Unraveling Arbuscular Mycorrhiza-Induced Changes in Plant Primary and Secondary Metabolome
Abstract
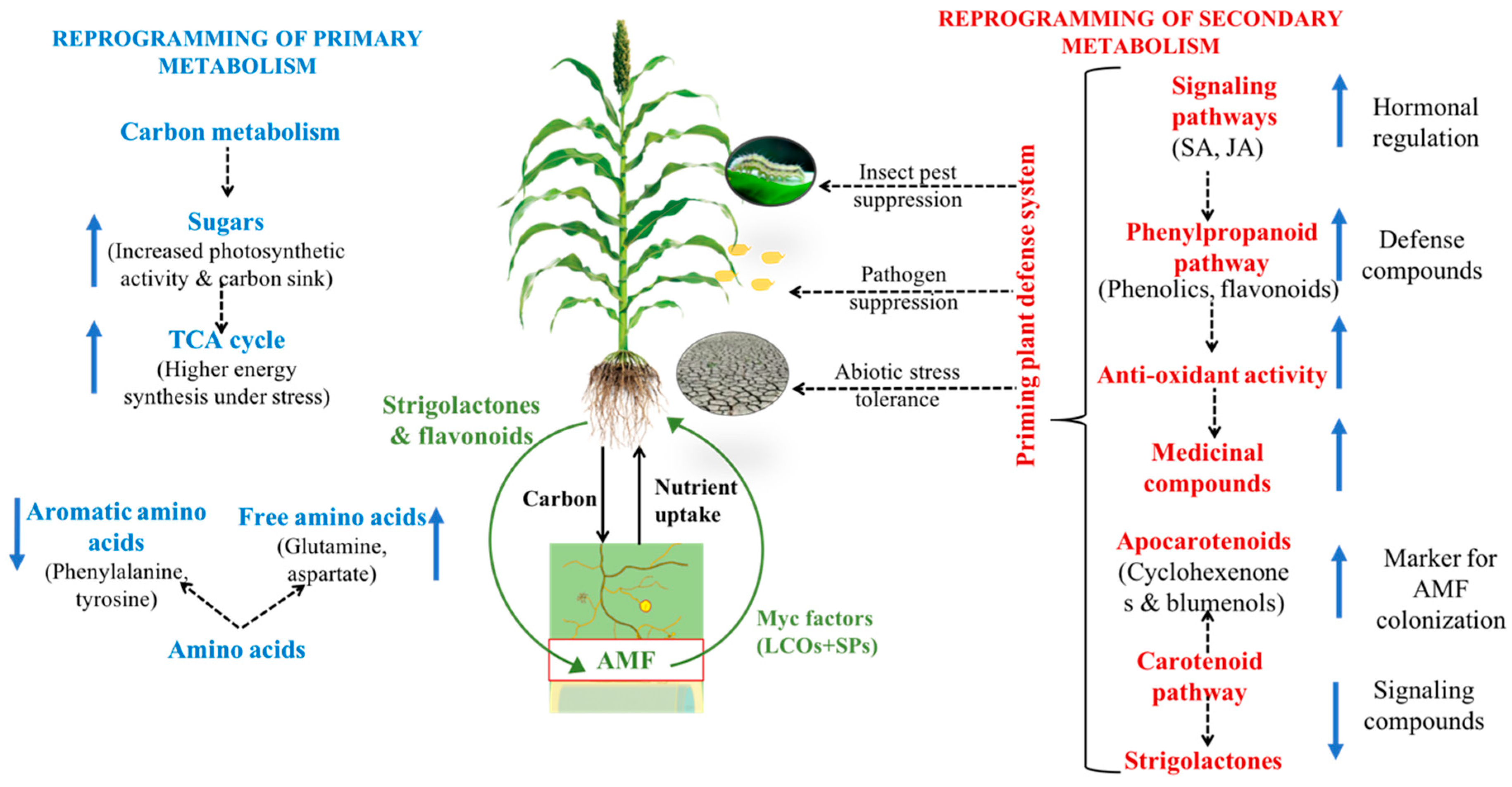
1. Introduction
2. Primary Metabolites
2.1. Sugars
2.2. Organic Acids
2.3. Amino Acids
2.4. Effect of AMF on Primary Metabolites under Biotic/Abiotic Stresses
| Primary Metabolites | Plant Parts | AMF Species | Plant Species | Enviromental Condition | Increase/Decrease | Reference |
|---|---|---|---|---|---|---|
| Sugars | ||||||
| Fructose | Roots | Glomus versiforme | Poncirus trifoliata | Well-watered | Increase | [109] |
| Glucose | Roots | Glomus versiforme | Poncirus trifoliata | Drought | Increase | [109] |
| Kestose | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Increase | [84] |
| Soluble sugars | Root + shoot | Glomus mossae | Maize | Salt stress | Increase | [61] |
| Soluble sugars | Leaves | Glomus mossae | Maize | Salt stress | Increase | [68] |
| Sucrose | Roots + leaves | Glomus versiforme | Poncirus trifoliata | Drought | Increase | [109] |
| Total sugars | Root | Glomus intraradices | Maize | Drought | Increase | [93] |
| Total sugars | Leaves | Glomus intraradices | Maize | Drought | Increase in drought sensitive and decrease in drought resistant variety | [93] |
| Trehalose | Roots | Glomus intraradices | Medicago truncatula | - | Increase | [70] |
| Sugar alcohols | ||||||
| Myo-inositol | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Increase | [84] |
| Pinitol | Roots | Funneliformis mossae | Triticum durum | Water stress | Decrease | [92] |
| Xilitol | Roots | * AMF mix and natural AMF inoculum | Triticum durum | N stress, P rich | Increase | [110] |
| Xylitol | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Increase | [84] |
| Organic acids | ||||||
| 2-methyl-malic acid | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Decrease | [84] |
| Acetic acid | Leaves | Glomus mossae | Maize | Salt stress | Increase | [68] |
| Citric acid | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Decrease | [84] |
| Citric acid | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Decrease in dicots and increase in monocot | [76] |
| Formic acid | Leaves | Glomus mossae | Maize | Salt stress | Decrease at highest salt concentration | [68] |
| Fumaric acid | Leaves | Glomus mossae | Maize | Salt stress | Increase | [68] |
| Fumaric acid | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Decrease in dicots and increase in monocot | [76] |
| Isocitric acid | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Partly Decrease in dicots and increase in monocot | [76] |
| Malic acid | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Decrease | [84] |
| Malic acid | Leaves | Glomus mossae | Maize | Salt stress | Increase | [68] |
| Malic acid | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Decrease in dicots and increase in monocot | [76] |
| Oxalic acid | Leaves | Glomus mossae | Maize | Salt stress | Increase | [68] |
| Succinic acid | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Decrease | [84] |
| Succinic acid | Leaves | Glomus mossae | Maize | Salt stress | Decrease at highest salt concentration | [68] |
| Succinic acid | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Partly decrease in dicots and increase in monocot | [76] |
| Amino acids | ||||||
| 4-amino-butanoic acid | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Decrease | [84] |
| Alanine | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Increase (mainly in dicots) | [76] |
| Alanine | Root | * AMF mix and natural AMF inoculum | Triticum durum | N stress, P rich | Decrease | [110] |
| Asparagine | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Decrease | [84] |
| Asparagine | Roots | * AMF mix and natural AMF inoculum | Triticum durum | N stress, P rich | Decrease in AMF mix | [110] |
| Asparagine | Roots | Glomus intraradices | Medicago truncatula | - | Increase | [70] |
| Aspartic acid | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Decrease | [84] |
| Aspartic acid | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Increase (mainly in dicots) | [76] |
| Aspartic acid | Roots | Funneliformis mossae and Rhizophagus irregularis (different treatments) | Solanum lycopersicum | Minimum P | Decrease | [64] |
| Aspartic acid | Roots | Glomus intraradices | Medicago truncatula | - | Increase | [70] |
| Glutamic acid | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Decrease | [84] |
| Glutamic acid | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Increase (mainly in dicots) | [76] |
| Glutamic acid | Roots | Funneliformis mossae and Rhizophagus irregularis (different treatments) | Solanum lycopersicum | Minimum P | Increase | [64] |
| Glutamic acid | Roots | Glomus intraradices | Medicago truncatula | - | Increase | [70] |
| Glutamine | Roots | * AMF mix and natural AMF inoculum | Triticum durum | N stress, P rich | Decrease in AMF mix | [110] |
| Glycine | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Decrease | [84] |
| Homoserine | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Increase (mainly in dicots) | [76] |
| Isoleucine | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Increase (mainly in dicots) | [76] |
| Leucine/Isoleucine | Roots | Funneliformis mossae and Rhizophagus irregularis (different treatments) | Solanum lycopersicum | Minimum P | Decrease | [64] |
| Ornithine | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Increase (mainly in dicots) | [76] |
| Phenylalanine | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Increase (mainly in dicots) | [76] |
