Abstract
There is a growing body of evidence that metabolic reprogramming contributes to the acquisition and maintenance of robustness associated with malignancy. The fine regulation of expression levels of amino acid and monocarboxylate transporters enables cancer cells to exhibit the metabolic reprogramming that is responsible for therapeutic resistance. Amino acid transporters characterized by xCT (SLC7A11), ASCT2 (SLC1A5), and LAT1 (SLC7A5) function in the uptake and export of amino acids such as cystine and glutamine, thereby regulating glutathione synthesis, autophagy, and glutaminolysis. CD44 variant, a cancer stem-like cell marker, stabilizes the xCT antiporter at the cellular membrane, and tumor cells positive for xCT and/or ASCT2 are susceptible to sulfasalazine, a system Xc(-) inhibitor. Inhibiting the interaction between LAT1 and CD98 heavy chain prevents activation of the mammalian target of rapamycin (mTOR) complex 1 by glutamine and leucine. mTOR signaling regulated by LAT1 is a sensor of dynamic alterations in the nutrient tumor microenvironment. LAT1 is overexpressed in various malignancies and positively correlated with poor clinical outcome. Metabolic reprogramming of glutamine occurs often in cancer cells and manifests as ASCT2-mediated glutamine addiction. Monocarboxylate transporters (MCTs) mediate metabolic symbiosis, by which lactate in cancer cells under hypoxia is exported through MCT4 and imported by MCT1 in less hypoxic regions, where it is used as an oxidative metabolite. Differential expression patterns of transporters cause functional intratumoral heterogeneity leading to the therapeutic resistance. Therefore, metabolic reprogramming based on these transporters may be a promising therapeutic target. This review highlights the pathological function and therapeutic targets of transporters including xCT, ASCT2, LAT1, and MCT.
1. Introduction
Metabolic reprogramming specific to cancer cells is one of the ten cancer hallmarks described by Drs. Hanahan and Weinberg in their review article published in 2011 [1]. Some of the most striking alterations of tumor cellular bioenergetics include activation of glycolysis, increase in glutaminolytic flux, upregulation of amino acid transporters and lipid metabolism, enhancement of mitochondrial biogenesis, and activation of the pentose phosphate pathway and macromolecule biosynthesis [2,3].
Increasing evidence strongly suggests that metabolic reprogramming is crucial for cancer stem-like cells to maintain unlimited self-renewal potential and hyper-adaptation to drastic changes in the tumor microenvironment [4,5]. Cancer stem cells have a robust phenotype, encompassing several characteristics such as a slow cell cycle, the ability to detoxify or promote the efflux of anti-cancer drugs, resistance to redox stress, and a rapid response to genotoxic damage—all of which contribute to the acquisition of chemoresistance [6,7,8,9]. For example, while non-cancer stem-like cells are susceptible to chemotherapy and undergo apoptosis, released cytokine prostaglandin E2 (PGE2) awakens the dormant cancer stem-like cells localized in the niche, which is the favorable microenvironment [10,11]. Proliferating cancer stem-like cells are likely to exhibit additional metabolic reprogramming, concomitant with the upregulation of mitochondrial oxidative phosphorylation (OXPHOS)-related molecules.
Due to the rapid proliferation, cancer cells have increased demand for amino acids in maintaining one-carbon metabolism, signal pathway, as well as the synthesis of nucleotide and protein [12,13]. The expression levels of amino acid transporters are closely associated with tumor size, pathological grade and distant metastasis [14,15,16]. Thus, increasing investigations have demonstrated the feasibility of amino acid transporters as a component of anti-cancer therapy.
This review highlights the pathological significance of amino acid transporters including xCT, ASCT2, and LAT1, as well as monocarboxylate transporters (MCTs) such as MCT1 and MCT4. Given that cancer cells exhibit the aerobic glycolysis termed “Warburg effect”, metabolites derived from glycolysis are important materials for amino acid production and macromolecule synthesis, which is required for robust tumor growth and proliferation.
2. xCT (SLC7A11)
System Xc(-) is composed of a light-chain subunit (xCT, also known as SLC7A11) and a heavy-chain subunit (CD98hc, also referred to as SLC3A2), and functions as a Na+-independent transporter that mediates the exchange of extracellular cystine for intracellular glutamate [17,18]. The availability of cysteine is a rate-limiting factor for the synthesis of the reduced form of glutathione (GSH), and the activity of system Xc(-) is crucial for the GSH-dependent anti-oxidant machinery. xCT interacts with the type II transmembrane protein CD98hc at the cell surface. CD98hc is separately and covalently linked to several light chains that function as amino acid transporters at the plasma membrane, including LAT1, LAT2, y+LAT1, y+LAT2, ASC-1, and xCT [19]. Mice lacking xCT expression appear healthy, but they have an increased plasma concentration of cystine compared with their wild-type littermates [20], suggesting that xCT-mediated cystine transport is required for cells exposed to severe redox stress. Transcription of the xCT gene is induced by depletion of cystine or redox stress due to electrophilic agents, and this effect is mediated by binding of the transcription factor Nrf2 to its response element in the promoter of the xCT gene [21]. Exposure of normal airway epithelial cells to cigarette smoking upregulates xCT [22] through the transient activation of the Nrf2 signaling pathway [23]. In many cases of non-small cell lung cancer (NSCLC), constitutive activation of Nrf2 in the nucleus caused by the loss-of-function KEAP1 genetic mutation prevents redox stress accumulation triggered by chemotherapy [24]. This is consistent with the poor 5-year overall survival of xCT-overexpressing NSCLC patients [22]. System Xc(-) is a main regulator of metabolic reprogramming with overarching effects on glucose metabolism, glutamine dependency, and intracellular GSH/glutathione disulfide redox balance in cancer stem-like cells. Furthermore, activating transcription factor 4, which plays an essential role in the response of cells to multiple types of stress [25], upregulates xCT expression [26,27]. These observations indicate that xCT contributes to the protection of cancer cells exposed to a high level of oxidative stress.
Stabilization of xCT at the plasma membrane of cancer cells is regulated by CD44v8-10 (an isoform that includes the sequence encoded by variant exons 8−10) and promotes GSH synthesis [6,28]. Small-interfering RNA-mediated depletion of CD44v8-10 downregulates xCT expression at the cellular membrane, thereby exhausting the intracellular cysteine pool without affecting the intracellular content of other amino acids. Therefore, CD44v8-10 plays a fundamental role in the GSH-dependent antioxidant system in cancer cells by modulating xCT-mediated cystine transport and consequently GSH synthesis. Alternative splicing of CD44 mRNA regulated by epithelial splicing regulatory protein 1 (ESRP1) induces CD44v8-10 expression in metastatic tumor-initiating cells [6,29]. Cancer cells positive for CD44v8-10 exhibit an enhanced capacity to defend against redox stress as a result of increased xCT-mediated cystine uptake and GSH synthesis. Such tumor-initiating cells with high levels of GSH are thus predominantly responsible for colonization of the pre-metastatic niche in the lungs, which contains neutrophils that generate oxidative stress [29,30].
A growing body of evidence suggests that the crosstalk between cancer cells and stromal cells plays an important role in metabolic reprogramming [4,31,32,33,34]. Metabolic interactions between chronic lymphocytic leukemia (CLL) cells and neighboring bone marrow-derived stromal cells (BMSCs) promote cancer cell survival. If cysteine is not provided by BMSCs, CLL cells, which lack the xCT transporter, are susceptible to redox stress. Neighboring stromal cells characterized by BMSCs can take up cystine via system Xc(-) and release cysteine [31]. A vast majority of cells, including tumor cells, do not depend on extracellular cysteine for GSH synthesis [31,35,36]. Instead, they take up cystine (two cysteine molecules joined by a disulfide bond), the more abundant and more stable oxidized form, and reduce it to cysteine in the cytoplasm [37]. By striking contrast, CLL cells aggressively import cysteine and use it for the synthesis of GSH, resulting in increased resistance to oxidative stress. Under these conditions, CLL cells can display enhanced therapeutic resistance to F-ara-A and oxaliplatin, two chemotherapeutic agents used clinically to treat CLL [31]. Blocking xCT activity in BMSCs with sulfasalazine (SSZ) suppresses CLL cell viability and improves the effectiveness of anticancer drugs.
