Gender-Specific Metabolomics Approach to Kidney Cancer
Abstract
:1. Introduction
2. Results
2.1. Serum 1H NMR Profiles of RCC Patients
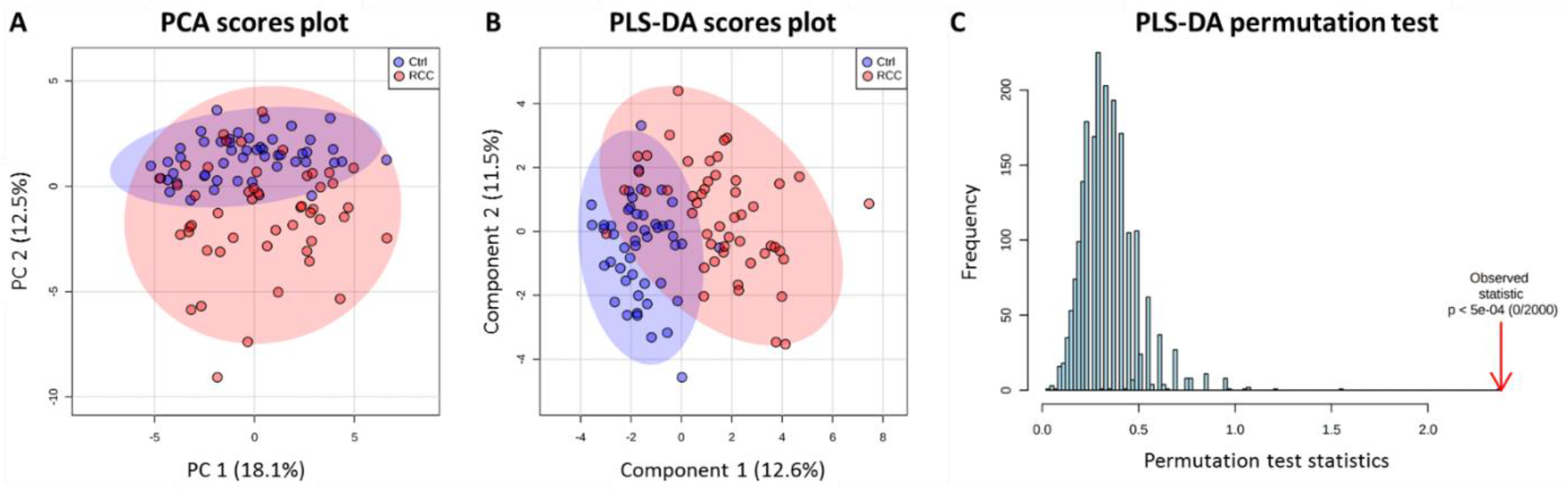
2.2. Discriminatory Potential of Serum in RCC Diagnosis Independent of Gender
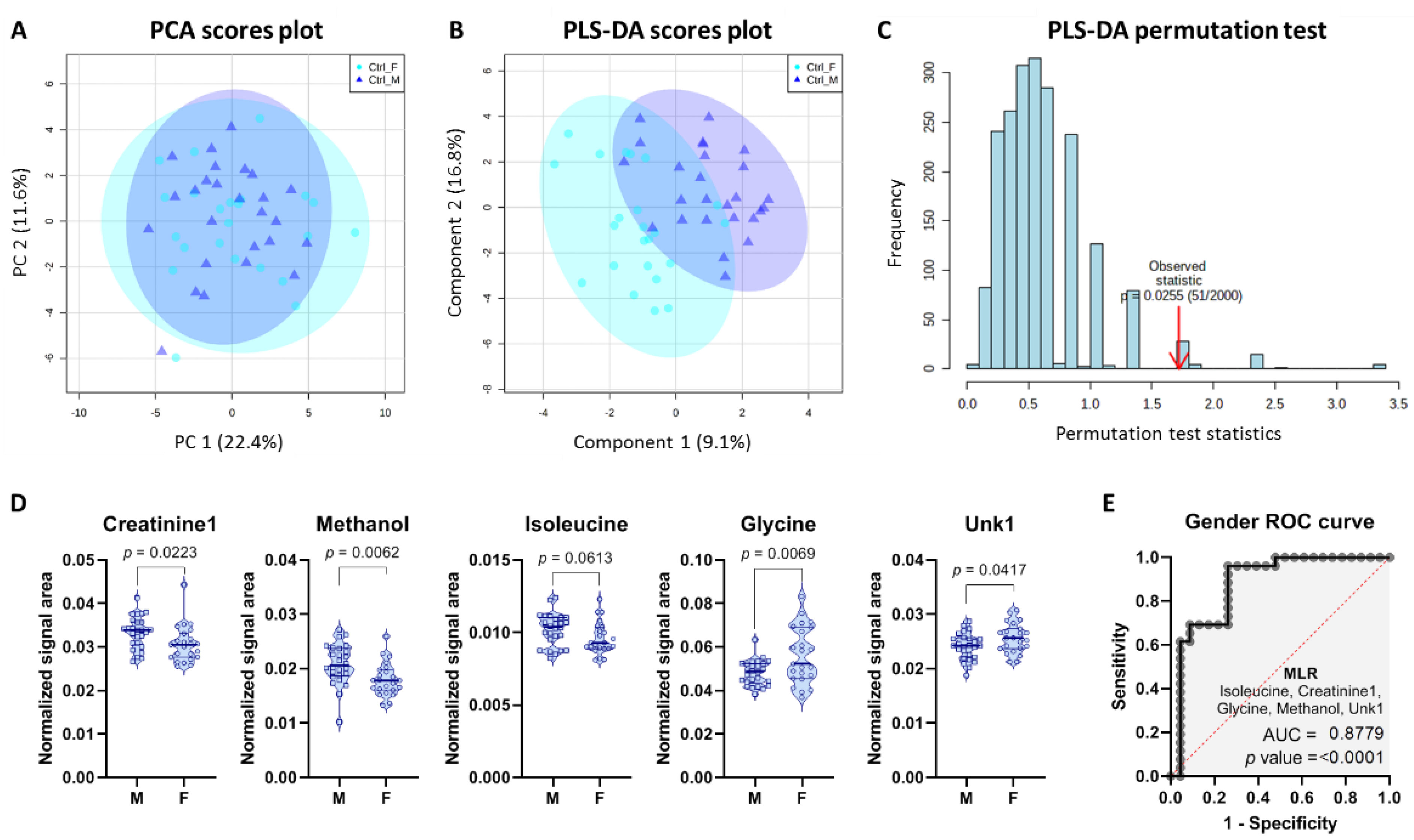
2.3. Gender-Specific Differences in Control Group
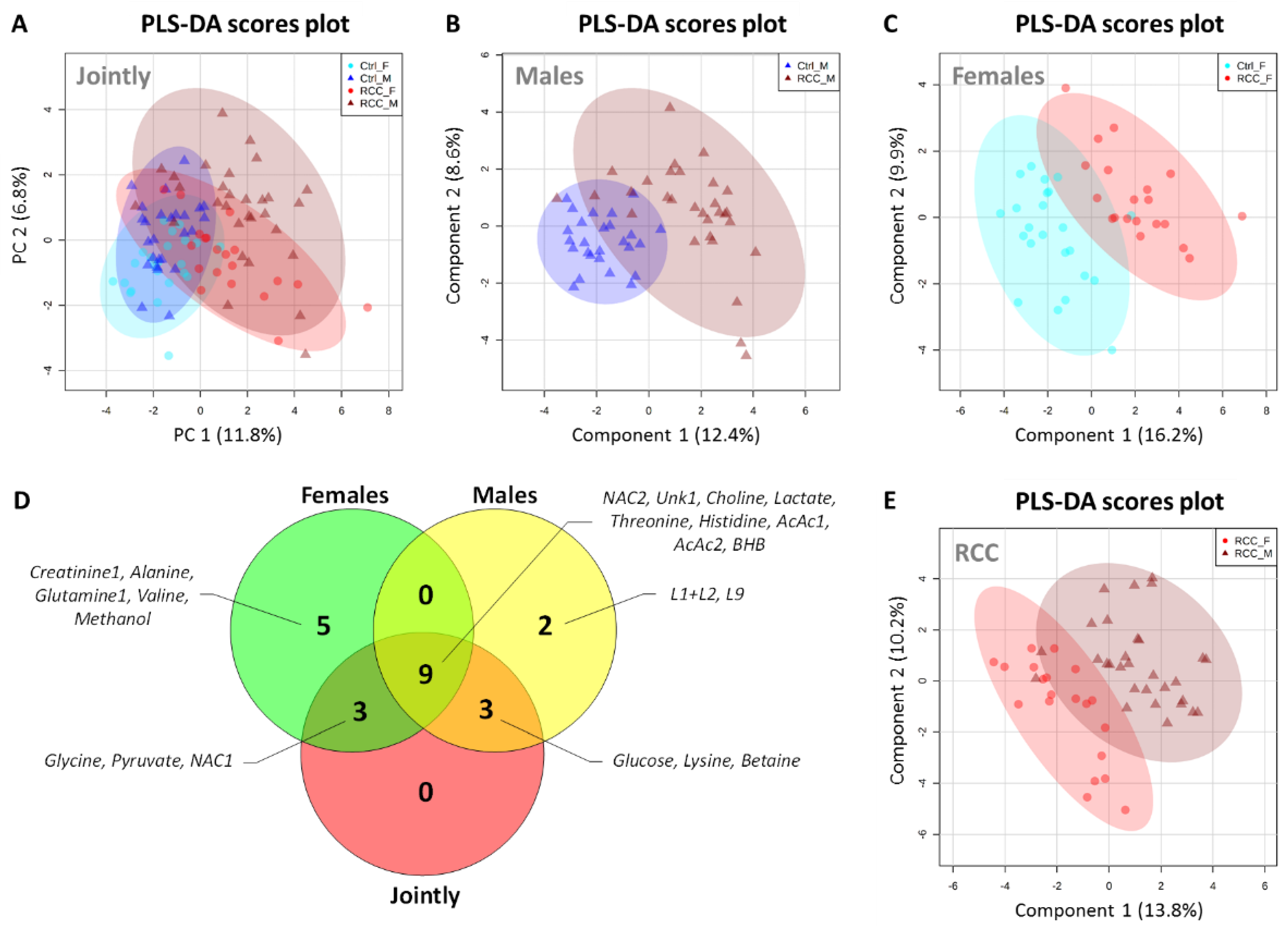
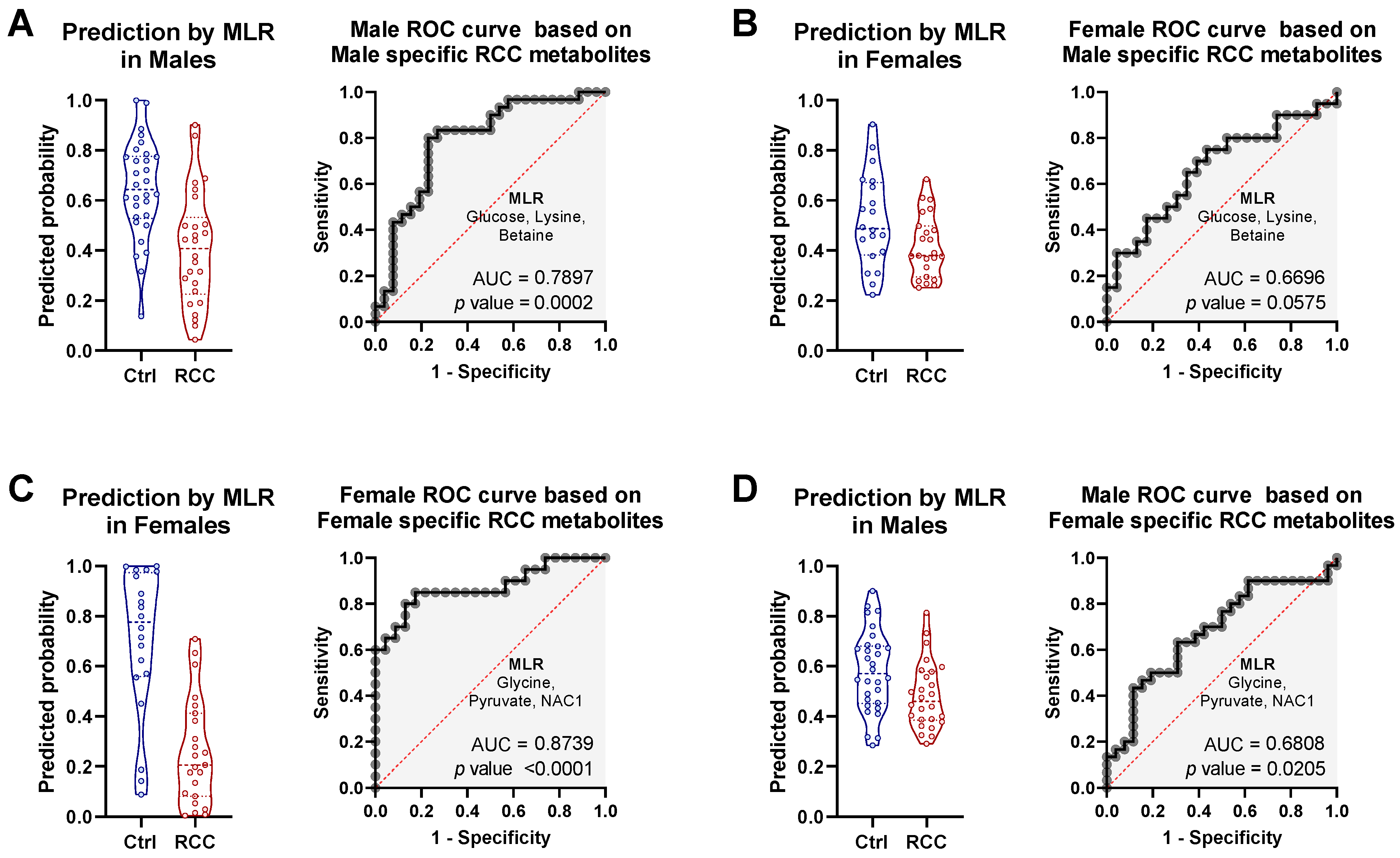
2.4. Gender-Specific Discriminatory Potential of Serum in RCC Diagnosis
2.5. Discriminatory Potential of Serum Metabolie Ratios
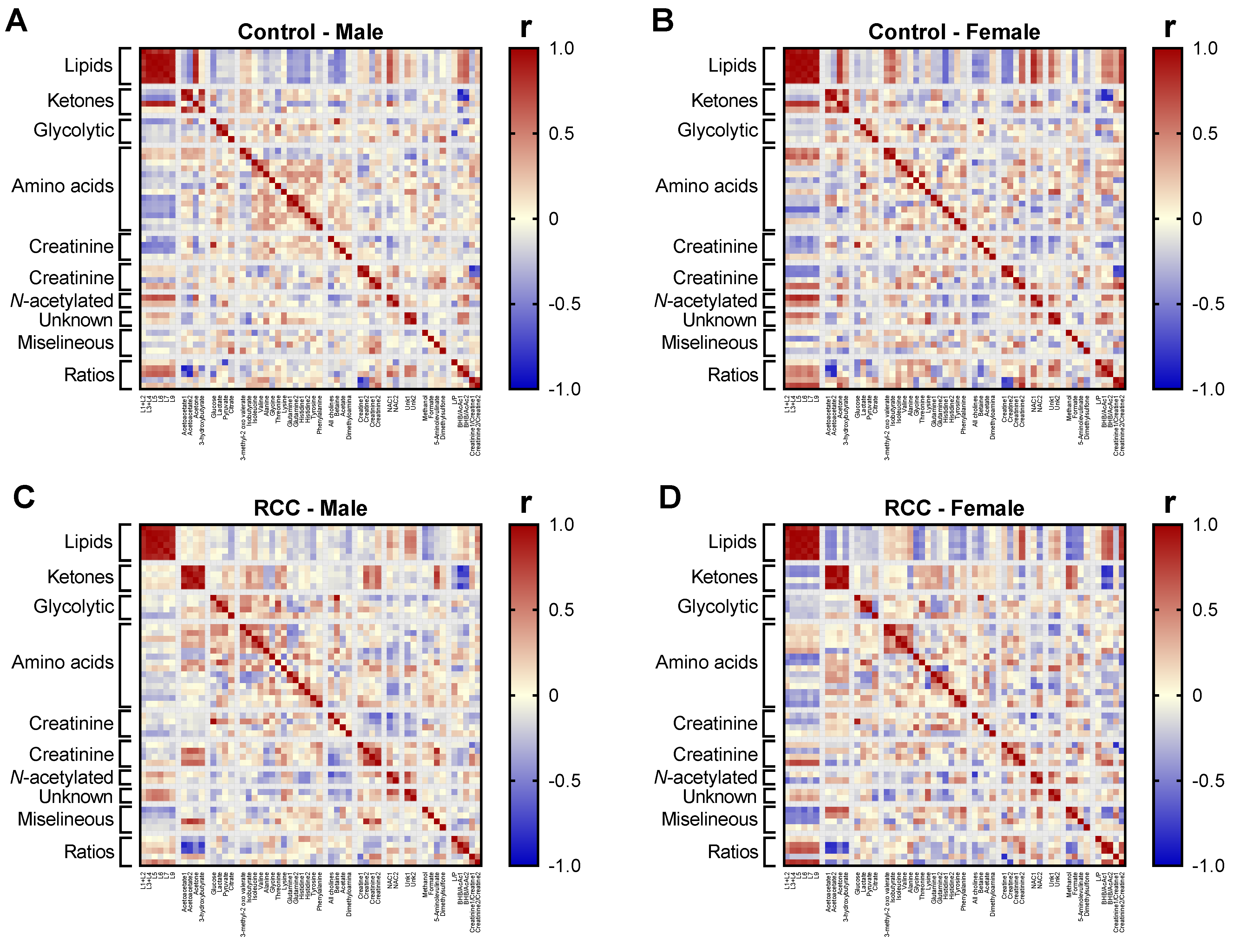
2.6. Gender-Sepcific Correlation Analysis
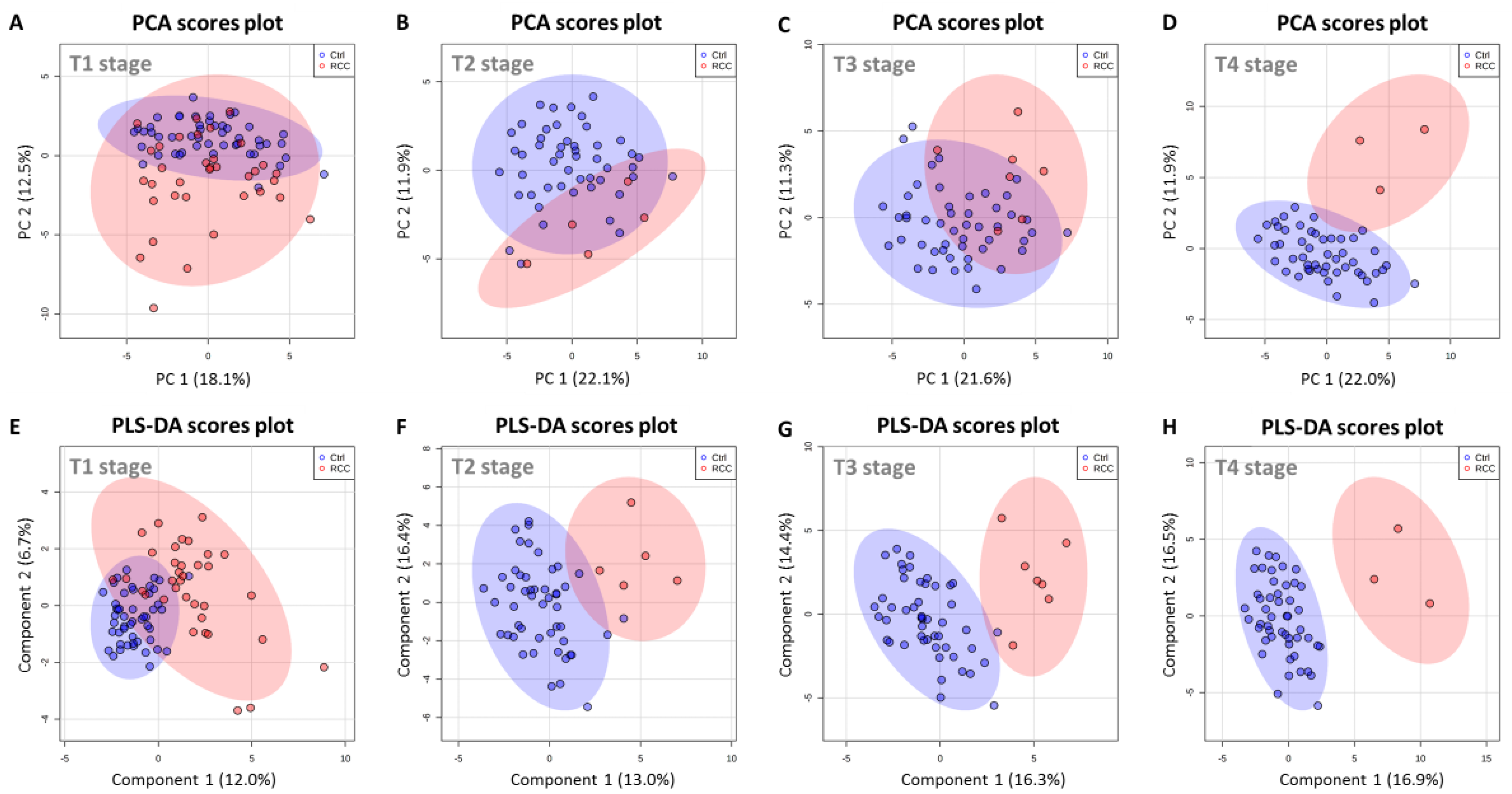
2.7. Serum Metabolic Profile Is Affected by T Stage in RCC Patients
3. Discussion
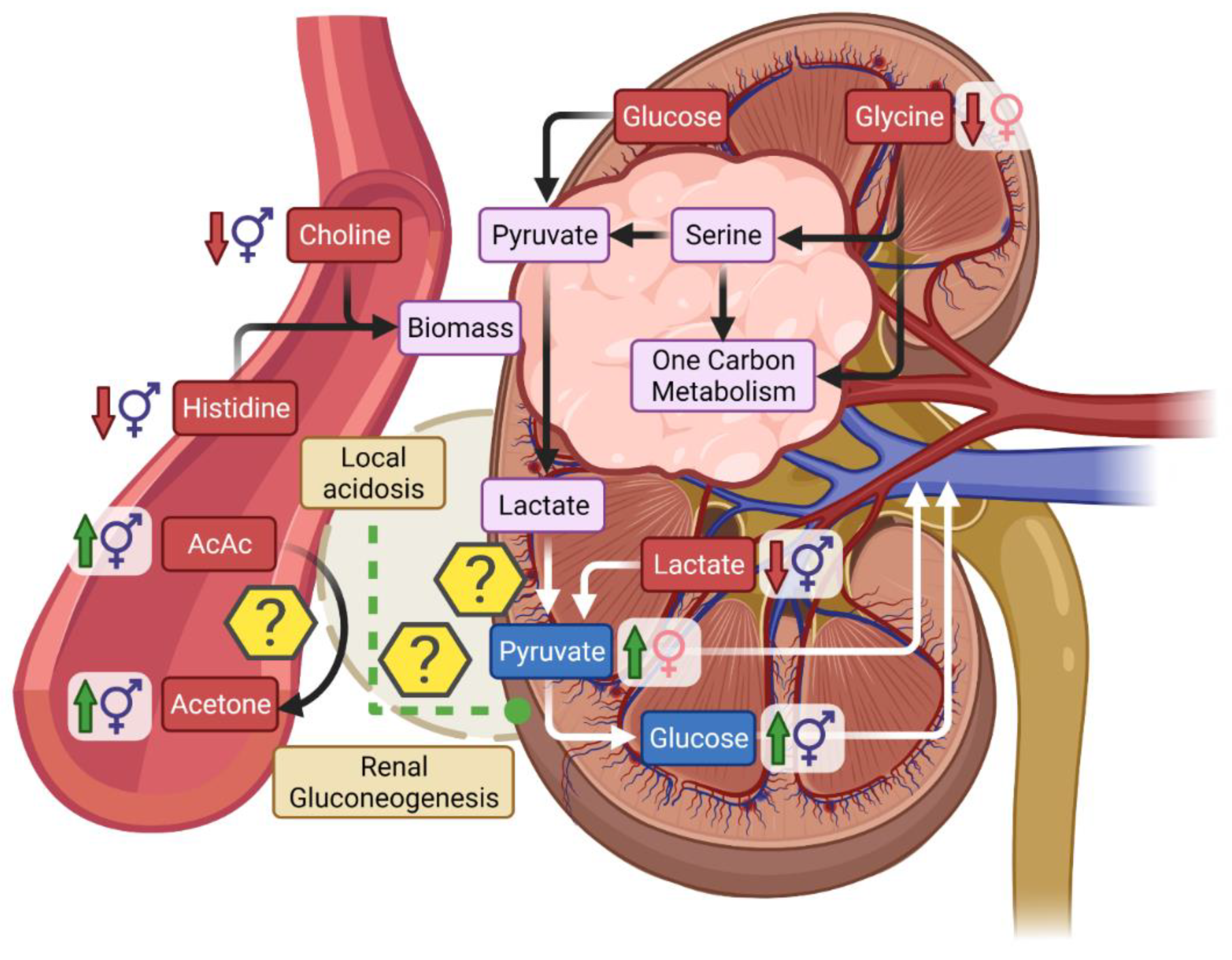
3.1. Metabolic Differences Associated with Gender and RCC
3.2. Low Circulating Lactate in Relationship with Warburg Effect in RCC
3.3. Ketones in RCC
3.4. Serum Creatinine Is a Poor Marker of Early RCC
4. Materials and Methods
4.1. Research Material Description
4.2. Sample Preparation
4.3. 1H NMR Spectroscopy Measurements and Metabolite Assignments
4.4. Data Processing and Chemometric Data Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muglia, V.F.; Prando, A. Renal cell carcinoma: Histological classification and correlation with imaging findings. Radiol. Bras. 2015, 48, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Linehan, W.M.; Bratslavsky, G.; Pinto, P.A.; Schmidt, L.S.; Neckers, L.; Bottaro, D.P.; Srinivasan, R. Molecular Diagnosis and Therapy of Kidney Cancer. Annu. Rev. Med. 2010, 61, 329–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terris, M.; Klaassen, Z.; Kabaria, R. Renal cell carcinoma: Links and risks. Int. J. Nephrol. Renov. Dis. 2016, 9, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Znaor, A.; Holcatova, I.; Fabianova, E.; Mates, D.; Wozniak, M.B.; Ferlay, J.; Scelo, G. Regional Geographic Variations in Kidney Cancer Incidence Rates in European Countries. Eur. Urol. 2015, 67, 1134–1141. [Google Scholar] [CrossRef]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2018, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chen, W.; Xia, Q.; Shi, O.; Chen, Q. Trends and projections of kidney cancer incidence at the global and national levels, 1990–2030: A Bayesian age-period-cohort modeling study. Biomark. Res. