Gut Microbiota Metabolism of Bile Acids Could Contribute to the Bariatric Surgery Improvements in Extreme Obesity
Abstract
:1. Introduction
2. Results
2.1. Clinical Data of the Volunteers
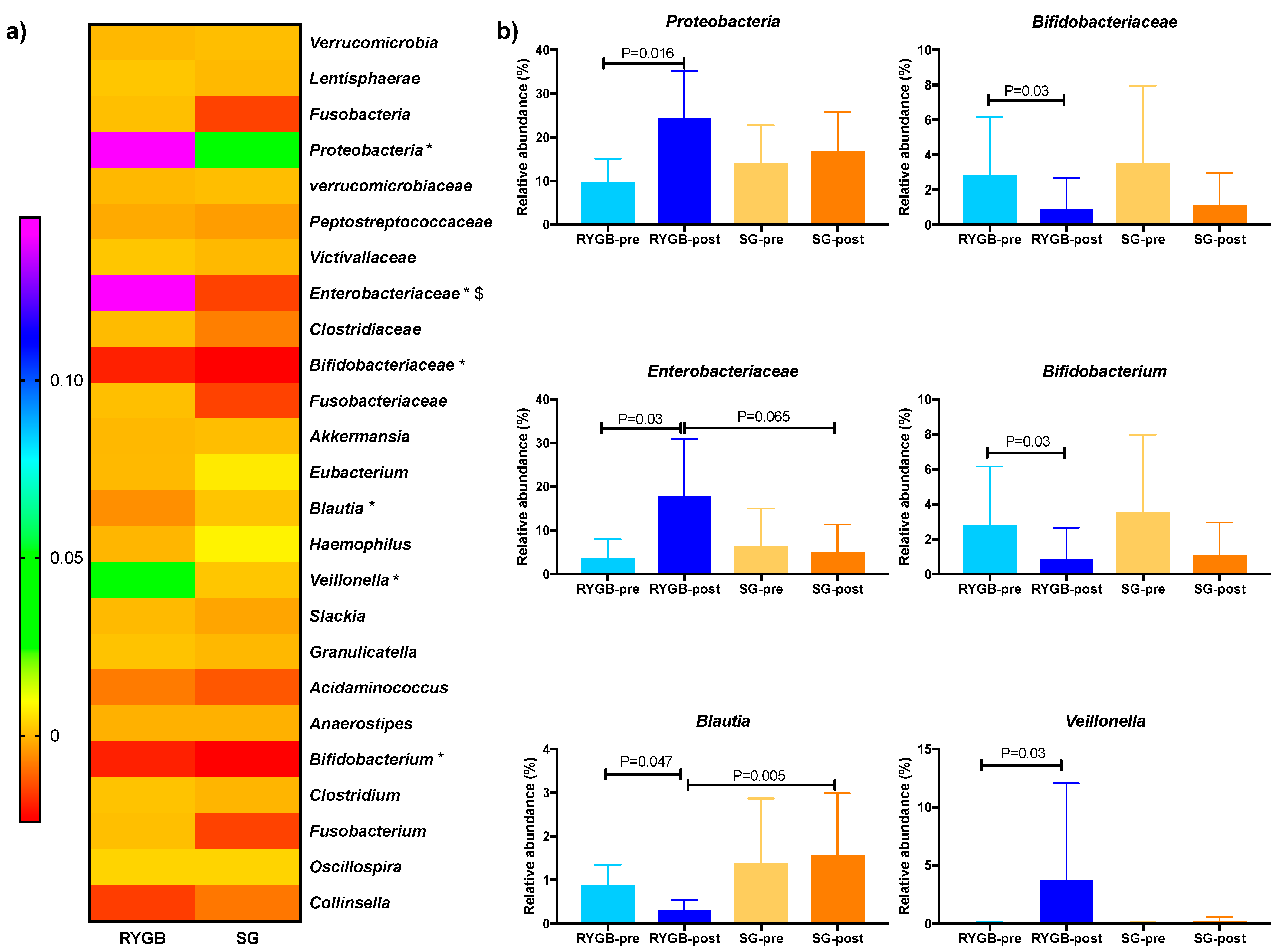
2.2. Gut Microbiota Results
2.3. Microbial Differential Abundance Analysis
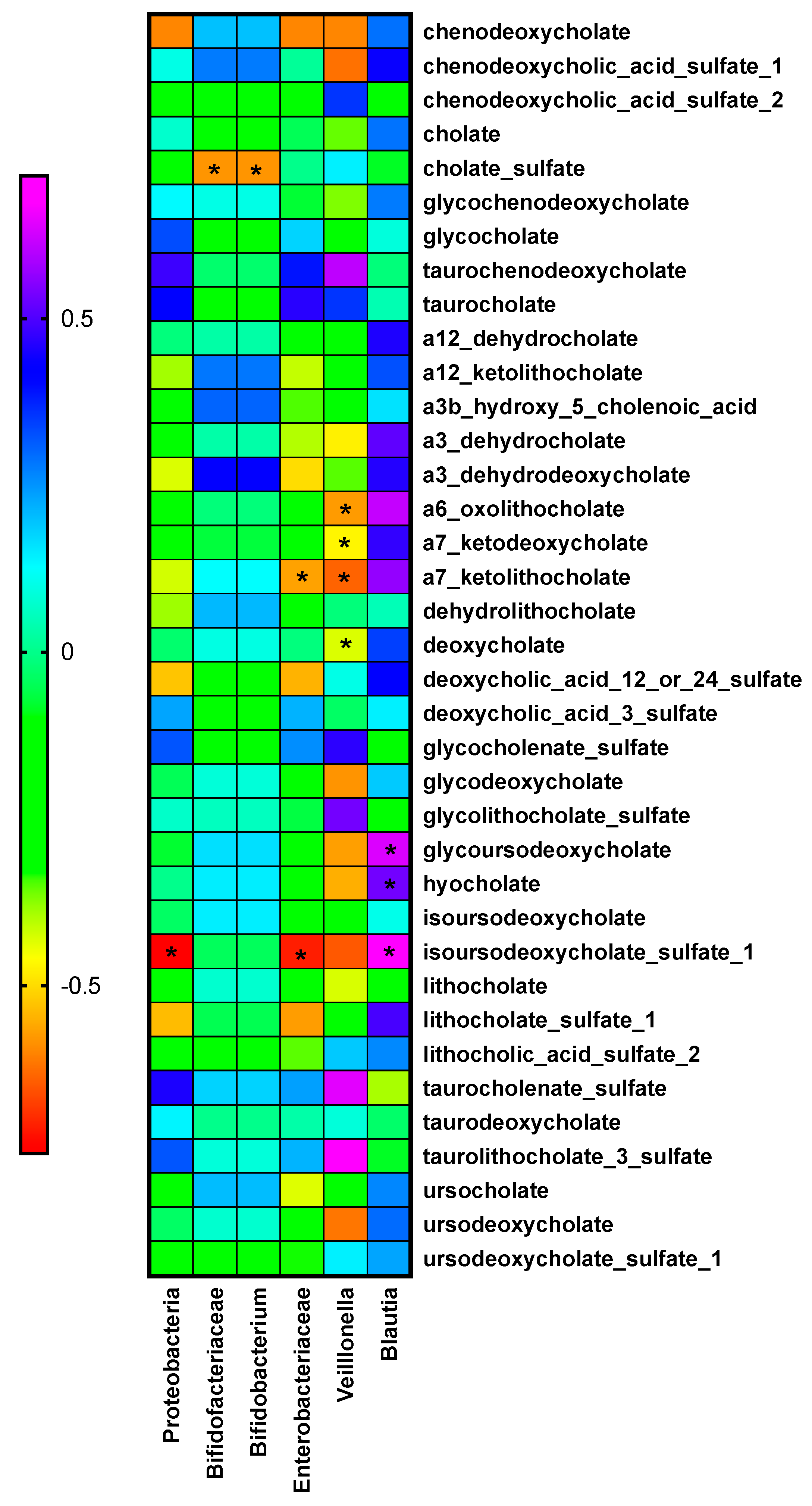
2.4. Bile Acids Profile
2.5. Relationship between Bacteria and Bile Acid Changes
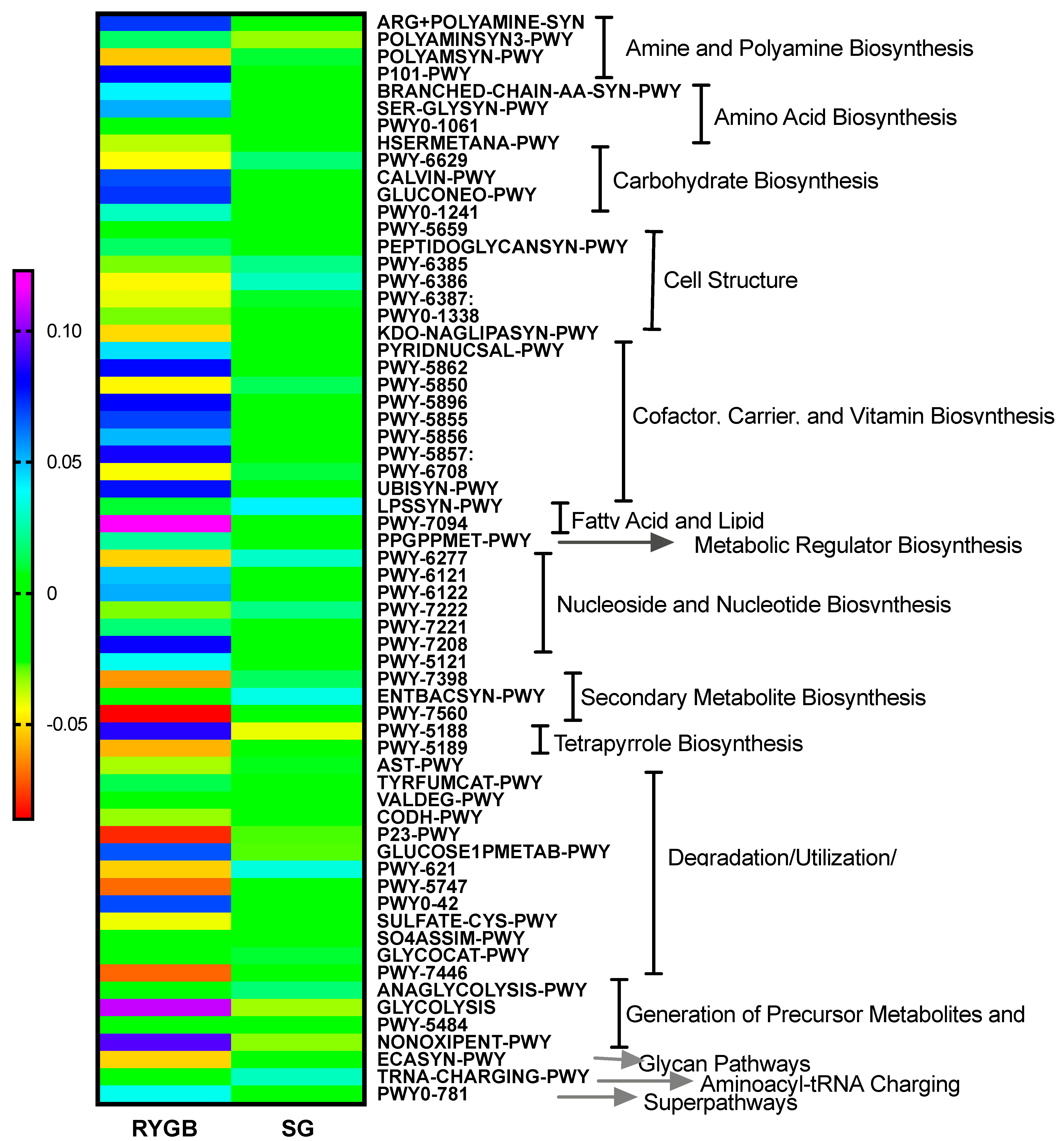
2.6. Microbiota Metabolic Pathways
2.7. Relationship between Biochemical and Anthropometric Variables and Bile Acid Changes
3. Discussion
4. Material and Methods
4.1. Anthropometric and Laboratory Measurements
4.2. Fecal Samples Analysis
4.3. Gut Microbiota Analysis
4.4. Sequence Data and Statistical Analysis
4.5. Metabolomics Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maciejewski, M.L.; Arterburn, D.E.; Van Scoyoc, L.; Smith, V.A.; Yancy, W.S., Jr.; Weidenbacher, H.J.; Livingston, E.H.; Olsen, M.K. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg. 2016, 151, 1046–1055. Available online: https://pubmed.ncbi.nlm.nih.gov/27579793 (accessed on 26 December 2020). [CrossRef]
- Celio, A.C.; Wu, Q.; Kasten, K.R.; Manwaring, M.L.; Pories, W.J.; Spaniolas, K. Comparative effectiveness of Roux-en-Y gastric bypass and sleeve gastrectomy in super obese patients. Surg. Endosc. 2017, 31, 317–323. [Google Scholar] [CrossRef]
- Pucci, A.; Batterham, R.L. Mechanisms underlying the weight loss effects of RYGB and SG: Similar, yet different. J. Endocrinol. Investig. 2019, 42, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Luijten, J.C.H.B.M.; Vugts, G.; Nieuwenhuijzen, G.A.P.; Luyer, M.D.P. The Importance of the Microbiome in Bariatric Surgery: A Systematic Review. Obes. Surg. 2019, 29, 2338–2349. [Google Scholar] [CrossRef]