SSZ, which is used for the treatment of rheumatoid arthritis and ulcerative colitis, inhibits system Xc(-), thereby targeting the CD44v8-10-xCT interaction [28,29,38]. SSZ, a well-characterized specific inhibitor of xCT-mediated cystine transport, suppresses CD44-driven tumor growth and activates p38 mitogen-activated protein kinase signaling (Figure 1). Ishimoto et al. showed that the combination of SSZ and cisplatin (CDDP) significantly decreases tumor proliferation compared with CDDP alone [28], suggesting that SSZ reduces the capacity of cancer stem-like cells to protect against redox stress and sensitizes them to available chemotherapeutic agents. In addition, SSZ inhibits the formation of metastatic lesions in the lungs derived from CD44v8-10-expressing breast cancer cells [29]. Alternative splicing of CD44 mRNA regulated by ESRP1 increases xCT-dependent resistance to redox stress, thereby allowing tumor cells to evade exogenous stress in the pre-metastatic niche. Cancer stem-like cells of head and neck squamous cell carcinoma (HNSCC) that survive treatment with cetuximab, an epidermal growth factor receptor (EGFR)-targeting agent, are sensitive to the induction of ferroptosis by SSZ [38,39]. CD44v8-10-expressing and undifferentiated cancer stem-like cells exhibit resistance to the monoclonal anti-EGFR antibody and sensitivity to SSZ [38]. This finding suggests that the Achilles’ heel of CD44v8-10-positive cancer stem-like cells may depend on the regulation of oxidative stress by xCT. Given the intratumoral heterogeneity in terms of the association between CD44v8-10 and xCT, combination therapy with SSZ and cetuximab may be an effective treatment.
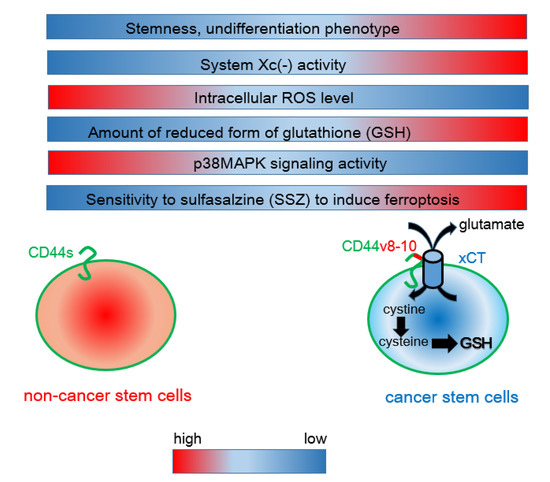
Figure 1.
Interaction between CD44 variant 8-10 (CD44v8-10) and the xCT antiporter determines the resistance to oxidative stress. Unlike mesenchymal cancer cells expressing CD44 standard (CD44s), CD44v8-10-positive cancer stem-like cells exhibit robust system Xc(-) activity, resulting in enhanced uptake of cystine. Given that cysteine is a rate-limiting factor for the synthesis of the reduced form of glutathione (GSH), CD44v8-10-expressing cancer cells show a phenotype of increased resistance to redox stress. Sulfasalazine (SSZ) inhibits the xCT transporter and is therefore effective for blocking the CD44v8-10-xCT-GSH axis.
3. ASCT2 (SLC1A5)
ASCT2, also known as SLC1A5, promotes the uptake of circulating glutamine into proliferating tumor cells [40]. ASCT2 is overexpressed in squamous cell carcinoma, adenocarcinoma, and neuroendocrine lung cancers [41]. In addition, overexpression of ASCT2 in oral squamous cell carcinoma is positively correlated with poor outcomes [42]. ASCT2 serves as an obligatory exchanger that imports a sodium-coupled amino acid substrate into cells and exports another sodium-coupled amino acid substrate with 1:1 stoichiometry [43,44]. ASCT2 is the primary transporter importing glutamine, and its inhibition attenuates tumor growth, which partially explains “glutamine addiction” [15,40,41]. Glutamine flux, which is dependent on the balance between the uptake of glutamine by ASCT2 and its subsequent export by LAT1 (SLC7A5), leads to high intracellular availability of essential amino acids (EAAs). Once in the cytoplasm, glutamine is a substrate of several glutamate-producing enzymes, such as mitochondrial glutaminase (GLS1), and cytosolic enzymes involved in the biosynthesis of nitrogenous metabolites. Glutamine-derived glutamate is likely to be transported back out of the cancer cell in exchange for cystine by system Xc(-) [45]. The combination of amino acid transporters plays a critical role in metabolic reprogramming and maintenance of the stem-like phenotype in cancer cells. For instance, in triple-negative breast cancer (TNBC) cells, the interaction between ASCT2 and xCT causes “glutamine addiction” [46,47]. System Xc(-) takes up cystine in exchange for glutamate for GSH synthesis, whereas ASCT2 imports glutamine in a cooperative manner [28,48] (Figure 2). Thus, targeted knockdown of ASCT2 inhibits GSH synthesis and induces ferroptosis mediated by the accumulation of intracellular redox stress [42].
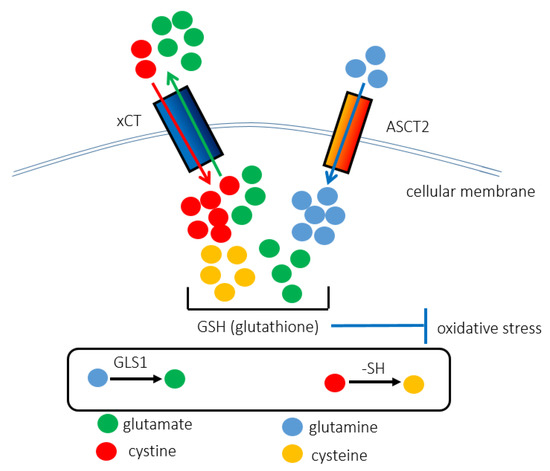
Figure 2.
The interaction between ASCT2 and xCT contributes to the synthesis of GSH, rendering cancer cells resistant to redox stress. The xCT (SLC7A11) antiporter is responsible for importing cystine instead of exporting glutamate. ASCT2 (SLC1A5) promotes the uptake of glutamine. Glutamine and cystine are chemically converted into glutamate and cysteine, respectively. As GSH is composed of cysteine, glutamate, and glycine, the cooperative function of ASCT2 and xCT underlies the anti-oxidant stress machinery. That is why ASCT2-targeted therapy increases intracellular redox stress levels and induces ferroptosis, iron-dependent cell death.
Cancer cells cannot survive in the absence of exogenous glutamine, and therefore exhibit “glutamine addiction”, which is orchestrated by the interaction between xCT and ASCT2 [4,40,49]. Glutamine taken up through ASCT2 is rapidly exported via the bidirectional amino acid transporter LAT1 in exchange for the uptake of extracellular EAA. Knockdown of ASCT2 in cancer cells impairs glutamine uptake and export, uptake of EAAs, and mTORC1 activation, which suggests that both uptake and export of glutamine are required for the activation of mTORC1 signaling [40,50]. Mounting evidence strongly suggests that glutamine is an essential substrate required for anabolic growth in mammalian cells. Investigation with the transaminase inhibitor amino-oxyacetic acid indicates that the main route of entry of glutamine-derived carbon into the tricarboxylic acid cycle (TCA cycle) in Myc-transformed cells is through the transamination reaction [51]. Oncogenic c-Myc induces the transcription of glutamine transporters, such as ASCT2 and LAT1, and the expression of glutamine-utilizing enzymes, such as GLS1 [52,53]. Following the transport of glutamine into the cell, the first step of glutaminolysis is the GLS1-mediated conversion of glutamine into glutamate. Glutamate is subsequently converted to α-ketoglutarate (α-KG) by either glutamate dehydrogenase (GDH) or aminotransferases. Glutaminolysis consists of a series of biochemical reactions by which glutamine is catabolized into metabolites including α-KG and glutamate [54]. In the TCA cycle, α-KG is catabolized to malate, which is transported into the cytoplasm and converted to pyruvate and ultimately to lactate [55]. Mechanistically, mTORC1 signaling promotes glutamine anaplerosis mediated by upregulation of GDH [56]. In addition to promoting glutamine uptake, c-Myc facilitates the metabolism of imported glutamine into glutamic acid and ultimately into lactic acid, pyruvate, and aspartate [57]. On the other hand, l-γ-glutamyl-p-nitroanilide, one of the inhibitors of ASCT2, can block glutamine uptake and inhibit glutamine-dependent mTOR activation [58,59]. In the absence of amino acids, mTOR signaling is refractory to the stimulation of growth factors. The uptake of glutamine mediated by ASCT2, followed by its rapid efflux via LAT1 in exchange for EAAs such as leucine, is the rate-limiting step for mTOR signaling activation in tumor cells [59]. The interaction between the glutamine antiporter ASCT2 and the heterodimeric LAT1/CD98hc bidirectional transporter is necessary for amino acid transport and activation of the mTORC1 signaling pathway leading to cellular growth and autophagy. The generation of α-KG from the catabolism of glutamine is essential for the activation of mTOR signaling in cervical carcinoma and osteosarcoma cells [60]. Targeting glutamine uptake and glutaminolysis in cancer patients could inhibit mTOR signaling, even in the presence of aberrant growth factor stimulation.
