Delayed Motor Milestones Achievement in Infancy Associates with Perturbations of Amino Acids and Lipid Metabolic Pathways
Abstract
1. Introduction
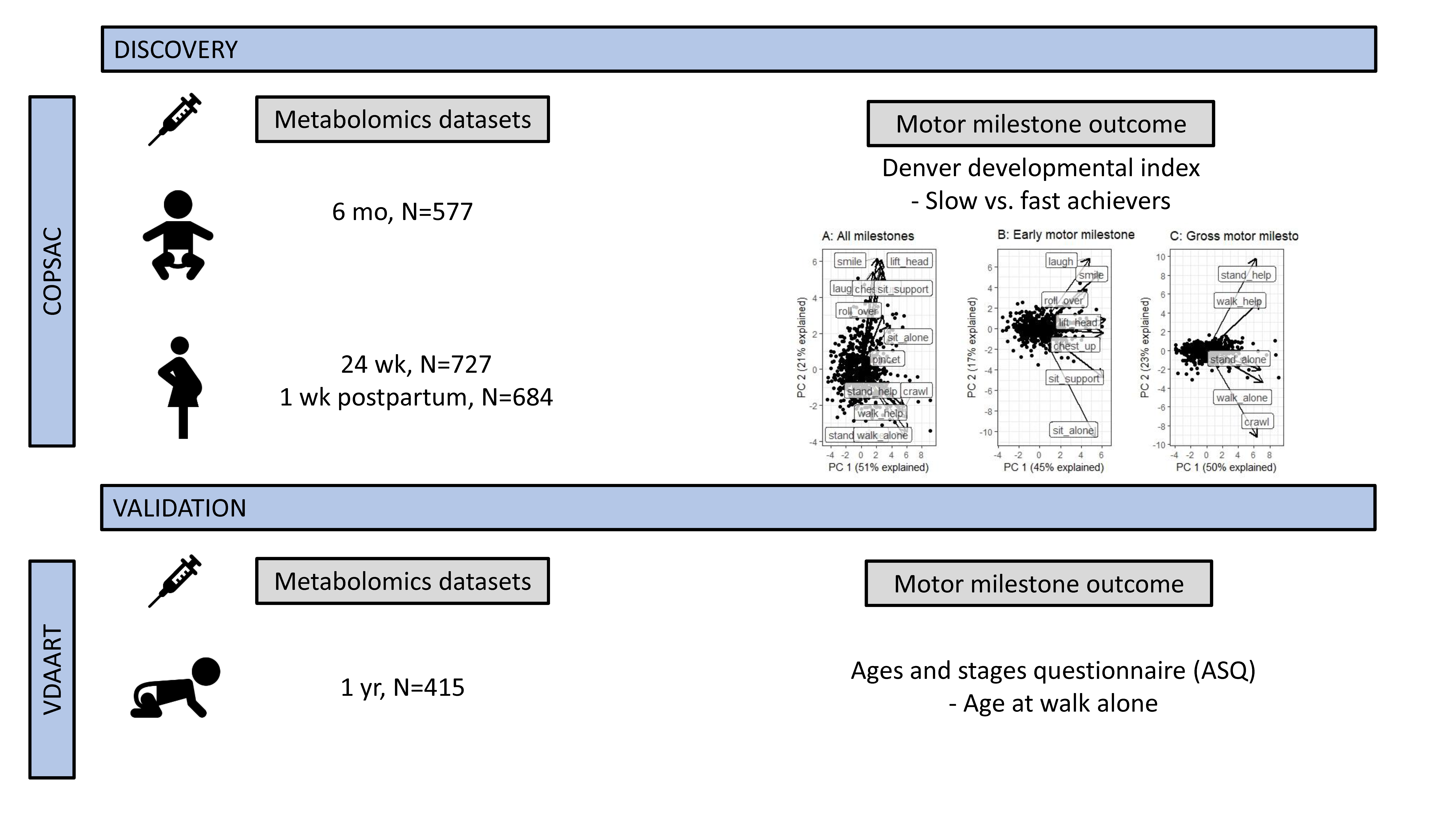
2. Results
2.1. Baseline Characteristics
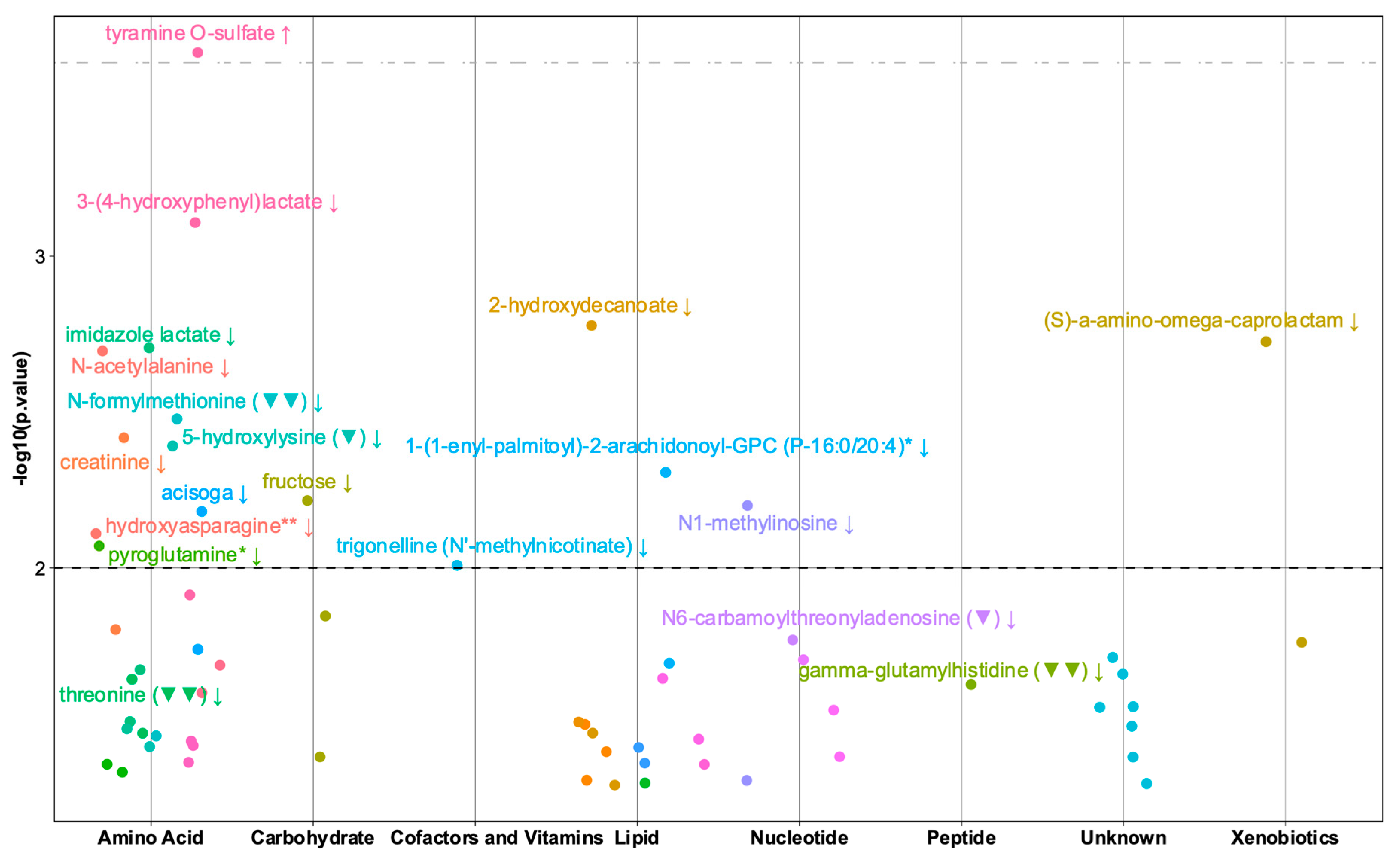
2.2. Gross-Motor Milestones
2.3. Validation in VDAART
2.4. Early Motor Milestones
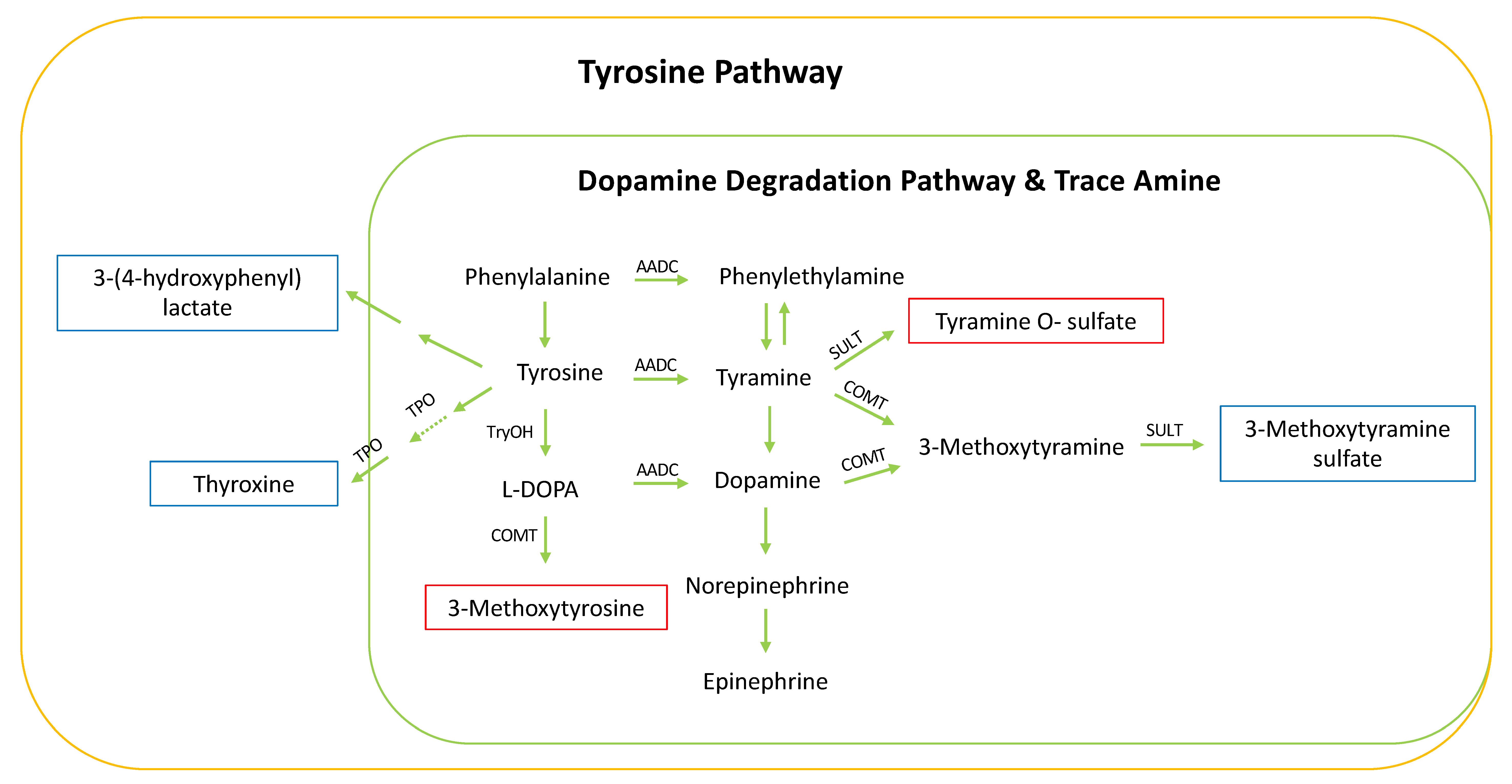
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Ethics
4.3. Developmental Milestones
4.4. Metabolomic Profiling
4.5. Covariates
4.6. Statistical Analysis
4.6.1. Data Pre-Processing
4.6.2. Univariate Analysis
4.6.3. Partial Least Squares Discriminant Analysis (PLS-DA)
4.6.4. Validation of Findings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stiles, J.; Jernigan, T.L. The Basics of Brain Development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef] [PubMed]
- WHO. Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference: Methods and Development; World Health Organization: Geneva, Switzerland, 2009; ISBN 9789241547635. [Google Scholar]
- Von Wendt, L.; Mäkinen, H.; Rantakallio, P. Psychomotor development in the first year and mental retardation—A prospective study. J. Intellect. Disabil. Res. 2008, 28, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Piek, J.; Dawson, L.; Smith, L.M.; Gasson, N. The role of early fine and gross motor development on later motor and cognitive ability. Hum. Mov. Sci. 2008, 27, 668–681. [Google Scholar] [CrossRef]
- Van Batenburg-Eddes, T.; Henrichs, J.; Schenk, J.J.; Sincer, I.; De Groot, L.; Hofman, A.; Jaddoe, V.W.V.; Verhulst, F.C.; Tiemeier, H. Early infant neuromotor assessment is associated with language and nonverbal cognitive function in toddlers: The Generation R Study. J. Dev. Behav. Pediatr. 2013, 34, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Hitzert, M.M.; Roze, E.; Van Braeckel, K.N.J.; Bos, A.F. Motor development in 3-month-old healthy term-born infants is associated with cognitive and behavioural outcomes at early school age. Dev. Med. Child Neurol. 2014, 56, 869–876. [Google Scholar] [CrossRef]