| Phenylalanine | Roots | * AMF mix and natural AMF inoculum | Triticum durum | N stress, P rich | Decrease in AMF mix | [110] |
| Phenylalanine | Roots | Funneliformis mossae and Rhizophagus irregularis (different treatments) | Solanum lycopersicum | Minimum P | Decrease | [64] |
| Serine | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Increase (mainly in dicots) | [76] |
| Threonine | Leaves | Rhizophagus irregularis | ** Monocot and dicots | - | Increase (mainly in dicots) | [76] |
| Tryptophan | Roots | Funneliformis mossae and Rhizophagus irregularis (different treatments) | Solanum lycopersicum | Minimum P | Decrease | [64] |
| Tyrosine | Roots | Funneliformis mossae and Rhizophagus irregularis (different treatments) | Solanum lycopersicum | Minimum P | Decrease | [64] |
| Fatty acids | ||||||
| Fatty acids and their esters | Roots | * AMF mix and natural AMF inoculum | Triticum durum | N stress, P rich | Decrease in AMF mix | [110] |
| Octadecanoic acid | Leaves, flowers | Glomus mossae | Lotus japonicus | - | Decrease | [84] |
| Oleic acid | Roots | Glomus intraradices | Medicago truncatula | - | Increase | [70] |
| Palmitic acid | Roots | Glomus intraradices | Medicago truncatula | - | Increase | [70] |
| Palmitvaccenic acid | Roots | Glomus intraradices | Medicago truncatula | - | Increase | [70] |
| Vaccenic acid | Roots | Glomus intraradices | Medicago truncatula | - | Increase | [70] |
| Proteins | Leaves | Gigaspora albida+ Acaulospora longula | Anadenanthera colubrina | Minimal P | Increase | [69] |
| Carbohydrates | ||||||
| 3-propylphosphoenolpyruvate | Roots | Funneliformis mossae | Triticum durum | Water stress | Increase | [92] |
| Carbohydrates | Leaves | Gigaspora albida+ Acaulospora longula | Anadenanthera colubrina | Minimal phosphorusP | Increase | [69] |
| Glucose-1,6-bisphosphate | Roots | Funneliformis mossae | Triticum durum | Water stress | Increase | [92] |
| Mannosylfructose-phosphate | Roots | Funneliformis mossae | Triticum durum | Water stress | Increase | [92] |
3. Secondary Metabolites
3.1. Carotenoid Pathway
3.2. Phenylpropanoid Pathway
3.3. Effect of AMF on Secondary Metabolites under Biotic/Abiotic Stresses
4. Plant Defense and Hormonal Regulation
5. Analytical Platforms for the Analysis of Plant Metabolome
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weller, D.M.; Thomashow, L.S. Current Challenges in Introducing Beneficial Microorganisms into the Rhizosphere. In Molecular Ecology of Rhizosphere Microorganisms; Wiley: Hoboken, NJ, USA, 2007; pp. 1–18. [Google Scholar]
- Nihorimbere, V.; Ongena, M.; Smargiassi, M.; Thonart, P. Beneficial effect of the rhizosphere microbial community for plant growth and health. Biotechnol. Agron. Soc. Environ. 2011, 15, 327–337. [Google Scholar]
- Sorty, A.M.; Bitla, U.M.; Meena, K.K.; Singh, N.P. Role of Microorganisms in Alleviating Abiotic Stresses. In Advances in Plant Microbiome and Sustainable Agriculture; Springer Science and Business Media: Berlin, Germany, 2018; pp. 115–128. [Google Scholar]
- Pineda, A.; Zheng, S.-J.; Van Loon, J.J.A.; Pieterse, C.M.; Dicke, M. Helping plants to deal with insects: The role of beneficial soil-borne microbes. Trends Plant Sci. 2010, 15, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Denison, R.F.; Bledsoe, C.; Kahn, M.; O’Gara, F.; Simms, E.L.; Thomashow, L.S. Cooperation in the rhizosphere and the “free rider” problem. Ecology 2003, 84, 838–845. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; A Dubery, I. The Chemistry of Plant–Microbe Interactions in the Rhizosphere and the Potential for Metabolomics to Reveal Signaling Related to Defense Priming and Induced Systemic Resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of soil and fertilizer phosphorus use: Reconciling changing concepts of soil phosphorus behaviour with agronomic information. FAO Fertil. Plant Nutr. Bull. 2008, 18, 128. [Google Scholar]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L.R. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Boil. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Jones, D.L. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Gilbert, G.A.; Vance, C.P.; Knight, J.D.; Allan, D. Acid phosphatase activity in phosphorus-deficient white lupin roots. Plant Cell Environ. 1999, 22, 801–810. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Genet. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Taylor, T.N.; Remy, W.; Haas, H.; Kerp, H. Fossil arbuscular mycorrhizae from the Early Devonian. Mycologia 1995, 87, 560–573. [Google Scholar] [CrossRef]
- Strullu-Derrien, C.; Kenrick, P.; Pressel, S.; Duckett, J.G.; Rioult, J.-P.; Strullu, D.-G. Fungal associations inHorneophyton lignerifrom the Rhynie Chert (c. 407 million year old) closely resemble those in extant lower land plants: Novel insights into ancestral plant-fungus symbioses. New Phytol. 2014, 203, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; George, E.; Marschner, H. Extension of the phosphorus depletion zone in VA-mycorrhizal white clover in a calcareous soil. Plant Soil 1991, 136, 41–48. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 2005, 168, 189–204. [Google Scholar] [CrossRef]
- Smith, S.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; p. 175. [Google Scholar]
- Smith, F.A.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Annu. Rev. Plant Boil. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Hodge, A.; Storer, K. Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems. Plant Soil 2014, 386, 1–19. [Google Scholar] [CrossRef]