Induction of redox stress by treatment with SSZ requires ASCT2-mediated glutamine uptake and the synthesis of α-KG mediated by GDH in CD44v8-10-expressing cancer stem-like cells in HNSCC [61]. Increased expression levels of xCT and ASCT2 are positively correlated with the undifferentiated phenotype in HNSCC. The transcriptional program regulated by c-Myc is likely to play a critical role in glutaminolysis in CD44v8-10-positive undifferentiated HNSCC cells. Unlike CDDP, SSZ eliminates CD44v8-10-positive undifferentiated cancer cells, especially HNSCC cells that are also positive for ASCT2 [61]. Therefore, xCT-targeted therapeutic strategies may be effective for the depletion of ASCT2-positive cancer stem-like cells. Indeed, plasma membrane proteins, including CD44v, xCT, and ASCT2, are widely expressed in several types of human malignancy, such as HNSCC and colorectal cancer, and their upregulation is associated with poor prognosis [62,63].
4. LAT1 (SLC7A5)
The L-type amino acid transporter family is a crucial route of entry of EAAs into cancer cells and comprises four members (LAT1–4). Among the four LAT transporters, LAT1 is predominantly overexpressed in a variety of cancers [64,65,66,67]. LAT1 (SLC7A5) contains 12 transmembrane domains and covalently binds to CD98hc, which incorporates LAT1 into the cellular membrane, resulting in its functional expression [68]. LAT1 mediates the uptake of neutral EAAs (leucine, isoleucine, phenylalanine, methionine, histidine, tryptophan, valine, and tyrosine) into cancer cells [69,70] in exchange for the efflux of intracellular substrates (EAAs and/or glutamine) [71,72], thus serving as an amino acid antiporter. The complex composed of LAT1 and CD98hc functions as an antiporter that imports branched amino acids such as leucine and exports glutamine [50]. Nicklin et al. used pharmacological inhibitors and small-interfering RNAs to show that the inhibition of LAT1-CD98hc prevents mTORC1 activation by glutamine and leucine without affecting glutamine uptake [59]. Further, glutamine acts upstream of leucine as an efflux solute, allowing the uptake of leucine through the LAT1-CD98hc antiporter.
LAT1 regulates the cellular uptake of exogenous leucine, thereby promoting mTORC1 activation in tumor cells [64,67,73]. Nutrient and growth factor pathways control cellular metabolism and protein synthesis by regulating mTORC1 activation [74,75]. Aberrant activation of mTORC1 is common in human malignancies [64,76], and mTORC1 signaling promotes tumor progression by stimulating signaling pathways that support cancer cell growth, autophagy, and resistance to apoptosis [75,77,78]. mTORC1 phosphorylates ULK1 under hypo-nutrient conditions, thereby preventing its activation by AMP-activated protein kinase (AMPK), a key activator of autophagy [76,79]. The relative activity of mTORC1 and AMPK in different cellular contexts largely determines the extent of autophagy induction. Among amino acids, leucine is the most effective activator of mTORC1 [74,76]. The expression level of LAT1 is regulated by activating transcription factor 4 (ATF4) depending on mTORC1 [80]. Lack of nutrition caused by amino acid deficiency activates ATF4, which induces amino-acid transport into cancer cells [80,81]. Increased ATF4 levels upregulate LAT1, causing an enhanced uptake of leucine and thereby activating mTORC1 and simultaneously inhibiting autophagy [80,82]. Indeed, the knockout of LAT1 or ATF4 can block amino acid uptake, prevent mTORC1 activation, and enhance autophagy.
The mTOR signaling pathway regulated by AMPK and LAT1 functions as a sensor of dynamic alterations in the nutrient tumor microenvironment [83,84]. DRAM1 binds amino acid transporters such as LAT1 and ASCT2, directing them to lysosomes and permitting efficient activation of mTORC1 [85]. The LAT1/ASCT2 and xCT/CD98hc complexes in cancer cells activate the mTORC1-SIRT4-GDH axis and anti-oxidant GSH synthesis, respectively [4,46,56]. The former pathway promotes the conversion of glutamate into α-KG, whereas the latter pathway regulates the level of redox stress. EpCAM, a functional marker of ovarian cancer stem cells, forms a complex with amino acid transporters including LAT1 and CD98hc [86,87,88], and the expression level of LAT1 is positively correlated with poor clinical outcomes in ovarian cancer, renal cell carcinoma, and pancreatic ductal adenocarcinoma [89,90,91]. LAT1 is markedly expressed in ovarian cancer, and positive LAT1 expression is an independent predictor of poor overall survival in patients with ovarian cancer [91]. By contrast, the expression of ASCT2 is not a significant prognostic factor of worse clinical outcomes in ovarian cancer, although positive expression of LAT1 is closely correlated with that of ASCT2 and CD98hc, and leads to significantly worse outcomes [91,92,93].
Increasing evidence suggests that LAT1 is involved in the metastatic potential of malignancies. LAT1 upregulation is positively correlated with metastasis in multiple cancers [82,89,94,95,96]. For example, lymph node metastasis-positive squamous cell carcinomas express LAT1, whereas a positive signal for LAT1 is not detected in metastasis-negative cells [95]. The transcriptional levels of LAT1 are significantly higher in renal cell carcinoma with metastasis [89]. Cells with high LAT1 expression tend to have more severe metastatic disease in gastric cancer [96]. LAT1 expression in neuroendocrine tumors is significantly associated with lymph node metastasis [94]. The functional significance of LAT1 in metastasis has been demonstrated. Knockdown of LAT1 inhibits the migration and invasion of gastric carcinoma and cholangiocarcinoma cells [97,98]. From the perspective of cancer treatment, heptane-2-carboxylic acid (BCH) inhibits proliferation and migration in human epithelial ovarian cancer cells without affecting Akt signaling [59,99]. These findings suggest that blockage of LAT1 is a promising strategy to prevent metastasis of cancer.
5. Monocarboxylate Transporters
A significant increase in the expression levels of MCT1 and MCT4 is a hallmark of several human malignancies, and high levels of these transporters lead to poor clinical outcome. For instance, increased MCT1 expression is detected in a wide range of malignancies, including glioma and neuroblastoma, as well as breast, colorectal, gastric, and cervical cancers [100,101,102,103,104,105]. MCT4 expression is highly elevated in renal cell carcinoma, as well as in cervical and prostate cancers [102,106,107]. Increased expression of MCT1 and MCT4 in cancer provides a therapeutic window for disabling these transporters with small-molecule inhibitors and/or by targeting their co-chaperone CD147 [108,109].