- Flensborg-Madsen, T.; Mortensen, E.L. Predictors of motor developmental milestones during the first year of life. Eur. J. Nucl. Med. Mol. Imaging 2016, 176, 109–119. [Google Scholar] [CrossRef]
- Kuklina, E.V.; Ramakrishnan, U.; Stein, A.D.; Barnhart, H.H.; Martorell, R. Growth and Diet Quality Are Associated with the Attainment of Walking in Rural Guatemalan Infants. J. Nutr. 2004, 134, 3296–3300. [Google Scholar] [CrossRef]
- Ghassabian, A.; Sundaram, R.; Bell, E.; Bello, S.C.; Kus, C.; Yeung, E.H. Gross Motor Milestones and Subsequent Development. Pediatrics 2016, 138, e20154372. [Google Scholar] [CrossRef]
- Manicolo, O.; Brotzmann, M.; Arx, P.H.-V.; Grob, A.; Weber, P. Gait in children with infantile/atypical autism: Age-dependent decrease in gait variability and associations with motor skills. Eur. J. Paediatr. Neurol. 2019, 23, 117–125. [Google Scholar] [CrossRef]
- Berry, M.D. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J. Neurochem. 2004, 90, 257–271. [Google Scholar] [CrossRef]
- Broadley, K.J. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. 2010, 125, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Beloborodova, N.; Bairamov, I.T.; Olenin, A.Y.; Shubina, V.S.; Teplova, V.V.; Fedotcheva, N. Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J. Biomed. Sci. 2012, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Ciloglu, F.; Peker, I.; Pehlivan, A.; Karacabey, K.; Ilhan, N.; Saygin, O.; Ozmerdivenli, R. Exercise intensity and its effects on thyroid hormones. Neuro Endocrinol. Lett. 2005, 26, 830–834. [Google Scholar] [PubMed]
- Clarke, M.C.; Tanskanen, A.; Huttunen, M.; Leon, D.A.; Murray, R.M.; Jones, P.B.; Cannon, M. Increased Risk of Schizophrenia from Additive Interaction Between Infant Motor Developmental Delay and Obstetric Complications: Evidence from a Population-Based Longitudinal Study. Am. J. Psychiatry 2011, 168, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Kapur, S. The Dopamine Hypothesis of Schizophrenia: Version III—The Final Common Pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef]