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Mancini, M.; Righetto, M.; Baggio, G. Gender-Related Approach to Kidney Cancer Management: Moving Forward. Int. J. Mol. Sci. 2020, 21, 3378. [Google Scholar] [CrossRef]
- Wiinberg, N.; Høegholm, A.; Christensen, H.R.; Bang, L.E.; Mikkelsen, K.L.; Nielsen, P.E.; Svendsen, T.L.; Kampmann, J.P.; Madsen, N.H.; Bentzon, M.W. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender*. Am. J. Hypertens. 1995, 8, 978–986. [Google Scholar] [CrossRef]
- Layton, A.T.; Sullivan, J.C. Recent advances in sex differences in kidney function. Am. J. Physiol. Physiol. 2019, 316, F328–F331. [Google Scholar] [CrossRef] [PubMed]
- Lucca, I.; Klatte, T.; Fajkovic, H.; De Martino, M.; Shariat, S.F. Gender differences in incidence and outcomes of urothelial and kidney cancer. Nat. Rev. Urol. 2015, 12, 585–592. [Google Scholar] [CrossRef]
- Hew, M.N.; Zonneveld, R.; Kümmerlin, I.P.E.D.; Opondo, D.; De La Rosette, J.J.M.C.H.; Laguna, M.P. Age and Gender Related Differences in Renal Cell Carcinoma in a European Cohort. J. Urol. 2012, 188, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Mph, K.D.M.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Scelo, G.; Li, P.; Chanudet, E.; Muller, D.C. Variability of Sex Disparities in Cancer Incidence over 30 Years: The Striking Case of Kidney Cancer. Eur. Urol. Focus 2018, 4, 586–590. [Google Scholar] [CrossRef]
- Gelfond, J.; Al-Bayati, O.; Kabra, A.; Iffrig, K.; Kaushik, D.; Liss, M.A. Modifiable risk factors to reduce renal cell carcinoma incidence: Insight from the PLCO trial. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 340.e1–340.e6. [Google Scholar] [CrossRef] [PubMed]