Post-translational stabilization of MCT1 has been observed under nutrient stress conditions [109]. It involves a poorly characterized mechanism associated with redox stress derived from OXPHOS in mitochondria [110]. Although glucose deprivation induces autophagy and activates canonical Wnt/β-catenin signaling, β-catenin downregulation decreases MCT1 expression in cancer cells, thereby positively coupling autophagy to high MCT1 expression [111]. In addition, MCT1, MCT4, and CD147 interact with the hyaluronate receptor CD44 in breast and prostate cancer cells [112,113]. In this complex, CD44 serves as a chaperone for MCT1 and MCT4, and impairment of CD44 signaling decreases the expression levels at the cellular membrane and impairs the activity of both MCT1 and MCT4 [113]. Depletion of the chaperone associated with MCT causes the inappropriate expression of MCT in intracellular vesicles, indicating that CD147 targets the transporters to the cellular membrane [114,115]. CD147 gene expression is induced by hypoxia [116], which accounts for hypoxia-inducible MCT1 expression. Increased stability of MCT1 mRNA is related to the loss of the MCT1 translation repressor microRNA (miR)-124, as observed in pancreatic ductal adenocarcinoma and medulloblastoma [117,118]. Loss of miR-342-3p, which acts downstream of the estrogen receptor, upregulates MCT1 expression in TNBC cells [119]. MCT4 expression is indirectly regulated by miR-1, which is regulated by decreased levels of Smad3-hypoxia-inducible factor 1 (HIF-1) signaling, ultimately leading to the downregulation of MCT4 in glycolytic cancer cells [120]. Surprisingly enough, miR-1 inhibits Warburg effect while Smad3 promotes it, mediated by its downstream effecter HIF-1α and glycolytic enzymes such as hexokinase 2 (HK2) and MCT4 [120]. These findings strongly suggest that the transcriptional levels of MCT1 and MCT4 are both directly and indirectly regulated by miRNAs.
Metabolic interaction between epithelial tumor cells and cancer-associated fibroblasts (CAFs) requires that each cell population expresses different subtypes of MCT. Epithelial cancer cells express MCT1, whereas CAFs lacking caveolin expression are positive for MCT4 [121,122]. Cancer cells synthesize pyruvate from lactate, thereby providing the TCA cycle with an intermediate metabolite. An extracellular space rich in lactate leads to an acidic microenvironment, which in turn contributes to the generation of pseudo-hypoxic conditions. In this emerging concept of reverse Warburg effect, MCT1-positive cancer cells play an important role in maintaining the hierarchy in tumor cellular society unlike MCT4-positive CAFs [4,33,34]. In tumor cells positive for MCT1, a second signaling activity of lactate is tightly linked to its positive regulation of amino acid metabolism. In those cells, inhibition of HIF prolylhydoxylases by lactate-derived pyruvate leads to stabilization and activation of both HIF-1α and HIF-2α [109,123]. Interestingly, HIF-2α plays a fundamental role in the regulation of expression levels of ASCT2 in MCT1-positive cancer cells [123]. HIF-2α activates c-Myc signaling, thereby promoting glutamine uptake and metabolism through the upregulation of the inward glutamine transporter ASCT2 and the glutamine-metabolizing enzyme GLS1 [123].
However, all the types of cancer cells do not necessarily exhibit the reverse the Warburg effect. Tumors that express high levels of MCT4 or those with the mesenchymal phenotype fail to exhibit the reverse Warburg effect. Instead, hierarchical metabolic heterogeneity occurs: while MCT4-positive cancer cells depend on aerobic glycolysis and secrete lactate mediated by MCT4, MCT1-positive cancer cells import lactate through MCT1 and exhibit OXPHOS in mitochondria. In addition, the amount of glucose uptake is low in MCT1-positive cancer cells as compared with that in MCT4-expressing cells [124,125]. This metabolic heterogeneity is referred to as metabolic symbiosis, and this type of lactate shuttle is also observed between neurons and astrocytes in normal brain tissues [126]. It is notable that normal and cancerous tissues share finely regulated mechanisms of metabolic symbiosis. Remarkably, well-oxygenated cancer cells, which express high levels of MCT1, efficiently produce metabolic intermediates, as well as ATP and lactate, by utilizing lactate derived from hypoxic tumor cells expressing high levels of MCT4 (Figure 3). Redox stress is a major hallmark of malignant neoplasms, which drive the robust metabolism in adjacent proliferating MCT1-positive cancer cells in which a high amount of mitochondria exists; this is mediated by the paracrine transfer of mitochondrial fuels characterized by lactate, pyruvate, and ketone bodies [124,125].
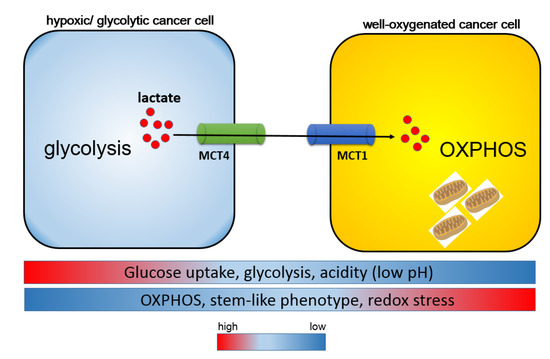
Figure 3.
Harmonious interaction between MCT1 and MCT4 is responsible for the heterogeneity of cancer metabolism. Metabolic symbiosis occurs between well-oxygenated/aerobic cancer cells and hypoxic/glycolytic cancer cells. Intra-tumoral heterogeneity induces a lactate shuttle between hypoxic and oxidative cancer cells driven by both MCT1 and MCT4. In contrast to MCT1-expressing cancer cells, glucose uptake is robust in MCT4-positive cells. MCT4-positive hypoxic cells are responsible for the formation of an acidic tumor microenvironment through aerobic glycolysis and the secretion of lactate, whereas MCT1-positive oxidative cells utilize lactate as a metabolic intermediate for the tricarboxylic acid cycle (TCA cycle) and oxidative phosphorylation (OXPHOS), and consequently exhibit a stem-like phenotype.
Genotoxic stress due to chemotherapy and/or irradiation, which increases oxidative stress, promotes a stem-like phenotype [8,127,128,129]. As cancer stem-like cells exhibit a rapidly proliferating and poorly differentiated phenotype, MCT1-positive cancer cells are likely to harbor a stem-like phenotype in the heterogeneous cellular society. Indeed, cancer cells follow a hierarchical model in which a subpopulation of cancer stem-like cells have a tumorigenic potential much greater than that of other differentiated cancer cells [6,130]. The activated mitochondrial metabolism in cancer stem-like cells provides enough energy not only for self-renewal by symmetric cell division, but also for invasive and metastatic phenotypes. Therefore, pharmacological blockage of MCT1 is useful for the treatment of malignancy because the suppression of MCT1 activity inhibits metabolic symbiosis, and MCT1-positive aerobic cancer stem-like cells can no longer take up lactate [125]. These findings suggest that MCT1-positive cells play an important role in maintaining the hierarchy in cancer cellular society, in contrast to MCT4-positive cells.
6. Conclusions
Mounting evidence helps us understand the reason why cancer cells develop metabolic phenotypes that differ from those of adjacent, non-malignant tissues, as well as when these phenotypes represent actionable therapeutic vulnerabilities. Aberrant proliferation of tumor cells is maintained by the adaptation to a nutrient microenvironment generated through alterations in energy metabolism specific to malignancy. As a consequence, metabolic reprogramming is one of the hallmarks of cancer cells in parallel with genomic instability, chronic inflammation in the tumor microenvironment, and escape from the immune surveillance. Although aerobic glycolysis, also known as the Warburg effect, is a characteristic metabolic feature of cancer cells, recent investigations show that other metabolic features driven by the interaction of amino acid transporters, in particular, glutamine addiction, metabolic symbiosis, and reverse Warburg effect, are responsible for therapeutic resistance. Thus, metabolic reprogramming orchestrated by transporters such as xCT, ASCT2, LAT1, and the MCT family is a therapeutic target as the Achilles’ heel of malignant neoplasms.
Funding
This review article was financially supported by the Japan Society for the Promotion of Science (20K16340).
Conflicts of Interest
There are no competing interest to be addressed.