- Kuipers, F.; Bloks, V.W.; Groen, A.K. Beyond intestinal soap--bile acids in metabolic control. Nat. Rev. Endocrinol. 2014, 10, 488–498. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. Available online: https://pubmed.ncbi.nlm.nih.gov/16299351/ (accessed on 26 December 2020). [CrossRef] [PubMed] [Green Version]
- Ramírez-Pérez, O.; Cruz-Ramón, V.; Chinchilla-López, P.; Méndez-Sánchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16, S21–S26. Available online: http://www.sciencedirect.com/science/article/pii/S1665268119310403 (accessed on 26 December 2020). [CrossRef]
- Sánchez-Alcoholado, L.; Gutiérrez-Repiso, C.; Gómez-Pérez, A.M.; García-Fuentes, E.; Tinahones, F.J.; Moreno-Indias, I. Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surg. Obes. Relat. Dis. 2019, 15, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, Z.; Wang, Y.; Dai, Y.; Zhang, X.; Hu, S. Role of Bile Acids in Bariatric Surgery. Front. Physiol. 2019, 10, 374. Available online: https://pubmed.ncbi.nlm.nih.gov/31001146 (accessed on 26 December 2020). [CrossRef]
- Geng, W.; Lin, J. Bacterial bile salt hydrolase: An intestinal microbiome target for enhanced animal health. Anim. Health Res. Rev. 2016, 17, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Urdaneta, V.; Casadesús, J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front. Med. 2017, 4, 163. [Google Scholar] [CrossRef]
- de Siqueira Cardinelli, C.; Torrinhas, R.S.; Sala, P.; Pudenzi, M.A.; Fernando, F.; Angolini, C.; Marques da Silva, M.; Machado, N.M.; Ravacci, G.; Eberlin, M.N.; et al. Fecal bile acid profile after Roux-en-Y gastric bypass and its association with the remission of type 2 diabetes in obese women: A preliminary study. Clin. Nutr. 2019, 38, 2906–2912. [Google Scholar] [CrossRef]