- Bolan, N. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 1991, 134, 189–207. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Q.; Pan, J.; Jiang, S.; Liu, Y.; Bahadur, A.; Peng, Z.; Yang, Y.; Feng, H. Effect of arbuscular mycorrhizal fungi on soil enzyme activity is coupled with increased plant biomass. Eur. J. Soil Sci. 2019, 71, 84–92. [Google Scholar] [CrossRef]
- Harrison, M.J. The Arbuscular Mycorrhizal Symbiosis. In Plant-Microbe Interactions; Springer Science and Business Medi: Berlin, Germany, 1997; pp. 1–34. [Google Scholar]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Tullio, M.; Rivera, C.M.; Rea, E. Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorus concentration. Boil. Fertil. Soils 2007, 44, 501–509. [Google Scholar] [CrossRef]
- Gianinazzi, S.; Gollotte, A.; Binet, M.-N.; Van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef]
- Jung, S.C.; Martínez-Medina, A.; López-Ráez, J.A.; Pozo, M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcon, C.; Aroca, R.; Azcón, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef] [PubMed]
- Selosse, M.-A.; Bessis, A.; Pozo, M.J. Microbial priming of plant and animal immunity: Symbionts as developmental signals. Trends Microbiol. 2014, 22, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Jayne, B.; Quigley, M. Influence of arbuscular mycorrhiza on growth and reproductive response of plants under water deficit: A meta-analysis. Mycorrhiza 2013, 24, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-H.; Zou, Y.-N.; Rahman, M.M.; Ni, Q.-D.; Wu, Q. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci. Rep. 2017, 7, 42389. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Morales, V.; Villegas, J.; Wendelin, S.; Vierheilig, H.; Eder, R.; Cárdenas-Navarro, R. Root colonisation by the arbuscular mycorrhizal fungus Glomus intraradices alters the quality of strawberry fruits (Fragaria × ananassa Duch.) at different nitrogen levels. J. Sci. Food Agric. 2010, 90, 1774–1782. [Google Scholar] [CrossRef]
- Baum, C.; El-Tohamy, W.; Gruda, N.S. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hortic. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Wilson, G.; Rice, C.W.; Rillig, M.C.; Springer, A.; Hartnett, D.C. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecol. Lett. 2009, 12, 452–461. [Google Scholar] [CrossRef]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Camenzind, T.; Cavagnaro, T.R.; Degrune, F.; Hohmann, P.; Lammel, D.R.; Mansour, I.; Roy, J.; Van Der Heijden, M.G.A.; et al. Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 2019, 222, 1171–1175. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Raju, P.S.; Clark, R.B.; Ellis, J.R.; Maranville, J.W. Effects of species of VA-mycorrhizal fungi on growth and mineral uptake of sorghum at different temperatures. Plant Soil 1990, 121, 165–170. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Emmett, B.D.; Levesque-Tremblay, V.; MacLean, A.M.; Sun, X.; Satterlee, J.W.; Fei, Z.; Harrison, M.J. Diverse Sorghum bicolor accessions show marked variation in growth and transcriptional responses to arbuscular mycorrhizal fungi. Plant Cell Environ. 2019, 42, 1758–1774. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, P.-L.; Bradley, R.L.; Maherali, H.; Klironomos, J. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.M.; Antunes, P.M.; Maherali, H.; Hart, M.M.; Klironomos, J. Evolutionary asymmetry in the arbuscular mycorrhizal symbiosis: Conservatism in fungal morphology does not predict host plant growth. New Phytol. 2017, 214, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Lanfranco, L.; Fiorilli, V.; Gutjahr, C. Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytol. 2018, 220, 1031–1046. [Google Scholar] [CrossRef] [PubMed]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal Rewards Stabilize Cooperation in the Mycorrhizal Symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef]
- Smith, F.A.; Grace, E.J.; Smith, F.A. More than a carbon economy: Nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol. 2009, 182, 347–358. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, S.E. How useful is the mutualism-parasitism continuum of arbuscular mycorrhizal functioning? Plant Soil 2013, 363, 7–18. [Google Scholar] [CrossRef]
- Ndoye, F.; Kane, A.; Bakhoum, N.; Sanon, A.; Fall, D.; Diouf, D.; Sylla, S.N.; Bâ, A.M.; Sy, M.O.; Noba, K. Response of Acacia senegal (L.) Willd. to inoculation with arbuscular mycorrhizal fungi isolates in sterilized and unsterilized soils in Senegal. Agrofor. Syst. 2013, 87, 941–952. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Leonard, R.T.; Menge, J.A. Membrane-Mediated Decrease in Root Exudation Responsible for Phorphorus Inhibition of Vesicular-Arbuscular Mycorrhiza Formation. Plant Physiol. 1981, 68, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Matsuzaki, K.-I.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.-C.; Roux, C.; Bécard, G.; Sejalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Boil. 2006, 4, e226. [Google Scholar] [CrossRef]
- Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.-P.; Vierheilig, H. Flavonoids and Strigolactones in Root Exudates as Signals in Symbiotic and Pathogenic Plant-Fungus Interactions. Molecules 2007, 12, 1290–1306. [Google Scholar] [CrossRef]
- Maillet, F.; Poinsot, V.; André, O.; Puech-Pagès, V.; Haouy, A.; Gueunier, M.; Cromer, L.; Giraudet, D.; Formey, D.; Niebel, A.; et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 2011, 469, 58–63. [Google Scholar] [CrossRef]
