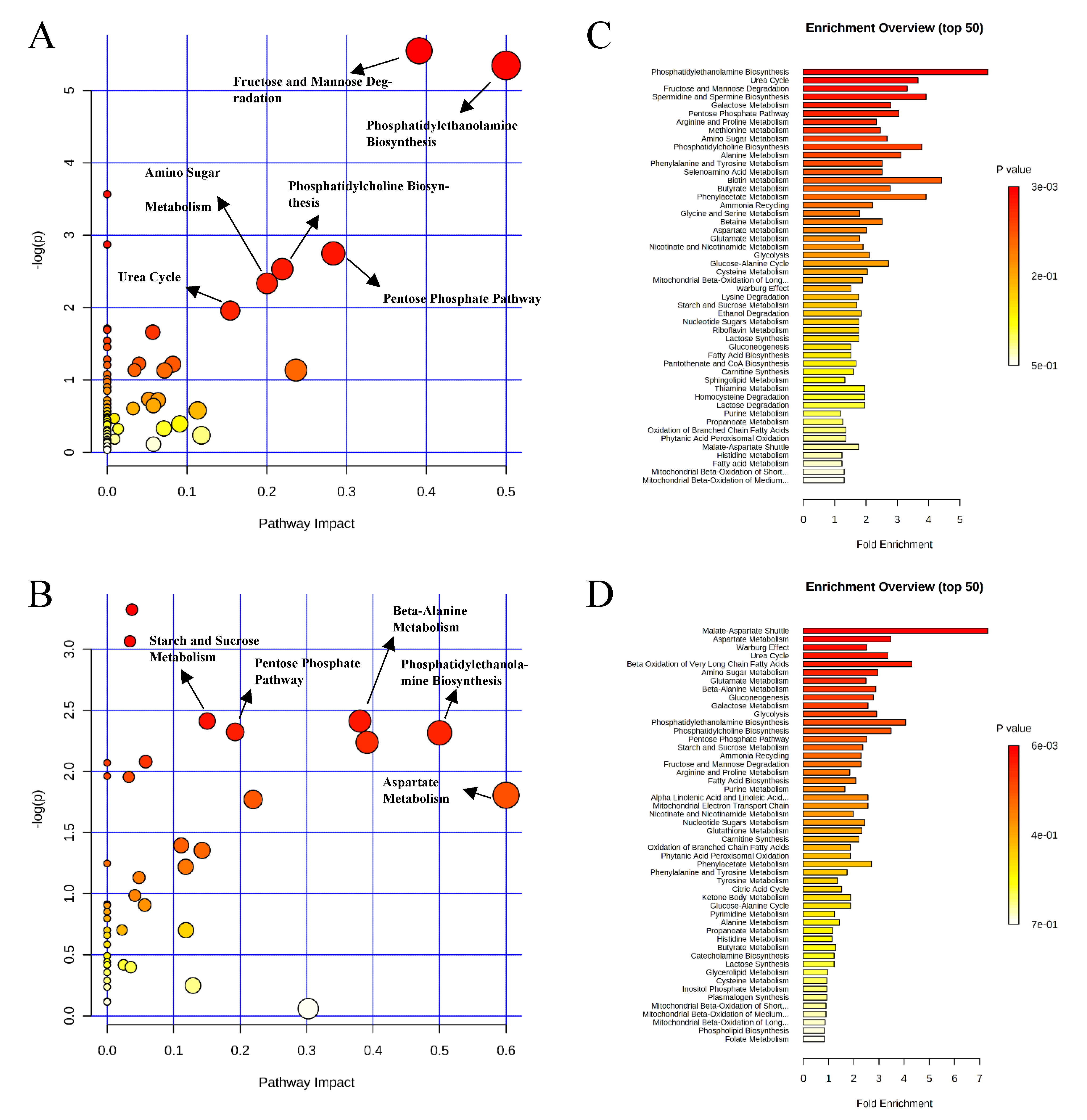
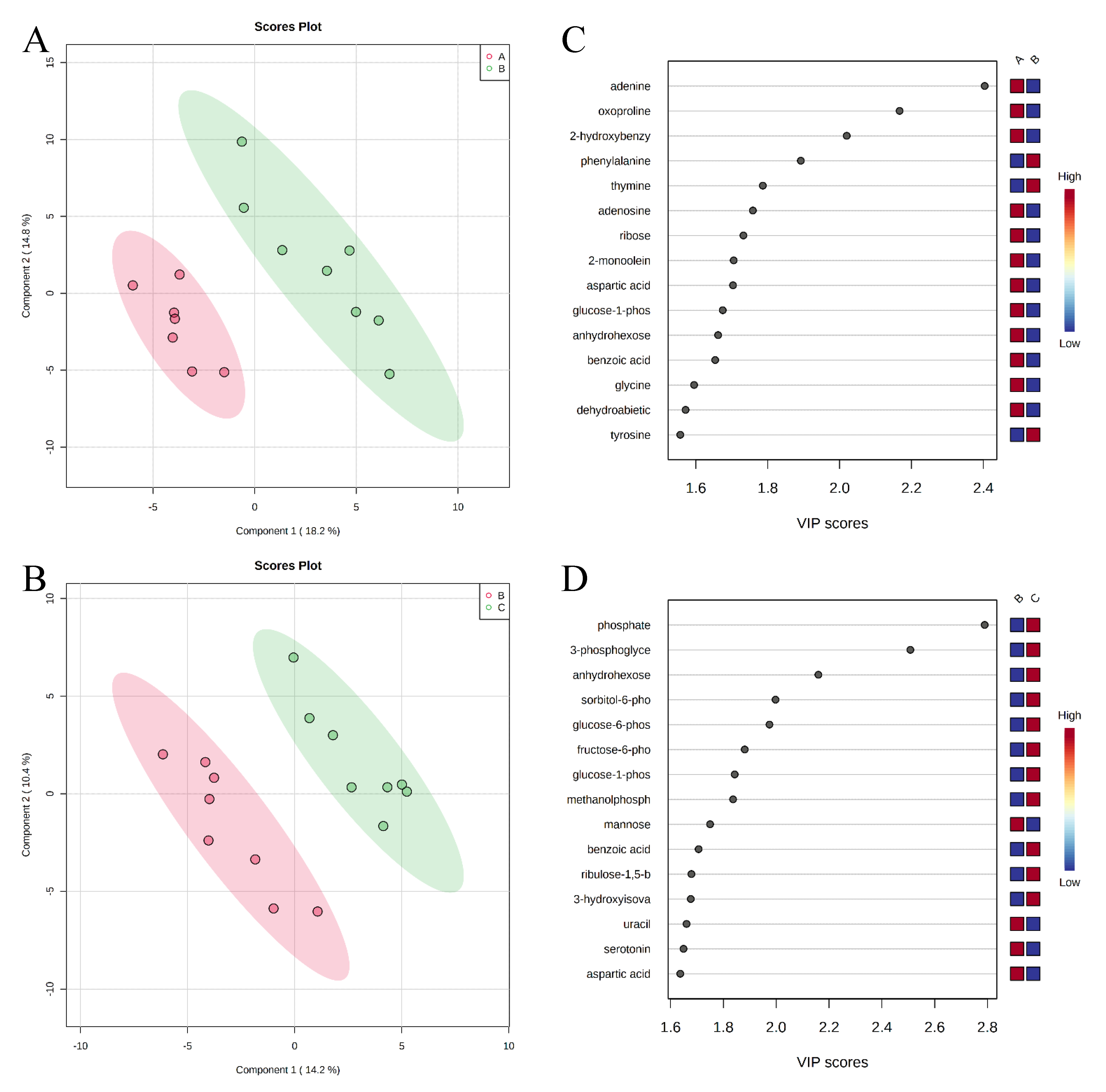
 , negative control (A),
, negative control (A),  , positive control (B),
, positive control (B),  , carbadox (C), and
, carbadox (C), and  , probiotic (D). Shaded areas in different colors represent the 95% confidence interval.