- Aron, M.; Nguyen, M.M.; Stein, R.J.; Gill, I.S. Impact of Gender in Renal Cell Carcinoma: An Analysis of the SEER Database. Eur. Urol. 2008, 54, 133–142. [Google Scholar] [CrossRef]
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2015, 387, 894–906. [Google Scholar] [CrossRef]
- Patel, H.D.; Johnson, M.H.; Pierorazio, P.M.; Sozio, S.; Sharma, R.; Iyoha, E.; Bass, E.; Allaf, M.E. Diagnostic Accuracy and Risks of Biopsy in the Diagnosis of a Renal Mass Suspicious for Localized Renal Cell Carcinoma: Systematic Review of the Literature. J. Urol. 2016, 195, 1340–1347. [Google Scholar] [CrossRef] [Green Version]
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Rahman, A.M.A. Metabolomics toward personalized medicine. Mass Spectrom. Rev. 2017, 38, 221–238. [Google Scholar] [CrossRef]
- Gao, H.; Dong, B.; Liu, X.; Xuan, H.; Huang, Y.; Lin, D. Metabonomic profiling of renal cell carcinoma: High-resolution proton nuclear magnetic resonance spectroscopy of human serum with multivariate data analysis. Anal. Chim. Acta 2008, 624, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Nizioł, J.; Ossoliński, K.; Tripet, B.P.; Copié, V.; Arendowski, A.; Ruman, T. Nuclear magnetic resonance and surface-assisted laser desorption/ionization mass spectrometry-based serum metabolomics of kidney cancer. Anal. Bioanal. Chem. 2020, 412, 5827–5841. [Google Scholar] [CrossRef]
- Zheng, H.; Ji, J.; Zhao, L.; Chen, M.; Shi, A.; Pan, L.; Huang, Y.; Zhang, H.; Dong, B.; Gao, H. Prediction and diagnosis of renal cell carcinoma using nuclear magnetic resonance-based serum metabolomics and self-organizing maps. Oncotarget 2016, 7, 59189–59198. [Google Scholar] [CrossRef] [Green Version]
- Zira, A.N.; Theocharis, S.E.; Mitropoulos, D.; Migdalis, V.; Mikros, E. 1H NMR Metabonomic Analysis in Renal Cell Carcinoma: A Possible Diagnostic Tool. J. Proteome Res. 2010, 9, 4038–4044. [Google Scholar] [CrossRef] [PubMed]