- Vierheilig, H.; Piché, Y. Signalling in Arbuscular Mycorrhiza: Facts and Hypotheses. In Advances in Experimental Medicine and Biology; Springer Science and Business Media: Berlin, Germany, 2002; Volume 505, pp. 23–39. [Google Scholar]
- Oldroyd, G.E.D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Genet. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Pozo, M.J.; Verhage, A.; García-Andrade, J.; Garcia, J.M.; Azcón-Aguilar, C. Priming Plant Defence Against Pathogens by Arbuscular Mycorrhizal Fungi. In Mycorrhizas—Functional Processes and Ecological Impact; Springer Science and Business Media: Berlin, Germany, 2008; pp. 123–135. [Google Scholar]
- Song, Y.; Ye, M.; Li, C.Y.; Wang, R.L.; Wei, X.C.; Luo, S.M.; Zeng, R. Priming of Anti-Herbivore Defense in Tomato by Arbuscular Mycorrhizal Fungus and Involvement of the Jasmonate Pathway. J. Chem. Ecol. 2013, 39, 1036–1044. [Google Scholar] [CrossRef]
- Bago, B.; Pfeffer, P.E.; Shachar-Hill, Y. Carbon Metabolism and Transport in Arbuscular Mycorrhizas. Plant Physiol. 2000, 124, 949–958. [Google Scholar] [CrossRef]
- Keymer, A.; Pimprikar, P.; Wewer, V.; Huber, C.; Brands, M.; Bucerius, S.L.; Delaux, P.-M.; Klingl, V.; Von Röpenack-Lahaye, E.; Wang, T.L.; et al. Lipid transfer from plants to arbuscular mycorrhiza fungi. eLife 2017, 6. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Boil. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Korenblum, E.; Aharoni, A. Phytobiome metabolism: Beneficial soil microbes steer crop plants’ secondary metabolism. Pest Manag. Sci. 2019, 75, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Schäfer, M.; Li, D.; Halitschke, R.; Dong, C.; McGale, E.; Paetz, C.; Song, Y.; Li, S.; Dong, J.; et al. Blumenols as shoot markers of root symbiosis with arbuscular mycorrhizal fungi. eLife 2018, 7, e37093. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, R.; Müller, C. Leaf metabolome in arbuscular mycorrhizal symbiosis. Curr. Opin. Plant Boil. 2015, 26, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Feng, G.; Li, X.; Tian, C.; Tang, C.; Rengel, Z. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 2002, 12, 185–190. [Google Scholar] [CrossRef]
- Whiteside, M.D.; Garcia, M.O.; Treseder, K.K. Amino Acid Uptake in Arbuscular Mycorrhizal Plants. PLoS ONE 2012, 7, e47643. [Google Scholar] [CrossRef]
- Luginbuehl, L.H.; Menard, G.N.; Kurup, S.; Van Erp, H.; Radhakrishnan, G.V.; Breakspear, A.; Oldroyd, G.E.D.; Eastmond, P.J. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef]
- Rivero, J.; Gamir, J.; Aroca, R.; Pozo, M.J.; Flors, V. Metabolic transition in mycorrhizal tomato roots. Front. Microbiol. 2015, 6, 598. [Google Scholar] [CrossRef]
- Shachar-Hill, Y.; Pfeffer, P.E.; Douds, D.; Osman, S.F.; Doner, L.W.; Ratcliffe, G. Partitioning of Intermediary Carbon Metabolism in Vesicular-Arbuscular Mycorrhizal Leek. Plant Physiol. 1995, 108, 7–15. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Douds, D.D.; Bécard, G.; Shachar-Hill, Y. Carbon Uptake and the Metabolism and Transport of Lipids in an Arbuscular Mycorrhiza. Plant Physiol. 1999, 120, 587–598. [Google Scholar] [CrossRef]
- Schubert, A.; Allara, P.; Morte, A. Cleavage of sucrose in roots of soybean (Glycine max) colonized by an arbuscular mycorrhizal fungus. New Phytol. 2004, 161, 495–501. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza 2010, 21, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Pedone-Bonfim, M.V.; A Lins, M.; Coelho, I.R.; Santana, A.S.; Da Silva, F.S.B.; Maia, L.C. Mycorrhizal technology and phosphorus in the production of primary and secondary metabolites in cebil (Anadenanthera colubrina (Vell.) Brenan) seedlings. J. Sci. Food Agric. 2012, 93, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Schliemann, W.; Ammer, C.; Strack, D. Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry 2008, 69, 112–146. [Google Scholar] [CrossRef] [PubMed]
- Schubert, A.; Wyss, P.; Wiemken, A. Occurrence of trehalose in vesicular-arbuscular mycorrhizal fungi and in mycorrhizal roots. J. Plant Physiol. 1992, 140, 41–45. [Google Scholar] [CrossRef]
- Ocon, A.; Hampp, R.; Requena, N. Trehalose turnover during abiotic stress in arbuscular mycorrhizal fungi. New Phytol. 2007, 174, 879–891. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Boil. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Li, M.; Guo, R.; Jiao, Y.; Jin, X.; Zhang, H.; Shi, L.-X. Comparison of Salt Tolerance in Soja Based on Metabolomics of Seedling Roots. Front. Plant Sci. 2017, 8, 1101. [Google Scholar] [CrossRef]
- Shtark, O.Y.; Puzanskiy, R.K.; Avdeeva, G.S.; Yurkov, A.P.; Smolikova, G.; Yemelyanov, V.V.; Kliukova, M.S.; Shavarda, A.L.; Kirpichnikova, A.A.; Zhernakov, A.I.; et al. Metabolic alterations in pea leaves during arbuscular mycorrhiza development. Peer J. 2019, 7, e7495. [Google Scholar] [CrossRef]
- Schweiger, R.; Baier, M.C.; Persicke, M.; Müller, C. High specificity in plant leaf metabolic responses to arbuscular mycorrhiza. Nat. Commun. 2014, 5, 3886. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, J.; Cao, Y.P. Phosphorus deficiency enhances root exudation of low-molecular weight organic acids and utilization of sparingly soluble inorganic phosphates by radish (Raghanus satiuvs L.) and rape (Brassica napus L.) plants. Plant Soil 1997, 196, 261–264. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Tawaraya, K.; Naito, M.; Wagatsuma, T. Solubilization of Insoluble Inorganic Phosphate by Hyphal Exudates of Arbuscular Mycorrhizal Fungi. J. Plant Nutr. 2006, 29, 657–665. [Google Scholar] [CrossRef]