, probiotic (D). Shaded areas in different colors represent the 95% confidence interval.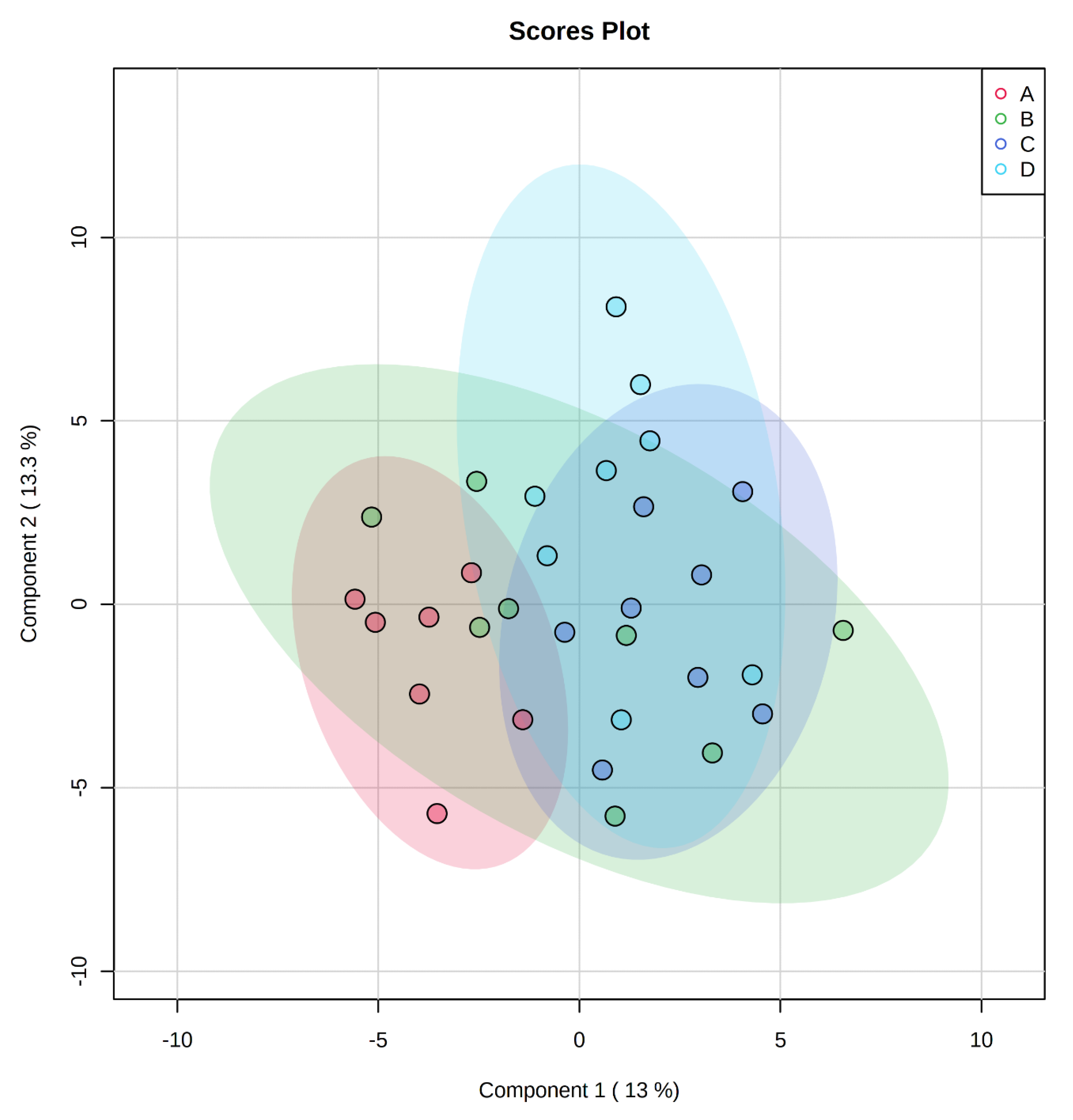

| Metabolites | Fold Change 1 | VIP 2 | FDR 3 |
|---|---|---|---|
| positive control vs. negative control | |||
| spermidine | 6.25 | 1.87 | 0.010 |
| cytidine | 3.45 | 1.65 | 0.030 |
| gluconic acid | 3.23 | 1.63 | 0.030 |
| gluconic acid lactone | 3.13 | 1.81 | 0.010 |
| gulonic acid | 2.94 | 1.43 | 0.057 |
| fructose | 2.94 | 1.51 | 0.044 |
| fructose-6-phosphate | 2.33 | 1.34 | 0.080 |
| mannose | 2.27 | 1.44 | 0.055 |
| sorbitol-6-phosphate | 2.17 | 1.22 | 0.104 |
| glucose | 2.04 | 1.30 | 0.086 |
| glucose-6-phosphate | 2.04 | 1.21 | 0.105 |
| adipic acid | 0.44 | 1.36 | 0.076 |
| pentadecanoic acid | 0.43 | 1.52 | 0.044 |
| lignoceric acid | 0.41 | 1.26 | 0.093 |
| glutaric acid | 0.38 | 1.55 | 0.044 |
| pyrophosphate | 0.38 | 1.17 | 0.118 |
| conduritol-beta-epoxide | 0.26 | 1.47 | 0.049 |
| adenosine-5-monophosphate | 0.20 | 1.53 | 0.044 |
| succinic acid | 0.17 | 1.44 | 0.055 |
| carbadox vs. probiotics | |||
| 1-monoolein | 0.50 | 2.64 | 0.052 |
| Metabolites | Fold Change 1 | VIP 2 | FDR 3 |
|---|---|---|---|
| negative control vs. positive control | |||
| adenine | 3.63 | 2.40 | 0.105 |
| positive control vs. carbadox | |||
| phosphate | 0.49 | 2.79 | 0.085 |
| carbadox vs. probiotics | |||
| 2-monoolein | 0.28 | 1.92 | 0.187 |
| lactic acid | 0.37 | 2.15 | 0.138 |
| maltose | 0.37 | 2.12 | 0.138 |
| adenine | 0.42 | 1.93 | 0.187 |
| aspartic acid | 0.50 | 1.92 | 0.187 |
| phosphate | 2.03 | 2.44 | 0.054 |
| Ingredient, % | Control, Phase I | Control, Phase II |
|---|---|---|
| Corn | 44.41 | 57.27 |
| Dried whey | 15.00 | 10.00 |
| Soybean meal | 18.00 | 22.00 |
| Fish meal | 10.00 | 7.00 |
| Lactose | 6.00 | - |
| Soy protein concentrate | 3.00 | - |
| Soybean oil | 2.00 | 2.00 |