- Paganelli, F.L.; Luyer, M.; Hazelbag, C.M.; Uh, H.W.; Rogers, M.R.C.; Adriaans, D.; Berbers, R.-M.; Hendrickx, A.P.A.; Viveen, M.C.; Groot, J.A.; et al. Roux-Y Gastric Bypass and Sleeve Gastrectomy directly change gut microbiota composition independent of surgery type. Sci. Rep. 2019, 9, 10979. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31358818 (accessed on 23 April 2020). [CrossRef] [PubMed]
- Bajaj, J.S.; Hylemon, P.B.; Ridlon, J.M.; Heuman, D.M.; Daita, K.; White, M.B.; Monteith, P.; Noble, N.A.; Sikaroodi, M.; Gillevet, P.M. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G675–G685. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sanefuji, M.; Morokuma, S.; Yoden, M.; Momoda, R.; Sonomoto, K.; Ogawa, M.; Kato, K.; Nakayama, J. The association between gut microbiota development and maturation of intestinal bile acid metabolism in the first 3 y of healthy Japanese infants. Gut Microbes 2020, 11, 205–216. Available online: https://pubmed.ncbi.nlm.nih.gov/31550982 (accessed on 23 April 2020). [CrossRef] [PubMed]
- Jahansouz, C.; Xu, H.; Hertzel, A.V.; Serrot, F.J.; Kvalheim, N.; Cole, A.; Abraham, A.; Luthra, G.; Ewing, K.; Leslie, D.B.; et al. Bile Acids Increase Independently From Hypocaloric Restriction After Bariatric Surgery. Ann. Surg. 2016, 264, 1022–1028. [Google Scholar] [CrossRef]
- Grill, J.P.; Manginot-Dürr, C.; Schneider, F.; Ballongue, J. Bifidobacteria and probiotic effects: Action of Bifidobacterium species on conjugated bile salts. Curr. Microbiol. 1995, 31, 23–27. [Google Scholar] [CrossRef]