Abbreviations
| α-KG | α-ketoglutarate |
| AMPK | AMP activated protein kinase |
| ATF4 | activating transcription factor 4 |
| BMSC | bone marrow-derived stromal cell |
| CAFs | cancer-associated fibroblasts |
| CD44v | CD44 variant |
| CD98hc | CD98 heavy chain |
| CLL | chronic lymphocytic leukemia |
| EAA | essential amino acids |
| GDH | glutamate dehydrogenase |
| HIF | hypoxia-inducible factor |
| GSH | glutathione |
| HNSCC | head and neck squamous cell carcinoma |
| MCT | monocarboxylate transporter |
| microRNA | miR |
| mTOR | the mammalian target of rapamycin |
| OXPHOS | oxidative phosphorylation |
| SSZ | sulfasalazine |
| TCA cycle | tricarboxylic acid cycle |
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.M.; Yeung, S.J.; Lee, M.-H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar] [PubMed]
- Yoshida, G.J. Metabolic reprogramming: The emerging concept and associated therapeutic strategies. J. Exp. Clin. Cancer Res. 2015, 34, 111. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-A.; Wang, C.-Y.; Hsieh, Y.-T.; Chen, Y.-J.; Wei, Y.-H. Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle 2014, 14, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J.; Saya, H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016, 107, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Makena, M.R.; Ranjan, A.; Thirumala, V.; Reddy, A.P. Cancer stem cells: Road to therapeutic resistance and strategies to overcome resistance. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165339. [Google Scholar] [CrossRef]
- Yoshida, G.J. The heterogeneity of cancer stem-like cells at the invasive front. Cancer Cell Int. 2017, 17, 23. [Google Scholar] [CrossRef][Green Version]
- Frank, N.Y.; Schatton, T.; Frank, M.H. The therapeutic promise of the cancer stem cell concept. J. Clin. Investig. 2010, 120, 41–50. [Google Scholar] [CrossRef]
- Kurtova, A.V.; Xiao, J.; Mo, Q.; Pazhanisamy, S.K.; Krasnow, R.; Lerner, S.P.; Chen, F.; Roh, T.T.; Lay, E.; Ho, P.L.; et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nat. Cell Biol. 2015, 517, 209–213. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The Cancer Stem Cell Niche: How Essential Is the Niche in Regulating Stemness of Tumor Cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.R.; Sabatini, D.M. Cancer Cell Metabolism: One Hallmark, Many Faces. Cancer Discov. 2012, 2, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S. Amino Acid Transporters as Targets for Cancer Therapy: Why, Where, When, and How. Int. J. Mol. Sci. 2020, 21, 6156. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Babu, E.; Ramachandran, S.; Ganapathy, V. Amino Acid transporters in cancer and their relevance to “glutamine addiction”: Novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015, 75, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wang, C.; Liu, G.; Bi, C.; Wang, X.; Zhou, Q.; Jin, H. SLC7A11/xCT in cancer: Biological functions and therapeutic implications. Am J Cancer Res 2020, 10, 3106–3126. [Google Scholar]
- Huang, Y.; Dai, Z.; Barbacioru, C.; Sadée, W. Cystine-Glutamate Transporter SLC7A11 in Cancer Chemosensitivity and Chemoresistance. Cancer Res. 2005, 65, 7446–7454. [Google Scholar] [CrossRef]
- Lo, M.; Wang, Y.Z.; Gout, P.W. The x(c)- cystine/glutamate antiporter: A potential target for therapy of cancer and other diseases. J. Cell Physiol. 2008, 215, 593–602. [Google Scholar] [CrossRef]
- Closs, E.I.; Wagner, C.A.; Palacin, M.; Endou, H.; Kanai, Y. CATs and HATs: The SLC7 family of amino acid transporters. Pflügers Arch. Eur. J. Physiol. 2004, 447, 532–542. [Google Scholar] [CrossRef]
- Sato, H.; Shiiya, A.; Kimata, M.; Maebara, K.; Tamba, M.; Sakakura, Y.; Makino, N.; Sugiyama, F.; Yagami, K.-I.; Moriguchi, T.; et al. Redox Imbalance in Cystine/Glutamate Transporter-deficient Mice. J. Biol. Chem. 2005, 280, 37423–37429. [Google Scholar] [CrossRef]
- Sasaki, H.; Sato, H.; Kuriyama-Matsumura, K.; Sato, K.; Maebara, K.; Wang, H.; Tamba, M.; Itoh, K.; Yamamoto, M.; Bannai, S. Electrophile Response Element-mediated Induction of the Cystine/Glutamate Exchange Transporter Gene Expression. J. Biol. Chem. 2002, 277, 44765–44771. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Qian, J.; Rahman, S.M.J.; Siska, P.J.; Zou, Y.; Harris, B.K.; Hoeksema, M.D.; Trenary, I.A.; Heidi, C.; Eisenberg, R.; et al. xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small cell lung cancer progression. Oncogene 2018, 37, 5007–5019. [Google Scholar] [CrossRef] [PubMed]
- Habib, E.; Linher-Melville, K.; Lin, H.-X.; Singh, G. Expression of xCT and activity of system xc− are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol. 2015, 5, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.K.; Hill, T., 3rd; Alexander, C.M. The involvement of NRF2 in lung cancer. Oxid. Med. Cell. Longev. 2013, 2013, 746432. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An Integrated Stress Response Regulates Amino Acid Metabolism and Resistance to Oxidative Stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Lewerenz, J.; Sato, H.; Albrecht, P.; Henke, N.; Noack, R.; Methner, A.; Maher, P. Mutation of ATF4 mediates resistance of neuronal cell lines against oxidative stress by inducing xCT expression. Cell Death Differ. 2011, 19, 847–858. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Basal Levels of eIF2α Phosphorylation Determine Cellular Antioxidant Status by Regulating ATF4 and xCT Expression. J. Biol. Chem. 2008, 284, 1106–1115. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 Variant Regulates Redox Status in Cancer Cells by Stabilizing the xCT Subunit of System xc− and Thereby Promotes Tumor Growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef]
- Yae, T.; Tsuchihashi, K.; Ishimoto, T.; Motohara, T.; Yoshikawa, M.; Yoshida, G.J.; Wada, T.; Masuko, T.; Mogushi, K.; Tanaka, H.; et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat. Commun. 2012, 3, 883. [Google Scholar] [CrossRef]
- Granot, Z.; Henke, E.; Comen, E.A.; King, T.A.; Norton, L.; Benezra, R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 2011, 20, 300–314. [Google Scholar] [CrossRef]
- Zhang, W.; Trachootham, D.; Liu, J.; Chen, G.; Pelicano, H.; Garcia-Prieto, C.; Lu, W.; Burger, J.A.; Croce, C.M.; Plunkett, W.; et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat. Cell Biol. 2012, 14, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Wilde, L.; Roche, M.; Domingo-Vidal, M.; Tanson, K.; Philp, N.; Curry, J.; Martinez-Outschoorn, U.E. Metabolic coupling and the Reverse Warburg Effect in cancer: Implications for novel biomarker and anticancer agent development. Semin. Oncol. 2017, 44, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J.; Azuma, A.; Miura, Y.; Orimo, A. Activated Fibroblast Program Orchestrates Tumor Initiation and Progression; Molecular Mechanisms and the Associated Therapeutic Strategies. Int. J. Mol. Sci. 2019, 20, 2256. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J. Regulation of heterogeneous cancer-associated fibroblasts: The molecular pathology of activated signaling pathways. J. Exp. Clin. Cancer Res. 2020, 39, 1–15. [Google Scholar] [CrossRef]