- Nizioł, J.; Ossoliński, K.; Tripet, B.P.; Copié, V.; Arendowski, A.; Ruman, T. Nuclear magnetic resonance and surface-assisted laser desorption/ionization mass spectrometry-based metabolome profiling of urine samples from kidney cancer patients. J. Pharm. Biomed. Anal. 2020, 193, 113752. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Barros, A.; Pinto, J.; Carvalho, M.; Pires-Luís, A.S.; Henrique, R.; Jeronimo, C.; Bastos, M.D.L.; Gil, A.M.; de Pinho, P.G. Nuclear Magnetic Resonance metabolomics reveals an excretory metabolic signature of renal cell carcinoma. Sci. Rep. 2016, 6, 37275. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Dong, B.; Jia, J.; Zhu, H.; Diao, C.; Yan, Z.; Huang, Y.; Li, X. Application of ex vivo 1H NMR metabonomics to the characterization and possible detection of renal cell carcinoma metastases. J. Cancer Res. Clin. Oncol. 2012, 138, 753–761. [Google Scholar] [CrossRef]
- Jing, L.; Guigonis, J.-M.; Borchiellini, D.; Durand, M.; Pourcher, T.; Ambrosetti, D. LC-MS based metabolomic profiling for renal cell carcinoma histologic subtypes. Sci. Rep. 2019, 9, 15635. [Google Scholar] [CrossRef]
- Arendowski, A.; Ossoliński, K.; Ossolińska, A.; Ossoliński, T.; Nizioł, J.; Ruman, T. Serum and urine analysis with gold nanoparticle-assisted laser desorption/ionization mass spectrometry for renal cell carcinoma metabolic biomarkers discovery. Adv. Med. Sci. 2021, 66, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Falegan, O.S.; Ball, M.W.; Shaykhutdinov, R.A.; Pieroraio, P.M.; Farshidfar, F.; Vogel, H.J.; Allaf, M.E.; Hyndman, M.E. Urine and Serum Metabolomics Analyses May Distinguish between Stages of Renal Cell Carcinoma. Metabolites 2017, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Nizioł, J.; Bonifay, V.; Ossoliński, K.; Ossoliński, T.; Ossolińska, A.; Sunner, J.; Beech, I.; Arendowski, A.; Ruman, T. Metabolomic study of human tissue and urine in clear cell renal carcinoma by LC-HRMS and PLS-DA. Anal. Bioanal. Chem. 2018, 410, 3859–3869. [Google Scholar] [CrossRef] [Green Version]
- Corkey, B.E.; Deeney, J.T. The Redox Communication Network as a Regulator of Metabolism. Front. Physiol. 2020, 11, 567796. [Google Scholar] [CrossRef]