- Liu, M.-L.; Zheng, P.; Liu, Z.; Xu, Y.; Mu, J.; Guo, J.; Huang, T.; Meng, H.-Q.; Xie, P. GC-MS based metabolomics identification of possible novel biomarkers for schizophrenia in peripheral blood mononuclear cells. Mol. BioSyst. 2014, 10, 2398–2406. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Baxmann, A.C.; Ahmed, M.S.; Marques, N.C.; Menon, V.B.; Pereira, A.B.; Mastroianni-Kirsztajn, G.; Heilberg, I.P. Influence of Muscle Mass and Physical Activity on Serum and Urinary Creatinine and Serum Cystatin C. Clin. J. Am. Soc. Nephrol. 2008, 3, 348–354. [Google Scholar] [CrossRef]
- Berton, R.; Conceição, M.S.; Libardi, C.; Canevarolo, R.R.; Gáspari, A.F.; Chacon-Mikahil, M.; Zeri, A.C.M.; Cavaglieri, C.R. Metabolic time-course response after resistance exercise: A metabolomics approach. J. Sports Sci. 2016, 35, 1211–1218. [Google Scholar] [CrossRef]
- Gjaltema, R.A.F.; Bank, R.A. Molecular insights into prolyl and lysyl hydroxylation of fibrillar collagens in health and disease. Crit. Rev. Biochem. Mol. Biol. 2016, 52, 74–95. [Google Scholar] [CrossRef]
- Askenasi, R. Urinary excretion of free hydroxylysine, peptide-bound hydroxylysine and hydroxylysyl glycosides in physiological conditions. Clin. Chim. Acta 1975, 59, 87–92. [Google Scholar] [CrossRef]
- Moro, L.; Modricky, C.; Stagni, N.; Vittur, F.; de Bernard, B. High-performance liquid chromatographic analysis of urinary hydroxylysyl glycosides as indicators of collagen turnover. Analyst 1984, 109, 1621. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, L.W.; Ford, J.D.; Segrest, J.P. The isolation of identical hydroxylysyl glycosides from hydrolysates of soluble collagen and from human urine. J. Biol. Chem. 1967, 242, 2570–2571. [Google Scholar]
- Powers, S.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S.; Watkins, S.M. Identification of a Lipokine, a Lipid Hormone Linking Adipose Tissue to Systemic Metabolism. Cell 2008, 134, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1357. [Google Scholar] [CrossRef]
- Hama, H. Fatty acid 2-Hydroxylation in mammalian sphingolipid biology. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2010, 1801, 405–414. [Google Scholar] [CrossRef]