- Klugh, K.R.; Cumming, J.R. Variations in organic acid exudation and aluminum resistance among arbuscular mycorrhizal species colonizing Liriodendron tulipifera. Tree Physiol. 2007, 27, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Giasson, P.; Karam, A.; Jaouich, A. Arbuscular Mycorrhizae and Alleviation of Soil Stresses on Plant Growth. In Mycorrhizae: Sustainable Agriculture and Forestry; Springer Science and Business Media: Berlin, Germany, 2008; pp. 99–134. [Google Scholar]
- Hildebrandt, T.; Nunes-Nesi, A.; Araujo, W.; Braun, H.-P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Gachomo, E.; Allen, J.W.; Pfeffer, P.E.; Govindarajulu, M.; Douds, D.D.; Jin, H.; Nagahashi, G.; Lammers, P.J.; Shachar-Hill, Y.; Bücking, H. Germinating spores ofGlomus intraradicescan use internal and exogenous nitrogen sources forde novobiosynthesis of amino acids. New Phytol. 2009, 184, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Fester, T.; Fetzer, I.; Buchert, S.; Lucas, R.; Rillig, M.C.; Härtig, C. Towards a systemic metabolic signature of the arbuscular mycorrhizal interaction. Oecologia 2011, 167, 913–924. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.A.; Camargos, L.S.; Schiavinato, M.A.; De Andrade, S.A.L. Mycorrhization alters foliar soluble amino acid composition and influences tolerance to Pb in Calopogonium mucunoides. Theor. Exp. Plant Physiol. 2014, 26, 211–216. [Google Scholar] [CrossRef]
- Rivero, J.; Álvarez, D.; Flors, V.; Azcón-Aguilar, C.; Pozo, M.J. Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol. 2018, 220, 1322–1336. [Google Scholar] [CrossRef]
- Maier, W.; Schmidt, J.; Nimtz, M.; Wray, V.; Strack, D. Secondary products in mycorrhizal roots of tobacco and tomato. Phytochemistry 2000, 54, 473–479. [Google Scholar] [CrossRef]
- Shao, Y.-D.; Zhang, D.-J.; Hu, X.-C.; Wu, Q.; Jiang, C.-J.; Gao, X.-B.; Kuča, K. Arbuscular Mycorrhiza Improves Leaf Food Quality of Tea Plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 47. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Chamoun, R.; Jabaji, S.H. Metabolic responses of willow (Salix purpurea L.) leaves to mycorrhization as revealed by mass spectrometry and 1H NMR spectroscopy metabolite profiling. Front. Plant Sci. 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Di Fossalunga, A.S.; Zouari, I.; Chalot, M.; Bonfante, P. The arbuscular mycorrhizal status has an impact on the transcriptome profile and amino acid composition of tomato fruit. BMC Plant Boil. 2012, 12, 44. [Google Scholar] [CrossRef]
- Torres, N.; Hilbert, G.; Antolín, M.C.; Goicoechea, N. Amino acids and flavonoids profiling in tempranillo berries can be modulated by the arbuscular mycorrhizal fungi. Plants 2019, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, L.; Carletti, P.; Badeck, F.; Rizza, F.; Morcia, C.; Ghizzoni, R.; Rouphael, Y.; Colla, G.; Terzi, V.; Lucini, L. Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol. Biochem. 2019, 137, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.S.; Charest, C.; Dwyer, L.M.; Hamilton, R.I. Effects of arbuscular mycorrhizae on leaf water potential, sugar content, and P content during drought and recovery of maize. Can. J. Bot. 1997, 75, 1582–1591. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Y.; Shi, Z.Y. Arbuscular Mycorrhiza Enhances Biomass Production and Salt Tolerance of Sweet Sorghum. Microorganisms 2019, 7, 289. [Google Scholar] [CrossRef]
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Phisalaphong, M.; Singh, H.P.; Cha-Um, S. Promoting water deficit tolerance and anthocyanin fortification in pigmented rice cultivar (Oryza sativa L. subsp. indica) using arbuscular mycorrhizal fungi inoculation. Physiol. Mol. Boil. Plants 2019, 25, 821–835. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Xu, G.; Zhou, L.; Li, Y. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For. 2018, 50, 593–604. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Zamana, S.; Seredin, T.M.; Poluboyarinov, P.A.; Sokolov, S.; Baranova, H.; Krivenkov, L.; Pietrantonio, L.; Caruso, G. Effect of Selenium Biofortification and Beneficial Microorganism Inoculation on Yield, Quality and Antioxidant Properties of Shallot Bulbs. Plants 2019, 8, 102. [Google Scholar] [CrossRef]
- Ramadan, M.E.-S. Mycorrhizal inoculation and phosphorus fertilizers to improve onion productivity in saline soil. Acta Sci. Pol. Hortorum Cultus 2019, 18, 57–66. [Google Scholar] [CrossRef]
- Chu, X.; Fu, J.; Sun, Y.; Xu, Y.; Miao, Y.; Xu, Y.; Hu, T. Effect of arbuscular mycorrhizal fungi inoculation on cold stress-induced oxidative damage in leaves of Elymus nutans Griseb. S. Afr. J. Bot. 2016, 104, 21–29. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Wirth, S.; Egamberdieva, D. Arbuscular mycorrhizal fungi alleviate salt stress in lupine (Lupinus termis Forsik) through modulation of antioxidant defense systems and physiological traits. Legum. Res. Int. J. 2016, 39, 198–207. [Google Scholar] [CrossRef]
- Sanmartín, C.; Garmendia, I.; Romano, B.; Díaz, M.; Palop, J.A.; Goicoechea, N. Mycorrhizal inoculation affected growth, mineral composition, proteins and sugars in lettuces biofortified with organic or inorganic selenocompounds. Sci. Hortic. 2014, 180, 40–51. [Google Scholar] [CrossRef]