- Vignoli, A.; Tenori, L.; Luchinat, C.; Saccenti, E. Age and Sex Effects on Plasma Metabolite Association Networks in Healthy Subjects. J. Proteome Res. 2017, 17, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ix, J.H.; Shlipak, M.G. The Promise of Tubule Biomarkers in Kidney Disease: A Review. Am. J. Kidney Dis. 2021, 78, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.A.; Ferreira, D.L.S.; Fraser, A.; Soares, A.L.G.; Howe, L.D.; Lawlor, D.A.; Carslake, D.; Smith, G.D.; O’Keeffe, L.M. Sex differences in systemic metabolites at four life stages: Cohort study with repeated metabolomics. BMC Med. 2021, 19, 1–13. [Google Scholar] [CrossRef]
- Krumsiek, J.; Mittelstrass, K.; Do, K.T.; Stückler, F.; Ried, J.; Adamski, J.; Peters, A.; Illig, T.; Kronenberg, F.; Friedrich, N.; et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics 2015, 11, 1815–1833. [Google Scholar] [CrossRef] [Green Version]
- Oja, S.S.; Kemppainen, K.; Mero, A.A. Serum amino acid concentrations in aging men and women. Amino Acids 2003, 24, 413–421. [Google Scholar] [CrossRef]
- Rogiers, V. Long chain nonesterified fatty acid patterns in plasma of healthy children and young adults in relation to age and sex. J. Lipid Res. 1981, 22, 1–6. [Google Scholar] [CrossRef]
- Ellul, S.; Wake, M.; A Clifford, S.; Lange, K.; Würtz, P.; Juonala, M.; Dwyer, T.; Carlin, J.B.; Burgner, D.P.; Saffery, R. Metabolomics: Population epidemiology and concordance in Australian children aged 11–12 years and their parents. BMJ Open 2019, 9, 106–117. [Google Scholar] [CrossRef]
- Saito, K.; Maekawa, K.; Kinchen, J.; Tanaka, R.; Kumagai, Y.; Saito, Y. Gender- and Age-Associated Differences in Serum Metabolite Profiles among Japanese Populations. Biol. Pharm. Bull. 2016, 39, 1179–1186. [Google Scholar] [CrossRef] [Green Version]
- Linehan, W.M.; Schmidt, L.S.; Crooks, D.R.; Wei, D.; Srinivasan, R.; Lang, M.; Ricketts, C.J. The Metabolic Basis of Kidney Cancer. Cancer Discov. 2019, 9, 1006–1021. [Google Scholar] [CrossRef] [Green Version]