- Dick, K.J.; Al-Mjeni, R.; Baskir, W.; Koul, R.; Simpson, M.; Patton, M.A.; Raeburn, S.; Crosby, A.H. A novel locus for an autosomal recessive hereditary spastic paraplegia (SPG35) maps to 16q21-q23. Neurology 2008, 71, 248–252. [Google Scholar] [CrossRef]
- Edvardson, S.; Hama, H.; Shaag, A.; Gomori, J.M.; Berger, I.; Soffer, D.; Korman, S.H.; Taustein, I.; Saada, A.; Elpeleg, O. Mutations in the Fatty Acid 2-Hydroxylase Gene Are Associated with Leukodystrophy with Spastic Paraparesis and Dystonia. Am. J. Hum. Genet. 2008, 83, 643–648. [Google Scholar] [CrossRef]
- Dick, K.J.; Eckhardt, M.; Paisán-Ruíz, C.; Alshehhi, A.A.; Proukakis, C.; Sibtain, N.A.; Maier, H.; Sharifi, R.; Patton, M.A.; Bashir, W.; et al. Mutation of FA2H underlies a complicated form of hereditary spastic paraplegia (SPG35). Hum. Mutat. 2010, 31, E1251–E1260. [Google Scholar] [CrossRef]
- Kruer, M.C.; Paisán-Ruíz, C.; Boddaert, N.; Yoon, M.Y.; Hama, H.; Gregory, A.; Malandrini, A.; Woltjer, R.L.; Munnich, A.; Gobin, S.; et al. Defective FA2H leads to a novel form of neurodegeneration with brain iron accumulation (NBIA). Ann. Neurol. 2010, 68, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Garone, C.; Pippucci, T.; Cordelli, D.M.; Zuntini, R.; Castegnaro, G.; Marconi, C.; Graziano, C.; Marchiani, V.; Verrotti, A.; Seri, M.; et al. FA2H-related disorders: A novel c.270+3A>T splice-site mutation leads to a complex neurodegenerative phenotype. Dev. Med. Child Neurol. 2011, 53, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.K.; Veijola, J.; Moilanen, K.; Miettunen, J.; Glahn, D.; Cannon, T.; Jones, P.B.; Isohanni, M. Infant motor development is associated with adult cognitive categorisation in a longitudinal birth cohort study. J. Child Psychol. Psychiatry 2006, 47, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.K.; Jones, P.B.; Kuh, D.; Richards, M. Infant developmental milestones and subsequent cognitive function. Ann. Neurol. 2007, 62, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Bedford, R.; Pickles, A.; Lord, C. Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder. Autism Res. 2015, 9, 993–1001. [Google Scholar] [CrossRef]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol. Autism 2016, 7, 47. [Google Scholar] [CrossRef]