- Goicoechea, N.; Baslam, M.; Erice, G.; Irigoyen, J.J. Increased photosynthetic acclimation in alfalfa associated with arbuscular mycorrhizal fungi (AMF) and cultivated in greenhouse under elevated COJ. Plant Physiol. 2014, 171, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, S.; Wang, R.; Li, S.; Liao, X. AM fungi and rhizobium regulate nodule growth, phosphorous (P) uptake, and soluble sugar concentration of soybeans experiencing P deficiency. J. Plant Nutr. 2016, 39, 1915–1925. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Xu, H. Influence of arbuscular mycorrhiza on lipid peroxidation and antioxidant enzyme activity of maize plants under temperature stress. Mycorrhiza 2009, 20, 325–332. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, W.; Wang, Y.; Zhang, L.; Huang, S.; Lin, J. Metabolomics Analysis Reveals the Alkali Tolerance Mechanism in Puccinellia tenuiflora Plants Inoculated with Arbuscular Mycorrhizal Fungi. Microorganisms 2020, 8, 327. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, N.; Wu, P.; Huo, H.; Xu, G.; Wu, G. Arbuscular mycorrhizal colonization alleviates Fusarium wilt in watermelon and modulates the composition of root exudates. Plant Growth Regul. 2015, 77, 77–85. [Google Scholar] [CrossRef]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef]
- Tchameni, S.N.; Nwaga, D.; Wakam, L.N.; Ngonkeu, E.L.M.; Fokom, R.; Kuaté, J.; Etoa, F.-X. Growth Enhancement, Amino Acid Synthesis and Reduction in Susceptibility Towards Phytophthora megakarya by Arbuscular Mycorrhizal Fungi Inoculation in Cocoa Plants. J. Phytopathol. 2012, 160, 220–228. [Google Scholar] [CrossRef]
- Wu, Q.; Xia, R.-X.; Zou, Y.-N.; Wang, G.-Y. Osmotic solute responses of mycorrhizal citrus (Poncirus trifoliata) seedlings to drought stress. Acta Physiol. Plant. 2007, 29, 543–549. [Google Scholar] [CrossRef]
- Saia, S.; Ruisi, P.; Fileccia, V.; Di Miceli, G.; Amato, G.; Martinelli, F. Metabolomics Suggests That Soil Inoculation with Arbuscular Mycorrhizal Fungi Decreased Free Amino Acid Content in Roots of Durum Wheat Grown under N-Limited, P-Rich Field Conditions. PLoS ONE 2015, 10, e0129591. [Google Scholar] [CrossRef] [PubMed]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Zhi-lin, Y.; Chuan-Chao, D.; Lian-Qing, C. Regulation and accumulation of secondary metabolites in plant-fungus symbiotic system. Afr. J. Biotechnol. 2007, 6, 1266–1271. [Google Scholar]
- Zamioudis, C.; Pieterse, C.M. Modulation of Host Immunity by Beneficial Microbes. Mol. Plant-Microbe Interact. 2012, 25, 139–150. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting Ready for Battle. Mol. Plant-Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi –From ecology to application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Arbuscular Mycorrhizal Fungi (AMF) Improved Growth and Nutritional Quality of Greenhouse-Grown Lettuce. J. Agric. Food Chem. 2011, 59, 5504–5515. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.H.; Ghalavand, A.; Mashhadi-Akbar-Boojar, M.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A. Increased Medicinal Contents of Purslane by Nitrogen and Arbuscular Mycorrhiza under Drought Stress. Commun. Soil Sci. Plant Anal. 2019, 51, 118–135. [Google Scholar] [CrossRef]
- Hill, E.M.; Robinson, L.A.; Abdul-Sada, A.; Vanbergen, A.J.; Hodge, A.; Hartley, S.E. Arbuscular Mycorrhizal Fungi and Plant Chemical Defence: Effects of Colonisation on Aboveground and Belowground Metabolomes. J. Chem. Ecol. 2018, 44, 198–208. [Google Scholar] [CrossRef]
- Chen, S.; Jin, W.; Liu, A.; Zhang, S.; Liu, D.; Wang, F.; Lin, X.; He, C. Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Sci. Hortic. 2013, 160, 222–229. [Google Scholar] [CrossRef]
- Baslam, M.; Goicoechea, N. Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza 2011, 22, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Peipp, H.; Maier, W.; Schmidt, J.; Wray, V.; Strack, D. Arbuscular mycorrhizal fungus-induced changes in the accumulation of secondary compounds in barley roots. Phytochemistry 1997, 44, 581–587. [Google Scholar] [CrossRef]
- Fester, T.; Maier, W.; Strack, D. Accumulation of secondary compounds in barley and wheat roots in response to inoculation with an arbuscular mycorrhizal fungus and co-inoculation with rhizosphere bacteria. Mycorrhiza 1999, 8, 241–246. [Google Scholar] [CrossRef]
- Vierheilig, H.; Gagnon, H.; Strack, D.; Maier, W. Accumulation of cyclohexenone derivatives in barley, wheat and maize roots in response to inoculation with different arbuscular mycorrhizal fungi. Mycorrhiza 2000, 9, 291–293. [Google Scholar] [CrossRef]
- Schliemann, W.; Kolbe, B.; Schmidt, J.; Nimtz, M.; Wray, V. Accumulation of apocarotenoids in mycorrhizal roots of leek (Allium porrum). Phytochemistry 2008, 69, 1680–1688. [Google Scholar] [CrossRef]
- Schliemann, W.; Schmidt, J.; Nimtz, M.; Wray, V.; Fester, T.; Strack, D. Accumulation of apocarotenoids in mycorrhizal roots of Ornithogalum umbellatum. Phytochemistry 2006, 67, 1196–1205. [Google Scholar] [CrossRef]
- Maier, W.; Hammer, K.; Dammann, U.; Schulz, B.; Strack, D. Accumulation of sesquiterpenoid cyclohexenone derivatives induced by an arbuscular mycorrhizal fungus in members of the Poaceae. Planta 1997, 202, 36–42. [Google Scholar] [CrossRef]
- Rozpądek, P.; Wężowicz, K.; Stojakowska, A.; Malarz, J.; Surówka, E.; Sobczyk, Ł.; Anielska, T.; Ważny, R.; Miszalski, Z.; Turnau, K. Mycorrhizal fungi modulate phytochemical production and antioxidant activity of Cichorium intybus L. (Asteraceae) under metal toxicity. Chemosphere 2014, 112, 217–224. [Google Scholar] [CrossRef]