- Keshari, K.R.; Sriram, R.; Koelsch, B.L.; Van Criekinge, M.; Wilson, D.M.; Kurhanewicz, J.; Wang, Z.J. Hyperpolarized 13C-Pyruvate Magnetic Resonance Reveals Rapid Lactate Export in Metastatic Renal Cell Carcinomas. Cancer Res. 2012, 73, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Inigo, M.; Deja, S.; Burgess, S.C. Ins and Outs of the TCA Cycle: The Central Role of Anaplerosis. Annu. Rev. Nutr. 2021, 41, 19–47. [Google Scholar] [CrossRef]
- Courtney, K.D.; Bezwada, D.; Mashimo, T.; Pichumani, K.; Vemireddy, V.; Funk, A.; Wimberly, J.; McNeil, S.S.; Kapur, P.; Lotan, Y.; et al. Isotope Tracing of Human Clear Cell Renal Cell Carcinomas Demonstrates Suppressed Glucose Oxidation In Vivo. Cell Metab. 2018, 28, 793–800.e2. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Qiu, B.; Lee, D.; Walton, Z.E.; Ochocki, J.D.; Mathew, L.K.; Mancuso, A.; Gade, T.P.F.; Keith, B.; Nissim, I.; et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature 2014, 513, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Gerich, J.E.; Meyer, C.; Woerle, H.J.; Stumvoll, M. Renal Gluconeogenesis: Its importance in human glucose homeostasis. Diabetes Care 2001, 24, 382–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aber, G.M.; Morris, L.O.; Housley, E.; Aber, L.O.M.G.M. Gluconeogenesis by the Human Kidney. Nature 1966, 212, 1589–1590. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R. Bench-to-bedside review: Lactate and the kidney. Crit. Care 2002, 6, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Baverel, G.; Bonnard, M.; de Castanet, E.D.; Pellet, M. Lactate and pyruvate metabolism in isolated renal tubules of normal dogs. Kidney Int. 1978, 14, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaduto, R.C.; Davis, E.J. The involvement of pyruvate cycling in the metabolism of aspartate and glycerate by the perfused rat kidney. Biochem. J. 1986, 237, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.; Hulikova, A. The chemistry, physiology and pathology of pH in cancer. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130099. [Google Scholar] [CrossRef] [Green Version]
- Palgon, N.; Greenstein, F.; Novetsky, A.D.; Lichter, S.M.; Rosen, Y. Hyperglycemia associated with renal cell carcinoma. Urology 1986, 28, 516–517. [Google Scholar] [CrossRef]