- Wang, H.; Liang, S.; Wang, M.; Gao, J.; Sun, C.; Wang, J.; Xia, W.; Wu, S.; Sumner, S.J.; Zhang, F.; et al. Potential serum biomarkers from a metabolomics study of autism. J. Psychiatry Neurosci. 2016, 41, 27–37. [Google Scholar] [CrossRef]
- West, P.R.; Amaral, D.G.; Bais, P.; Smith, A.M.; Egnash, L.A.; Ross, M.E.; Palmer, J.A.; Fontaine, B.R.; Conard, K.R.; Corbett, B.A.; et al. Metabolomics as a Tool for Discovery of Biomarkers of Autism Spectrum Disorder in the Blood Plasma of Children. PLoS ONE 2014, 9, e112445. [Google Scholar] [CrossRef]
- Hagenbeek, F.A.; Kluft, C.; Hankemeier, T.; Bartels, M.; Draisma, H.H.; Middeldorp, C.M.; Berger, R.; Noto, A.; Lussu, M.; Pool, R.; et al. Discovery of biochemical biomarkers for aggression: A role for metabolomics in psychiatry. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2016, 171, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Vissing, N.H.; Carson, C.G.; Bischoff, A.L.; Følsgaard, N.V.; Kreiner-Møller, E.; Chawes, B.L.K.; Stokholm, J.; Brix, S.; Bjarnadóttir, E.; et al. Deep phenotyping of the unselected COPSAC2010 birth cohort study. Clin. Exp. Allergy 2013, 43, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Bjarnadóttir, E.; Stokholm, J.; Chawes, B.; Thorsen, J.; Mora-Jensen, A.-R.C.; Deleuran, M.; Bønnelykke, K.; Lauritzen, L.; Bisgaard, H. Determinants of neurodevelopment in early childhood—Results from the Copenhagen prospective studies on asthma in childhood (COPSAC 2010) mother–child cohort. Acta Paediatr. 2019, 108, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Rago, D.; Rasmussen, M.A.; Lee-Sarwar, K.A.; Weiss, S.T.; Lasky-Su, J.; Stokholm, J.; Bønnelykke, K.; Chawes, B.L.; Bisgaard, H. Fish-oil supplementation in pregnancy, child metabolomics and asthma risk. EBioMedicine 2019, 46, 399–410. [Google Scholar] [CrossRef]