- Bonifácio, V.D.B.; Pereira, S.A.; Serpa, J.; Vicente, J.B. Cysteine metabolic circuitries: Druggable targets in cancer. Br. J. Cancer 2020, 1–18. [Google Scholar] [CrossRef]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- McBean, G.J.; Flynn, J. Molecular mechanisms of cystine transport. Biochem. Soc. Trans. 2001, 29, 717–722. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tsuchihashi, K.; Ishimoto, T.; Yae, T.; Motohara, T.; Sugihara, E.; Onishi, N.; Masuko, T.; Yoshizawa, K.; Kawashiri, S.; et al. xCT Inhibition Depletes CD44v-Expressing Tumor Cells That Are Resistant to EGFR-Targeted Therapy in Head and Neck Squamous Cell Carcinoma. Cancer Res. 2013, 73, 1855–1866. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 1–16. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Hassanein, M.; Hoeksema, M.D.; Shiota, M.; Qian, J.; Harris, B.K.; Chen, H.; Clark, J.E.; Alborn, W.E.; Eisenberg, R.; Massion, P.P. SLC1A5 Mediates Glutamine Transport Required for Lung Cancer Cell Growth and Survival. Clin. Cancer Res. 2013, 19, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, W.; Ling, Z.; Hu, Q.; Fan, Z.; Cheng, B.; Tao, X. ASCT2 overexpression is associated with poor survival of OSCC patients and ASCT2 knockdown inhibited growth of glutamine-addicted OSCC cells. Cancer Med. 2020, 9, 3489–3499. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Pochini, L.; Galluccio, M.; Indiveri, C. The glutamine/amino acid transporter (ASCT2) reconstituted in liposomes: Transport mechanism, regulation by ATP and characterization of the glutamine/glutamate antiport. Biochim. Biophys. Acta (BBA) Biomembr. 2007, 1768, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Pochini, L.; Console, L.; Losso, M.A.; Indiveri, C. The Human SLC1A5 (ASCT2) Amino Acid Transporter: From Function to Structure and Role in Cell Biology. Front. Cell Dev. Biol. 2018, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhang, Y.; Zhuang, L.; Gan, B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond) 2018, 38, 12. [Google Scholar] [CrossRef]
- Timmerman, L.A.; Holton, T.; Yuneva, M.; Louie, R.J.; Padro, M.; Daemen, A.; Hu, M.; Chan, D.A.; Ethier, S.P.; van Veer, L.J.; et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 2013, 24, 450–465. [Google Scholar] [CrossRef]
- Cao, M.D.; Lamichhane, S.; Lundgren, S.; Bofin, A.M.; Fjøsne, H.; Giskeødegård, G.F.; Bathen, T.F. Metabolic characterization of triple negative breast cancer. BMC Cancer 2014, 14, 941. [Google Scholar] [CrossRef]
- McGivan, J.D.; Bungard, C.I. The transport of glutamine into mammalian cells. Front Biosci. 2007, 12, 874–882. [Google Scholar] [CrossRef]
- Eagle, H. Nutrition Needs of Mammalian Cells in Tissue Culture. Science 2006, 122, 501–504. [Google Scholar] [CrossRef]
- Fuchs, B.C.; Bode, B.P. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime? Semin. Cancer Biol. 2005, 15, 254–266. [Google Scholar] [CrossRef]
- Moreadith, R.W.; Lehninger, A.L. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J. Biol. Chem. 1984, 259, 6215–6221. [Google Scholar] [PubMed]
- Nagarajan, A.; Malvi, P.; Wajapeyee, N. Oncogene-Directed Alterations in Cancer Cell Metabolism. Trends Cancer 2016, 2, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and Cancer Metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Venneti, S.; Nagrath, D. Glutaminolysis: A Hallmark of Cancer Metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A. Glutamine and cancer. J. Nutr. 2001, 131, 2539S–2542S. [Google Scholar] [CrossRef] [PubMed]
- Csibi, A.; Fendt, S.-M.; Li, C.; Poulogiannis, G.; Choo, A.Y.; Chapski, D.J.; Jeong, S.M.; Dempsey, J.M.; Parkhitko, A.; Morrison, T.; et al. The mTORC1 Pathway Stimulates Glutamine Metabolism and Cell Proliferation by Repressing SIRT4. Cell 2013, 153, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.R.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.-Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [Google Scholar] [CrossRef]
- Esslinger, C.S.; Cybulski, K.A.; Rhoderick, J.F. Ngamma-aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg. Med. Chem. 2005, 13, 1111–1118. [Google Scholar] [CrossRef]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional Transport of Amino Acids Regulates mTOR and Autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef]
- Duran, R.V.; Oppliger, W.; Robitaille, A.M.; Heiserich, L.; Skendaj, R.; Gottlieb, E.; Hall, M.N. Glutaminolysis activates Rag-mTORC1 signaling. Mol. Cell 2012, 47, 349–358. [Google Scholar] [CrossRef]
- Okazaki, S.; Umene, K.; Yamasaki, J.; Suina, K.; Otsuki, Y.; Yoshikawa, M.; Minami, Y.; Masuko, T.; Kawaguchi, S.; Nakayama, H.; et al. Glutaminolysis-related genes determine sensitivity to xCT-targeted therapy in head and neck squamous cell carcinoma. Cancer Sci. 2019, 110, 3453–3463. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Kaira, K.; Ohshima, Y.; Ishioka, N.S.; Shino, M.; Sakakura, K.; Takayasu, Y.; Takahashi, K.; Tominaga, H.; Oriuchi, N.; et al. Prognostic significance of amino-acid transporter expression (LAT1, ASCT2, and xCT) in surgically resected tongue cancer. Br. J. Cancer 2014, 110, 2506–2513. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Huang, T.; Li, W.; Wang, X.; Wu, X.; Liu, S.; Yang, W.; Shi, Q.; Li, H.; Hou, F. Prognostic Value of CD44 and Its Isoforms in Advanced Cancer: A Systematic Meta-Analysis With Trial Sequential Analysis. Front. Oncol. 2019, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Holst, J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015, 5, 1281–1294. [Google Scholar]
- Bodoor, K.; Almomani, R.; Alqudah, M.; Haddad, Y.; Samouri, W. LAT1 (SLC7A5) Overexpression in Negative Her2 Group of Breast Cancer: A Potential Therapy Target. Asian Pac. J. Cancer Prev. 2020, 21, 1453–1458. [Google Scholar] [CrossRef]
- Barollo, S.; Bertazza, L.; Watutantrige-Fernando, S.; Censi, S.; Cavedon, E.; Galuppini, F.; Pennelli, G.; Fassina, A.; Citton, M.; Rubin, B.; et al. Overexpression of L-Type Amino Acid Transporter 1 (LAT1) and 2 (LAT2): Novel Markers of Neuroendocrine Tumors. PLoS ONE 2016, 11, e0156044. [Google Scholar] [CrossRef]
- Yoshida, G.J. Beyond the Warburg Effect: N-Myc Contributes to Metabolic Reprogramming in Cancer Cells. Front. Oncol. 2020, 10, 791. [Google Scholar] [CrossRef]
- Nakamura, E.; Sato, M.; Yang, H.; Miyagawa, F.; Harasaki, M.; Tomita, K.; Matsuoka, S.; Noma, A.; Iwai, K.; Minato, N. 4F2 (CD98) Heavy Chain Is Associated Covalently with an Amino Acid Transporter and Controls Intracellular Trafficking and Membrane Topology of 4F2 Heterodimer. J. Biol. Chem. 1999, 274, 3009–3016. [Google Scholar] [CrossRef]
- Kanai, Y.; Segawa, H.; Miyamoto, K.-I.; Uchino, H.; Takeda, E.; Endou, H. Expression Cloning and Characterization of a Transporter for Large Neutral Amino Acids Activated by the Heavy Chain of 4F2 Antigen (CD98). J. Biol. Chem. 1998, 273, 23629–23632. [Google Scholar] [CrossRef]
- Mastroberardino, L.; Spindler, B.; Pfeiffer, R.; Skelly, P.J.; Loffing, J.; Shoemaker, C.B.; Verrey, F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nat. Cell Biol. 1998, 395, 288–291. [Google Scholar] [CrossRef]