- Loomba, R.; Ling, L.; Dinh, D.M.; DePaoli, A.M.; Lieu, H.D.; Harrison, S.A.; Sanyal, A.J. The Commensal Microbe Veillonella as a Marker for Response to an FGF19 Analog in NASH. Hepatology 2021, 73, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. Available online: https://pubmed.ncbi.nlm.nih.gov/24625896 (accessed on 23 April 2020). [CrossRef] [PubMed] [Green Version]
- Li, K.; Zou, J.; Li, S.; Guo, J.; Shi, W.; Wang, B.; Han, X.; Zhang, H.; Zhang, P.; Miao, Z.; et al. Farnesoid X receptor contributes to body weight-independent improvements in glycemic control after Roux-en-Y gastric bypass surgery in diet-induced obese mice. Mol. Metab. 2020, 37, 100980. Available online: https://pubmed.ncbi.nlm.nih.gov/32305491 (accessed on 23 April 2020). [CrossRef]
- Liou, A.P.; Paziuk, M.; Luevano, J.-M.J.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra41. [Google Scholar] [CrossRef] [Green Version]
- Browning, M.G.; Pessoa, B.M.; Khoraki, J.; Campos, G.M. Changes in Bile Acid Metabolism, Transport, and Signaling as Central Drivers for Metabolic Improvements After Bariatric Surgery. Curr. Obes. Rep. 2019, 8, 175–184. [Google Scholar] [CrossRef]
- Kaska, L.; Sledzinski, T.; Chomiczewska, A.; Dettlaff-Pokora, A.; Swierczynski, J. Improved glucose metabolism following bariatric surgery is associated with increased circulating bile acid concentrations and remodeling of the gut microbiome. World J. Gastroenterol. 2016, 22, 8698–8719. Available online: https://pubmed.ncbi.nlm.nih.gov/27818587 (accessed on 23 April 2020). [CrossRef] [PubMed]
- Van den Bossche, L.; Hindryckx, P.; Devisscher, L.; Devriese, S.; Van Welden, S.; Holvoet, T.; Vilchez-Vargas, R.; Vital, M.; Pieper, D.H.; Vanden Bussche, J.; et al. Ursodeoxycholic Acid and Its Taurine- or Glycine-Conjugated Species Reduce Colitogenic Dysbiosis and Equally Suppress Experimental Colitis in Mice. Appl. Environ. Microbiol. 