- Sumner, L.; Amberg, A.; Barrett, D.A.; Beale, M.H.; Beger, R.D.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Vinding, R.K.; Sejersen, T.S.; Chawes, B.L.; Bønnelykke, K.; Buhl, T.; Bisgaard, H.; Stokholm, J. Cesarean Delivery and Body Mass Index at 6 Months and Into Childhood. Pediatrics 2017, 139, e20164066. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Lange, N.E.; Carey, V.J.; Brown, S.; Laranjo, N.; Harshfield, B.J.; O’Connor, G.T.; Sandel, M.; Strunk, R.C.; Bacharier, L.B.; et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): Rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp. Clin. Trials 2014, 38, 37–50. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinding, R.K.; Rago, D.; Kelly, R.S.; Gürdeniz, G.; Rasmussen, M.A.; Stokholm, J.; Bønnelykke, K.; Litonjua, A.A.; Weiss, S.T.; Lasky-Su, J.; et al. Delayed Motor Milestones Achievement in Infancy Associates with Perturbations of Amino Acids and Lipid Metabolic Pathways. Metabolites 2020, 10, 337. https://doi.org/10.3390/metabo10090337
Vinding RK, Rago D, Kelly RS, Gürdeniz G, Rasmussen MA, Stokholm J, Bønnelykke K, Litonjua AA, Weiss ST, Lasky-Su J, et al. Delayed Motor Milestones Achievement in Infancy Associates with Perturbations of Amino Acids and Lipid Metabolic Pathways. Metabolites. 2020; 10(9):337. https://doi.org/10.3390/metabo10090337
Chicago/Turabian StyleVinding, Rebecca Kofod, Daniela Rago, Rachel S. Kelly, Gözde Gürdeniz, Morten Arendt Rasmussen, Jakob Stokholm, Klaus Bønnelykke, Augusto A. Litonjua, Scott T. Weiss, Jessica Lasky-Su, and et al. 2020. "Delayed Motor Milestones Achievement in Infancy Associates with Perturbations of Amino Acids and Lipid Metabolic Pathways" Metabolites 10, no. 9: 337. https://doi.org/10.3390/metabo10090337
APA StyleVinding, R. K., Rago, D., Kelly, R. S., Gürdeniz, G., Rasmussen, M. A., Stokholm, J., Bønnelykke, K., Litonjua, A. A., Weiss, S. T., Lasky-Su, J., Bisgaard, H., & Chawes, B. L. (2020). Delayed Motor Milestones Achievement in Infancy Associates with Perturbations of Amino Acids and Lipid Metabolic Pathways. Metabolites, 10(9), 337. https://doi.org/10.3390/metabo10090337