| Limestone | 0.56 | 0.70 |
| L-Lysine·HCl | 0.21 | 0.23 |
| DL-Methionine | 0.08 | 0.05 |
| L-Threonine | 0.04 | 0.05 |
| Salt | 0.40 | 0.40 |
| Vit-mineral, Sow 6 2 | 0.30 | 0.30 |
| Total | 100.00 | 100.00 |
| Calculated energy and nutrient | ||
| Metabolizable energy, kcal/kg | 3463 | 3429 |
| Net energy, kcal/kg | 2601 | 2575 |
| Crude protein, % | 22.27 | 20.80 |
| Arg,3 % | 1.23 | 1.15 |
| His,3 % | 0.49 | 0.47 |
| Ile,3 % | 0.83 | 0.76 |
| Leu,3 % | 1.62 | 1.55 |
| Lys,3 % | 1.35 | 1.23 |
| Met,3 % | 0.45 | 0.39 |
| Thr,3 % | 0.79 | 0.73 |
| Trp,3 % | 0.23 | 0.21 |
| Val,3 % | 0.91 | 0.84 |
| Met + Cys,3 % | 0.74 | 0.68 |
| Phe + Tyr,3 % | 1.45 | 1.38 |
| Ca, % | 0.80 | 0.70 |
| Total P, % | 0.68 | 0.59 |
| Digestible P, % | 0.47 | 0.37 |
| Analyzed nutrient, as-is | ||
| Dry matter, % | 90.70 | 89.90 |
| Crude protein, % | 23.13 | 21.30 |
| ADF, % | 7.26 | 9.35 |
| NDF, % | 2.54 | 3.60 |
| Ca, % | 0.96 | 0.88 |
| P, % | 0.71 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Liu, Y.; Ji, P. Metabolomic Profile of Weaned Pigs Challenged with E. coli and Supplemented with Carbadox or Bacillus subtilis. Metabolites 2021, 11, 81. https://doi.org/10.3390/metabo11020081
He Y, Liu Y, Ji P. Metabolomic Profile of Weaned Pigs Challenged with E. coli and Supplemented with Carbadox or Bacillus subtilis. Metabolites. 2021; 11(2):81. https://doi.org/10.3390/metabo11020081
Chicago/Turabian StyleHe, Yijie, Yanhong Liu, and Peng Ji. 2021. "Metabolomic Profile of Weaned Pigs Challenged with E. coli and Supplemented with Carbadox or Bacillus subtilis" Metabolites 11, no. 2: 81. https://doi.org/10.3390/metabo11020081
APA StyleHe, Y., Liu, Y., & Ji, P. (2021). Metabolomic Profile of Weaned Pigs Challenged with E. coli and Supplemented with Carbadox or Bacillus subtilis. Metabolites, 11(2), 81. https://doi.org/10.3390/metabo11020081