- Meier, C.; Ristic, Z.; Klauser, S.; Verrey, F. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 2002, 21, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, O.; Kanai, Y.; Chairoungdua, A.; Kim, D.K.; Segawa, H.; Nii, T.; Cha, S.H.; Matsuo, H.; Fukushima, J.-I.; Fukasawa, Y.; et al. Human L-type amino acid transporter 1 (LAT1): Characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta (BBA) Biomembr. 2001, 1514, 291–302. [Google Scholar] [CrossRef]
- Salisbury, T.B.; Arthur, S. The Regulation and Function of the L-Type Amino Acid Transporter 1 (LAT1) in Cancer. Int. J. Mol. Sci. 2018, 19, 2373. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J Cell Sci 2009, 122, 3589–3594. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Manning, B.D. mTORC1 signaling and the metabolic control of cell growth. Curr. Opin. Cell Biol. 2017, 45, 72–82. [Google Scholar] [CrossRef]
- Yoshida, G.J. Therapeutic strategies of drug repositioning targeting autophagy to induce cancer cell death: From pathophysiology to treatment. J. Hematol. Oncol. 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Kato, H.; Nakajima, S.; Saito, Y.; Takahashi, S.; Katoh, R.; Kitamura, M. mTORC1 serves ER stress-triggered apoptosis via selective activation of the IRE1–JNK pathway. Cell Death Differ. 2011, 19, 310–320. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Chen, R.; Zou, Y.; Mao, D.; Sun, D.; Gao, G.; Shi, J.; Liu, X.; Zhu, C.; Yang, M.; Ye, W.; et al. The general amino acid control pathway regulates mTOR and autophagy during serum/glutamine starvation. J. Cell Biol. 2014, 206, 173–182. [Google Scholar] [CrossRef]
- Park, Y.; Reyna-Neyra, A.; Philippe, L.; Thoreen, C. mTORC1 Balances Cellular Amino Acid Supply with Demand for Protein Synthesis through Post-transcriptional Control of ATF4. Cell Rep. 2017, 19, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Anzai, N. Novel therapeutic approaches targeting L-type amino acid transporters for cancer treatment. World J. Gastrointest. Oncol. 2017, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Loewith, R.J.; Hall, M.N. TOR Signaling in Growth and Metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, C.; Yoshino, K.-I.; Yonezawa, K. mTOR integrates amino acid- and energy-sensing pathways. Biochem. Biophys. Res. Commun. 2004, 313, 443–446. [Google Scholar] [CrossRef]
- Beaumatin, F.; O’Prey, J.; Barthet, V.J.; Zunino, B.; Parvy, J.-P.; Bachmann, A.M.; O’Prey, M.; Kania, E.; Gonzalez, P.S.; MacIntosh, R.; et al. mTORC1 Activation Requires DRAM-1 by Facilitating Lysosomal Amino Acid Efflux. Mol. Cell 2019, 76, 163–176. [Google Scholar] [CrossRef]
- Yoshida, G.J.; Saya, H. EpCAM expression in the prostate cancer makes the difference in the response to growth factors. Biochem. Biophys. Res. Commun. 2014, 443, 239–245. [Google Scholar] [CrossRef]
- Xu, D.; Hemler, M.E. Metabolic Activation-related CD147-CD98 Complex. Mol. Cell. Proteom. 2005, 4, 1061–1071. [Google Scholar] [CrossRef]
- Tayama, S.; Motohara, T.; Narantuya, D.; Li, C.; Fujimoto, K.; Sakaguchi, I.; Tashiro, H.; Saya, H.; Nagano, O.; Katabuchi, H. The impact of EpCAM expression on response to chemotherapy and clinical outcomes in patients with epithelial ovarian cancer. Oncotarget 2017, 8, 44312–44325. [Google Scholar] [CrossRef]
- Betsunoh, H.; Fukuda, T.; Anzai, N.; Nishihara, D.; Mizuno, T.; Yuki, H.; Masuda, A.; Yamaguchi, Y.; Abe, H.; Yashi, M.; et al. Increased expression of system large amino acid transporter (LAT)-1 mRNA is associated with invasive potential and unfavorable prognosis of human clear cell renal cell carcinoma. BMC Cancer 2013, 13, 509. [Google Scholar] [CrossRef]
- Yanagisawa, N.; Ichinoe, M.; Mikami, T.; Nakada, N.; Hana, K.; Koizumi, W.; Endou, H.; Okayasu, I. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J. Clin. Pathol. 2012, 65, 1019–1023. [Google Scholar] [CrossRef]
- Kaira, K.; Nakamura, K.; Hirakawa, T.; Imai, H.; Tominaga, H.; Oriuchi, N.; Nagamori, S.; Kanai, Y.; Tsukamoto, N.; Oyama, T.; et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) expression in patients with ovarian tumors. Am. J. Transl. Res. 2015, 7, 1161–1171. [Google Scholar] [PubMed]
- Kaira, K.; Arakawa, K.; Shimizu, K.; Oriuchi, N.; Nagamori, S.; Kanai, Y.; Oyama, T.; Takeyoshi, I. Relationship between CD147 and expression of amino acid transporters (LAT1 and ASCT2) in patients with pancreatic cancer. Am. J. Transl. Res. 2015, 7, 356–363. [Google Scholar] [PubMed]
- Yazawa, T.; Shimizu, K.; Kaira, K.; Nagashima, T.; Ohtaki, Y.; Atsumi, J.; Obayashi, K.; Nagamori, S.; Kanai, Y.; Oyama, T.; et al. Clinical significance of coexpression of L-type amino acid transporter 1 (LAT1) and ASC amino acid transporter 2 (ASCT2) in lung adenocarcinoma. Am. J. Transl. Res. 2015, 7, 1126–1139. [Google Scholar] [PubMed]
- Kaira, K.; Oriuchi, N.; Imai, H.; Shimizu, K.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Kawashima, O.; Iijima, H.; Ishizuka, T.; et al. Expression of L-type amino acid transporter 1 (LAT1) in neuroendocrine tumors of the lung. Pathol. Res. Pr. 2008, 204, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Oriuchi, N.; Imai, H.; Shimizu, K.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Tanaka, S.; Ishizuka, T.; Kanai, Y.; et al. Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I–III nonsmall cell lung cancer. Br. J. Cancer 2008, 98, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Ichinoe, M.; Yanagisawa, N.; Mikami, T.; Hana, K.; Nakada, N.; Endou, H.; Okayasu, I.; Murakumo, Y. L-type amino acid transporter 1 (LAT1) expression in lymph node metastasis of gastric carcinoma: Its correlation with size of metastatic lesion and Ki-67 labeling. Pathol. Res. Pr. 2015, 211, 533–538. [Google Scholar] [CrossRef]
- Shi, L.; Luo, W.; Huang, W.; Huang, S.; Huang, G. Downregulation of L-type amino acid transporter 1 expression inhibits the growth, migration and invasion of gastric cancer cells. Oncol. Lett. 2013, 6, 106–112. [Google Scholar] [CrossRef]
- Janpipatkul, K.; Suksen, K.; Borwornpinyo, S.; Jearawiriyapaisarn, N.; Hongeng, S.; Piyachaturawat, P.; Chairoungdua, A. Downregulation of LAT1 expression suppresses cholangiocarcinoma cell invasion and migration. Cell. Signal. 2014, 26, 1668–1679. [Google Scholar] [CrossRef]
- Kaji, M.; Kabir-Salmani, M.; Anzai, N.; Jin, C.J.; Akimoto, Y.; Horita, A.; Sakamoto, A.; Kanai, Y.; Sakurai, H.; Iwashita, M. Properties of L-Type Amino Acid Transporter 1 in Epidermal Ovarian Cancer. Int. J. Gynecol. Cancer 2010, 20, 329–336. [Google Scholar] [CrossRef]
- Miranda-Gonçalves, V.; Honavar, M.; Pinheiro, C.; Martinho, O.; Pires, M.M.; Pinheiro, C.; Cordeiro, M.; Bebiano, G.; Costa, P.; Palmeirim, I.; et al. Monocarboxylate transporters (MCTs) in gliomas: Expression and exploitation as therapeutic targets. Neuro-Oncology 2012, 15, 172–188. [Google Scholar] [CrossRef]