2017, 83, e02766-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bairaktari, E.T.; Seferiadis, K.I.; Elisaf, M.S. Evaluation of Methods for the Measurement of Low-Density Lipoprotein Cholesterol. J. Cardiovasc. Pharmacol. Ther. 2005, 10, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2 paper supplementary information: High resolution sample inference from amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; E Midford, P.; Ong, Q.; Ong, W.K.; et al. The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 2017, 46, D633–D639. [Google Scholar] [CrossRef] [Green Version]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. Available online: https://pubmed.ncbi.nlm.nih.gov/25061070 (accessed on 30 April 2020). [CrossRef] [PubMed] [Green Version]


| RYGB | SG | |||
|---|---|---|---|---|
| Pre-Surgery | Post-Surgery | Pre-Surgery | Post-Surgery | |
| Gender (M/F) | 3/5 | 4/4 | ||
| Age, years | 47.12 ± 8.02 | 46.75 ± 12.71 | ||
| Weight, kg | 116.85 ± 17.03 | 96.66 ± 16.16 * | 127.01 ± 24.90 | 104.21 ± 15.84 * |
| BMI, kg/m2 | 43.87 ± 6,58 | 36.16 ± 5.64 * | 45.49 ± 4.99 | 37.64 ± 4.65 * |
| Waist, cm | 131.63 ± 13,05 | 115.25 ± 10.31 * | 135.00 ± 11.82 | 118.71 ± 9.98 * |
| Hip, cm | 133.88 ± 15.08 | 121.75 ± 13.85 * | 136.94 ± 10.66 | 123.71 ± 10.63 * |
| HbA1C, % | 7.49 ± 1.77 | 5.53 ± 0,26 * | 6.38 ± 1.43 | 5.55 ± 0.56 * |
| HOMA-IR | 7.33 ± 3.50 | 2.81 ± 2.11 * | 9.20 ± 6.69 | 3.00 ± 1.91 |
| Insulin, mU/mL | 22.28 ± 11.17 | 11.61 ± 7.17 * | 33.29 ± 24.29 | 14.72 ± 5.70 * |
| Glucose, mg/dL | 130.13 ± 38.88 | 93.86 ± 9.26 * | 108.88 ± 38.16 | 85.71 ± 14.30 |
| SBP, mmHg | 136.63 ± 11.33 | 127.33 ± 20.39 | 144.50 ± 22.42 | 141.38 ± 16.12 |