- Gapp, J.; Anwar, M.F.; Parekh, J.; Griffin, T. New onset hyperglycemia attributed to renal cell carcinoma. Intractable Rare Dis. Res. 2018, 7, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [Green Version]
- Deja, S.; Kucejova, B.; Fu, X.; Browning, J.; Young, J.; Burgess, S. In Vivo Estimation of Ketogenesis Using Metabolic Flux Analysis—Technical Aspects and Model Interpretation. Metabolites 2021, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.M.; Williamson, D.H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 1980, 60, 143–187. [Google Scholar] [CrossRef]
- Weidemann, M.J.; Krebs, H.A. The fuel of respiration of rat kidney cortex. Biochem. J. 1969, 112, 149–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, O.E.; Felig, P.; Morgan, A.P.; Wahren, J.; Cahill, G.F. Liver and kidney metabolism during prolonged starvation. J. Clin. Investig. 1969, 48, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, H.; Kong, X.; Wang, K.; Mao, X.; Yan, X.; Wang, Y.; Liu, S.; Zhang, X.; Li, J.; et al. Proteomics analysis reveals diabetic kidney as a ketogenic organ in type 2 diabetes. Am. J. Physiol. Metab. 2011, 300, E287–E295. [Google Scholar] [CrossRef] [PubMed]
- Deja, S.; Porebska, I.; Kowal, A.; Zabek, A.; Barg, W.; Pawelczyk, K.; Stanimirova, I.; Daszykowski, M.; Korzeniewska, A.; Jankowska, R.; et al. Metabolomics provide new insights on lung cancer staging and discrimination from chronic obstructive pulmonary disease. J. Pharm. Biomed. Anal. 2014, 100, 369–380. [Google Scholar] [CrossRef]
- Dawiskiba, T. Serum and urine metabolomic fingerprinting in diagnostics of inflammatory bowel diseases. World J. Gastroenterol. 2014, 20, 163–174. [Google Scholar] [CrossRef]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Reson. 2010, 202, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
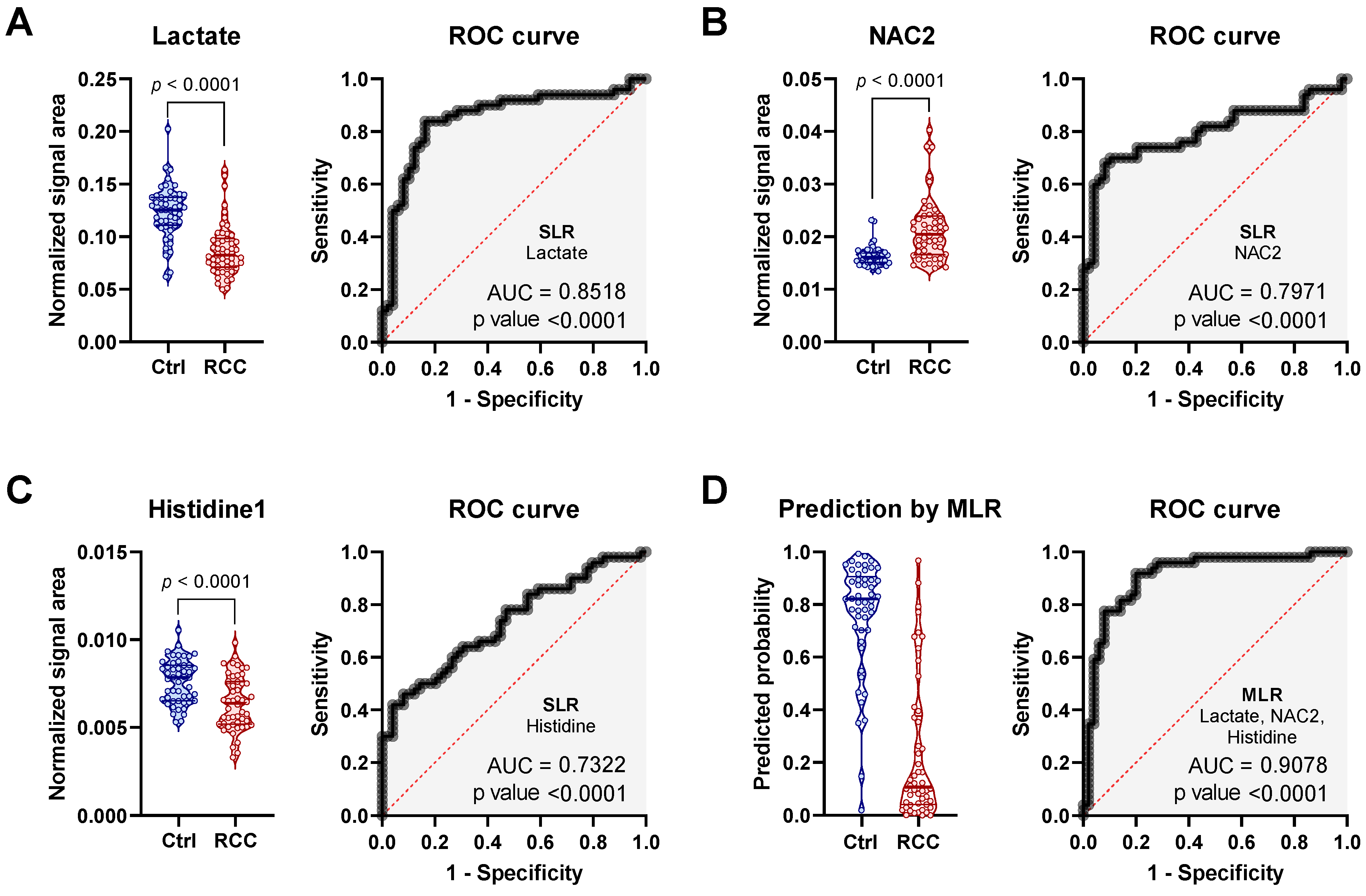


| Metabolite | VIP a | Change (%) b | AUC c | p Value c |
|---|---|---|---|---|
| Lactate | 2.4590 | −29.2% | 0.8518 | <0.0001 |