- Fang, J.; Quinones, Q.J.; Holman, T.L.; Morowitz, M.J.; Wang, Q.; Zhao, H.; Sivo, F.; Maris, J.M.; Wahl, M.L. The H+-Linked Monocarboxylate Transporter (MCT1/SLC16A1): A Potential Therapeutic Target for High-Risk Neuroblastoma. Mol. Pharmacol. 2006, 70, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Longatto-Filho, A.; Ferreira, L.; Pereira, S.M.M.; Etlinger, D.; Moreira, M.A.R.; Jubé, L.F.; Queiroz, G.S.; Schmitt, F.; Baltazar, F. Increasing Expression of Monocarboxylate Transporters 1 and 4 Along Progression to Invasive Cervical Carcinoma. Int. J. Gynecol. Pathol. 2008, 27, 568–574. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.T.T.; Pinheiro, C.; Longatto-Filho, A.; Brito, M.J.; Martinho, O.; Matos, D.; Carvalho, A.L.; Vazquez, V.D.L.; Silva, T.B.; Neto, C.S.; et al. Co-expression of monocarboxylate transporter 1 (MCT1) and its chaperone (CD147) is associated with low survival in patients with gastrointestinal stromal tumors (GISTs). J. Bioenerg. Biomembr. 2012, 44, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Longatto-Filho, A.; Scapulatempo, C.; Ferreira, L.; Martins, S.; Pellerin, L.; Rodrigues, M.; Alves, V.A.F.; Schmitt, F.; Baltazar, F. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008, 452, 139–146. [Google Scholar] [CrossRef]
- Pinheiro, C.; Albergaria, A.; Paredes, J.; Sousa, B.; Dufloth, R.; Vieira, D.; Schmitt, F.; Baltazar, F. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology 2010, 56, 860–867. [Google Scholar] [CrossRef]
- Pértega-Gomes, N.; Vizcaíno, J.R.; Miranda-Gonçalves, V.; Pinheiro, C.; Silva, J.; Pereira, H.; Monteiro, P.; Henrique, R.; Reis, R.M.; Lopes, C.; et al. Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer. BMC Cancer 2011, 11, 312. [Google Scholar] [CrossRef]
- Gerlinger, M.; Santos, C.R.; Spencer-Dene, B.; Martinez, P.; Endesfelder, D.; Burrell, R.A.; Vetter, M.; Jiang, M.; Saunders, R.E.; Kelly, G.; et al. Genome-wide RNA interference analysis of renal carcinoma survival regulators identifies MCT4 as a Warburg effect metabolic target. J. Pathol. 2012, 227, 146–156. [Google Scholar] [CrossRef]
- Doherty, J.R.; Cleveland, J.L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Investig. 2013, 123, 3685–3692. [Google Scholar] [CrossRef]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef]
- De Saedeleer, C.; Porporato, P.; Copetti, T.; Escuredo, J.P.; Payen, V.; Brisson, L.; Feron, O.; Sonveaux, P. Glucose deprivation increases monocarboxylate transporter 1 (MCT1) expression and MCT1-dependent tumor cell migration. Oncogene 2014, 33, 4060–4068. [Google Scholar] [CrossRef]
- Fan, Q.; Yang, L.; Zhang, X.; Ma, Y.; Li, Y.; Dong, L.; Zong, Z.; Hua, X.; Su, D.; Li, H.; et al. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/beta-catenin signaling pathway activation in hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 2018, 37, 9. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Chen, H.; Madigan, M.C.; Cozzi, P.J.; Beretov, J.; Xiao, W.; Delprado, W.J.; Russell, P.J.; Li, Y. Co-expression of CD147 (EMMPRIN), CD44v3-10, MDR1 and monocarboxylate transporters is associated with prostate cancer drug resistance and progression. Br. J. Cancer 2010, 103, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Slomiany, M.G.; Grass, G.D.; Robertson, A.D.; Yang, X.Y.; Maria, B.L.; Beeson, C.; Toole, B.P. Hyaluronan, CD44, and Emmprin Regulate Lactate Efflux and Membrane Localization of Monocarboxylate Transporters in Human Breast Carcinoma Cells. Cancer Res. 2009, 69, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.; Wilson, M.; Heddle, C.; Brown, M.; Barclay, A.; Halestrap, A.P. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000, 19, 3896–3904. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.M.; Castorino, J.J.; Wang, D.; Philp, N.J. Monocarboxylate Transporter 4 Regulates Maturation and Trafficking of CD147 to the Plasma Membrane in the Metastatic Breast Cancer Cell Line MDA-MB-231. Cancer Res. 2007, 67, 4182–4189. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Fei, F.; Chen, Y.; Xu, L.; Zhang, Z.; Huang, Q.; Zhang, H.; Yang, H.; Chen, Z.; Xing, J. Hypoxia upregulates CD147 through a combined effect of HIF-1α and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors. Carcinogenesis 2012, 33, 1598–1607. [Google Scholar] [CrossRef]
- Sprowl-Tanio, S.; Habowski, A.N.; Pate, K.T.; McQuade, M.M.; Wang, K.; Edwards, R.A.; Grun, F.; Lyou, Y.; Waterman, M.L. Lactate/pyruvate transporter MCT-1 is a direct Wnt target that confers sensitivity to 3-bromopyruvate in colon cancer. Cancer Metab. 2016, 4, 1–18. [Google Scholar] [CrossRef]
- Li, K.K.W.; Pang, J.C.-S.; Ching, A.K.-K.; Wong, C.K.; Kong, X.; Wang, Y.; Zhou, L.; Chen, Z.; Ng, H. miR-124 is frequently down-regulated in medulloblastoma and is a negative regulator of SLC16A1. Hum. Pathol. 2009, 40, 1234–1243. [Google Scholar] [CrossRef]
- Romero-Cordoba, S.; Rodriguez-Cuevas, S.; Bautista-Pina, V.; Maffuz-Aziz, A.; D’Ippolito, E.; Cosentino, G.; Baroni, S.; Iorio, M.V.; Hidalgo-Miranda, A. Loss of function of miR-342-3p results in MCT1 over-expression and contributes to oncogenic metabolic reprogramming in triple negative breast cancer. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Z.; Zou, K.; Cheng, Y.; Yang, M.; Chen, H.; Wang, H.; Zhao, J.; Chen, P.; He, L.; et al. MiR-1 suppresses tumor cell proliferation in colorectal cancer by inhibition of Smad3-mediated tumor glycolysis. Cell Death Dis. 2017, 8, e2761. [Google Scholar] [CrossRef]
- Pavlides, S.; Tsirigos, A.; Vera, I.; Frank, P.; Casimiro, M.C.; Addya, S.; Sotgia, F.; Flomenberg, N.; Wang, C.; Fortina, P.; et al. Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “reverse Warburg effect”: A transcriptional informatics analysis with validation. Cell Cycle 2010, 9, 2201–2219. [Google Scholar] [CrossRef] [PubMed]
- Pértega-Gomes, N.; Vizcaíno, J.R.; Attig, J.; Jurmeister, S.; Lopes, C.; Baltazar, F. A lactate shuttle system between tumour and stromal cells is associated with poor prognosis in prostate cancer. BMC Cancer 2014, 14, 352. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escuredo, J.; Dadhich, R.K.; Dhup, S.; Cacace, A.; Van Hée, V.F.; De Saedeleer, C.J.; Sboarina, M.; Rodriguez, F.; Fontenille, M.-J.; Brisson, L.; et al. Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle 2016, 15, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Tumor metabolism: Cancer cells give and take lactate. J. Clin. Investig. 2008, 118, 3835–3837. [Google Scholar] [CrossRef] [PubMed]
- Sonveaux, P.; Végran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.M. Inhibition of Lactate Dehydrogenase to Treat Epilepsy. N. Engl. J. Med. 2015, 373, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Cahu, J.; Bustany, S.; Sola, B. Senescence-associated secretory phenotype favors the emergence of cancer stem-like cells. Cell Death Dis. 2012, 3, e446. [Google Scholar] [CrossRef]
- Ogasawara, M.; Zhang, H. Redox Regulation and Its Emerging Roles in Stem Cells and Stem-Like Cancer Cells. Antioxid. Redox Signal. 2009, 11, 1107–1122. [Google Scholar] [CrossRef]
- Yoshida, G.J.; Saya, H. Inversed relationship between CD44 variant and c-Myc due to oxidative stress-induced canonical Wnt activation. Biochem. Biophys. Res. Commun. 2014, 443, 622–627. [Google Scholar] [CrossRef]
- Rich, J.N. Cancer stem cells: Understanding tumor hierarchy and heterogeneity. Medicine (Baltimore) 2016, 95, S2–S7. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).