- Devi, M.C.; Reddy, M. Phenolic acid metabolism of groundnut (Arachis hypogaea L.) plants inoculated with VAM fungus and Rhizobium. Plant Growth Regul. 2002, 37, 151–156. [Google Scholar] [CrossRef]
- Eftekhari, M.; Alizadeh, M.; Ebrahimi, P. Evaluation of the total phenolics and quercetin content of foliage in mycorrhizal grape (Vitis vinifera L.) varieties and effect of postharvest drying on quercetin yield. Ind. Crop. Prod. 2012, 38, 160–165. [Google Scholar] [CrossRef]
- Rojas-Andrade, R.; Cerda-García-Rojas, C.M.; Frías-Hernández, J.; Dendooven, L.; Olalde-Portugal, V.; Ramos-Valdivia, A. Changes in the concentration of trigonelline in a semi-arid leguminous plant (Prosopis laevigata) induced by an arbuscular mycorrhizal fungus during the presymbiotic phase. Mycorrhiza 2003, 13, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.K.; Charles, M.T.; Boulanger, R.; Tweddell, R.J.; Désilets, H. Effect of mycorrhization on the accumulation of rishitin and solavetivone in potato plantlets challenged with Rhizoctonia solani. Mycorrhiza 2003, 13, 333–336. [Google Scholar] [CrossRef]
- Caser, M.; Victorino, Í.M.M.; Demasi, S.; Berruti, A.; Donno, D.; Lumini, E.; Bianciotto, V.; Scariot, V. Saffron Cultivation in Marginal Alpine Environments: How AMF Inoculation Modulates Yield and Bioactive Compounds. Agronomy 2018, 9, 12. [Google Scholar] [CrossRef]
- Toussaint, J.-P.; Smith, F.A.; Smith, S.E. Arbuscular mycorrhizal fungi can induce the production of phytochemicals in sweet basil irrespective of phosphorus nutrition. Mycorrhiza 2007, 17, 291–297. [Google Scholar] [CrossRef]
- Adolfsson, L.; Nziengui, H.; De Abreu, I.N.; Šimura, J.; Beebo, A.; Herdean, A.; Aboalizadeh, J.; Široká, J.; Moritz, T.; Novák, O.; et al. Enhanced Secondary- and Hormone Metabolism in Leaves of Arbuscular Mycorrhizal Medicago truncatula. Plant Physiol. 2017, 175, 392–411. [Google Scholar] [CrossRef]
- Li, C.; Swofford, C.A.; Sinskey, A.J. Modular engineering for microbial production of carotenoids. Metab. Eng. Commun. 2020, 10, e00118. [Google Scholar] [CrossRef]
- Shumskaya, M.; Wurtzel, E.T. The carotenoid biosynthetic pathway: Thinking in all dimensions. Plant Sci. 2013, 208, 58–63. [Google Scholar] [CrossRef]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a Novel Carotenoid-Derived Plant Hormone. Annu. Rev. Plant Boil. 2015, 66, 161–186. [Google Scholar] [CrossRef]
- Mayzlish-Gati, E.; De-Cuyper, C.; Goormachtig, S.; Beeckman, T.; Vuylsteke, M.; Brewer, P.B.; Beveridge, C.A.; Yermiyahu, U.; Kaplan, Y.; Enzer, Y.; et al. Strigolactones Are Involved in Root Response to Low Phosphate Conditions in Arabidopsis. Plant Physiol. 2012, 160, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, V.; Wang, J.Y.; Bonfante, P.; Lanfranco, L.; Al-Babili, S. Apocarotenoids: Old and New Mediators of the Arbuscular Mycorrhizal Symbiosis. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Strack, D.; Fester, T. Isoprenoid metabolism and plastid reorganization in arbuscular mycorrhizal roots. New Phytol. 2006, 172, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Fester, T.; Wray, V.; Nimtz, M.; Strack, D. Is stimulation of carotenoid biosynthesis in arbuscular mycorrhizal roots a general phenomenon? Phytochemistry 2005, 66, 1781–1786. [Google Scholar] [CrossRef]
- Fontana, A.; Reichelt, M.; Hempel, S.; Gershenzon, J.; Unsicker, S.B. The Effects of Arbuscular Mycorrhizal Fungi on Direct and Indirect Defense Metabolites of Plantago lanceolata L. J. Chem. Ecol. 2009, 35, 833–843. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, S. Biosynthesis and Regulation of Phenylpropanoids in Plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- A Dixon, R.; Achnine, L.; Kota, P.; Liu, C.-J.; Reddy, M.S.S.; Wang, L. The phenylpropanoid pathway and plant defence-a genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Scervino, J.M.; Ponce, M.A.; Erra-Bassells, R.; Vierheilig, H.; Ocampo, J.A.; Godeas, A. Flavonoids exhibit fungal species and genus specific effects on the presymbiotic growth of Gigaspora and Glomus. Mycol. Res. 2005, 109, 789–794. [Google Scholar] [CrossRef]
- Catford, J.G.; Staehelin, C.; LaRose, G.; Piché, Y.; Vierheilig, H. Systemically suppressed isoflavonoids and their stimulating effects on nodulation and mycorrhization in alfalfa split-root systems. Plant Soil 2006, 285, 257–266. [Google Scholar] [CrossRef]
- Harrison, M.J. Isoflavonoid Accumulation and Expression of Defense Gene Transcripts During the Establishment of Vesicular-Arbuscular Mycorrhizal Associations in Roots of Medicago truncatula. Mol. Plant-Microbe Interact. 1993, 6, 643. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Flors, V.; García, J.M.; Pozo, M.J. AM symbiosis alters phenolic acid content in tomato roots. Plant Signal. Behav. 2010, 5, 1138–1140. [Google Scholar] [CrossRef]
- Jaiti, F.; Meddich, A.; El Hadrami, I. Effectiveness of arbuscular mycorrhizal fungi in the protection of date palm (Phoenix dactylifera L.) against bayoud disease. Physiol. Mol. Plant Pathol. 2007, 71, 166–173. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Da Silva, F.S.B.; Maia, L.C. Mycorrhization and phosphorus may be an alternative for increasing the production of metabolites in Myracrodruon urundeuva. Theor. Exp. Plant Physiol. 2018, 30, 297–302. [Google Scholar] [CrossRef]
- Wu, Q.; Zou, Y.-N.; Xia, R.X. Effects of water stress and arbuscular mycorrhizal fungi on reactive oxygen metabolism and antioxidant production by citrus (Citrus tangerine) roots. Eur. J. Soil Boil. 2006, 42, 166–172. [Google Scholar] [CrossRef]