| NAC2 | 2.1876 | +30.7% | 0.7971 | <0.0001 |
| Threonine | 2.0266 | −20.8% | 0.7951 | <0.0001 |
| Histidine | 1.7171 | −16.2% | 0.7322 | <0.0001 |
| Unk1 | 1.7071 | +10.9% | 0.7318 | <0.0001 |
| BHB | 1.5806 | +18.8% | 0.7298 | <0.0001 |
| AcAc1 | 1.5401 | +41.4% | 0.7155 | 0.0002 |
| AcAc2 | 1.4778 | +46.4% | 0.7049 | 0.0004 |
| Pyruvate | 1.3728 | +21.9% | 0.698 | 0.0007 |
| Choline | 1.3658 | −12.8% | 0.6869 | 0.0013 |
| Lysine | 1.1665 | +4.2% | 0.6796 | 0.0021 |
| NAC1 | 1.1630 | +8.6% | 0.6563 | 0.0074 |
| Jointly | Male | Female | |
|---|---|---|---|
| No. patients | 50 | 30 | 20 |
| Mean age | 64.4 | 64.3 | 64.7 |
| (range) Median BMI Diabetes | (32–87) - 5 | (32–82) 24.8 2 | (51–87) 23.6 3 |
| Tumor stage (pT) | |||
| pT1 | 35 | 20 | 15 |
| pT2 | 5 | 2 | 3 |
| pT3 | 7 | 5 | 2 |
| pT4 | 3 | 3 | 0 |
| RCC subtype | |||
| Clear cell RCC | 41 | 23 | 18 |
| Papillary RCC | 5 | 5 | 0 |
| Chromophobe RCC | 4 | 2 | 2 |
| Fuhrman Grade | |||
| 1 | 18 | 10 | 8 |
| 2 | 21 | 14 | 7 |
| 3 | 10 | 6 | 4 |
| 4 | 1 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deja, S.; Litarski, A.; Mielko, K.A.; Pudełko-Malik, N.; Wojtowicz, W.; Zabek, A.; Szydełko, T.; Młynarz, P. Gender-Specific Metabolomics Approach to Kidney Cancer. Metabolites 2021, 11, 767. https://doi.org/10.3390/metabo11110767
Deja S, Litarski A, Mielko KA, Pudełko-Malik N, Wojtowicz W, Zabek A, Szydełko T, Młynarz P. Gender-Specific Metabolomics Approach to Kidney Cancer. Metabolites. 2021; 11(11):767. https://doi.org/10.3390/metabo11110767
Chicago/Turabian StyleDeja, Stanisław, Adam Litarski, Karolina Anna Mielko, Natalia Pudełko-Malik, Wojciech Wojtowicz, Adam Zabek, Tomasz Szydełko, and Piotr Młynarz. 2021. "Gender-Specific Metabolomics Approach to Kidney Cancer" Metabolites 11, no. 11: 767. https://doi.org/10.3390/metabo11110767
APA StyleDeja, S., Litarski, A., Mielko, K. A., Pudełko-Malik, N., Wojtowicz, W., Zabek, A., Szydełko, T., & Młynarz, P. (2021). Gender-Specific Metabolomics Approach to Kidney Cancer. Metabolites, 11(11), 767. https://doi.org/10.3390/metabo11110767