| DBP, mmHg | 80.00 ± 8.33 | 75.00 ± 9.34 | 85.88 ± 5.38 | 85.25 ± 6.50 |
| Cholesterol, mg/dL | 196.88 ± 39.94 | 151.71 ± 36.70 * | 184.88 ± 28.39 | 174.43 ± 34.80 |
| HDL-chol, mg/dL | 45.00 ± 16.89 | 41.86 ± 11.45 | 42.63 ± 8.40 | 41.29 ± 11.25 |
| LDL-chol, mg/dL | 114.68 ± 29.95 | 86.63 ± 34.59 | 112.31 ± 20.45 | 111.51 ± 26.04 |
| TG, mg/dL | 186.00 ± 82.50 | 116.14 ± 48.13 * | 228.75 ± 276.57 | 108.14 ± 38.32 |
| GOT, U/L | 27.25 ± 11.82 | 21.14 ± 7.22 | 23.25 ± 8.12 | 17.71 ± 4.85 |
| GPT, U/L | 42.25 ± 19.03 | 28.71 ± 9.41 | 30.75 ± 17.30 | 20.83 ± 5.15 * |
| GGT, U/L | 48.50 ± 7.78 | 21.57 ± 12.53 | 36.33 ± 13.01 | 20.86 ± 7.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocaña-Wilhelmi, L.; Martín-Núñez, G.M.; Ruiz-Limón, P.; Alcaide, J.; García-Fuentes, E.; Gutiérrez-Repiso, C.; Tinahones, F.J.; Moreno-Indias, I. Gut Microbiota Metabolism of Bile Acids Could Contribute to the Bariatric Surgery Improvements in Extreme Obesity. Metabolites 2021, 11, 733. https://doi.org/10.3390/metabo11110733
Ocaña-Wilhelmi L, Martín-Núñez GM, Ruiz-Limón P, Alcaide J, García-Fuentes E, Gutiérrez-Repiso C, Tinahones FJ, Moreno-Indias I. Gut Microbiota Metabolism of Bile Acids Could Contribute to the Bariatric Surgery Improvements in Extreme Obesity. Metabolites. 2021; 11(11):733. https://doi.org/10.3390/metabo11110733
Chicago/Turabian StyleOcaña-Wilhelmi, Luis, Gracia María Martín-Núñez, Patricia Ruiz-Limón, Juan Alcaide, Eduardo García-Fuentes, Carolina Gutiérrez-Repiso, Francisco J. Tinahones, and Isabel Moreno-Indias. 2021. "Gut Microbiota Metabolism of Bile Acids Could Contribute to the Bariatric Surgery Improvements in Extreme Obesity" Metabolites 11, no. 11: 733. https://doi.org/10.3390/metabo11110733