- Avio, L.; Maggini, R.; Ujvári, G.; Incrocci, L.; Giovannetti, M.; Turrini, A. Phenolics content and antioxidant activity in the leaves of two artichoke cultivars are differentially affected by six mycorrhizal symbionts. Sci. Hortic. 2020, 264, 109153. [Google Scholar] [CrossRef]
- Da Silva, F.A.; Maia, L.C.; Silva, F.S.B. Arbuscular mycorrhizal fungi as biotechnology alternative to increase concentrate of secondary metabolites in Zea mays L. Braz. J. Bot. 2018, 42, 189–193. [Google Scholar] [CrossRef]
- Eid, K.E.; Abbas, M.H.; Mekawi, E.M.; Elnagar, M.M.; Abdelhafez, A.A.; Amin, B.H.; Mohamed, I.; Ali, M.M. Arbuscular mycorrhiza and environmentally biochemicals enhance the nutritional status of Helianthus tuberosus and induce its resistance against Sclerotium rolfsii. Ecotoxicol. Environ. Saf. 2019, 186, 109783. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.; Jing, G.; Li, C.; Ma, F. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 2020, 149, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Mao, Q.; Yan, Y.; Wu, M.; Wang, Y.; Ren, J.; Guo, P.; Liu, A.; Chen, S. Role of Melatonin in Arbuscular Mycorrhizal Fungi-Induced Resistance to Fusarium Wilt in Cucumber. Phytopathology 2020, 110, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.-M.; Zhang, Y.-C.; Liu, L.-P.; Zou, Y.-N.; Wu, Q.; Kuca, K. Mycorrhiza Regulates Signal Substance Levels and Pathogen Defense Gene Expression to Resist Citrus Canker. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1161–1167. [Google Scholar] [CrossRef]
- Li, Y.; Duan, T.; Nan, Z. Arbuscular mycorrhizal fungus alleviates alfalfa leaf spots caused by Phoma medicaginis revealed by RNA-seq analysis. J. Appl. Microbiol. 2019, 14387. [Google Scholar] [CrossRef]
- Song, Y.; Wang, M.; Zeng, R.; Groten, K.; Baldwin, I.T. Priming and filtering of antiherbivore defences among Nicotiana attenuata plants connected by mycorrhizal networks. Plant Cell Environ. 2019, 42, 2945–2961. [Google Scholar] [CrossRef]
- Sendek, A.; Karakoç, C.; Wagg, C.; Domínguez-Begines, J.; Couto, G.M.D.; Van Der Heijden, M.G.A.; Naz, A.A.; Lochner, A.; Chatzinotas, A.; Klotz, S.; et al. Drought modulates interactions between arbuscular mycorrhizal fungal diversity and barley genotype diversity. Sci. Rep. 2019, 9, 9650. [Google Scholar] [CrossRef]
- Gbongue, L.-R.; Lalaymia, I.; Zeze, A.; Delvaux, B.; Declerck, S. Increased Silicon Acquisition in Bananas Colonized by Rhizophagus irregularis MUCL 41833 Reduces the Incidence of Pseudocercospora fijiensis. Front. Plant Sci. 2019, 9, 1977. [Google Scholar] [CrossRef]
- Berdeni, D.; Cotton, T.E.A.; Daniell, T.J.; Bidartondo, M.I.; Cameron, D.D.; Evans, K.L. The Effects of Arbuscular Mycorrhizal Fungal Colonisation on Nutrient Status, Growth, Productivity, and Canker Resistance of Apple (Malus pumila). Front. Microbiol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Bernaola, L.; Cosme, M.; Schneider, R.W.; Stout, M. Belowground Inoculation With Arbuscular Mycorrhizal Fungi Increases Local and Systemic Susceptibility of Rice Plants to Different Pest Organisms. Front. Plant Sci. 2018, 9, 1461. [Google Scholar] [CrossRef]
- Omomowo, I.O.; Fadiji, A.E.; Omomowo, O.I. Assessment of bio-efficacy of Glomus versiforme and Trichoderma harzianum in inhibiting powdery mildew disease and enhancing the growth of cowpea. Ann. Agric. Sci. 2018, 63, 9–17. [Google Scholar] [CrossRef]
- Zhang, R.-Q.; Zhu, H.; Zhao, H.-Q.; Yao, Q. Arbuscular mycorrhizal fungal inoculation increases phenolic synthesis in clover roots via hydrogen peroxide, salicylic acid and nitric oxide signaling pathways. J. Plant Physiol. 2013, 170, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Aseel, D.G.; Rashad, Y.; Hammad, S.M. Arbuscular Mycorrhizal Fungi Trigger Transcriptional Expression of Flavonoid and Chlorogenic Acid Biosynthetic Pathways Genes in Tomato against Tomato Mosaic Virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Wang, J.; Chang, W.; Fan, X.; Sui, X.; Song, F. Proteomics Analysis of E. angustifolia Seedlings Inoculated with Arbuscular Mycorrhizal Fungi under Salt Stress. Int. J. Mol. Sci. 2019, 20, 788. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Gleadow, R.; Cavagnaro, T.R. Age versus stage: Does ontogeny modify the effect of phosphorus and arbuscular mycorrhizas on above- and below-ground defence in forage sorghum? Plant Cell Environ. 2013, 37, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zou, Y.-N.; Wu, Q.; Kuča, K. Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environ. Exp. Bot. 2020, 171, 103926. [Google Scholar] [CrossRef]
- Ghanbarzadeh, Z.; Mohsenzadeh, S.; Rowshan, V.; Moradshahi, A. Evaluation of the growth, essential oil composition and antioxidant activity of Dracocephalum moldavica under water deficit stress and symbiosis with Claroideoglomus etunicatum and Micrococcus yunnanensis. Sci. Hortic. 2019, 256, 108652. [Google Scholar] [CrossRef]
- Garcia-Garrido, J.M.; Ocampo, J.A. Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. J. Exp. Bot. 2002, 53, 1377–1386. [Google Scholar] [CrossRef]
- Fernández, I.; Merlos, M.; López-Ráez, J.A.; Martínez-Medina, A.; Ferrol, N.; Azcón-Aguilar, C.; Bonfante, P.; Flors, V.; Pozo, M.J. Defense Related Phytohormones Regulation in Arbuscular Mycorrhizal Symbioses Depends on the Partner Genotypes. J. Chem. Ecol. 2014, 40, 791–803. [Google Scholar] [CrossRef]
- Medina, M.H. Root colonization by arbuscular mycorrhizal fungi is affected by the salicylic acid content of the plant. Plant Sci. 2003, 164, 993–998. [Google Scholar] [CrossRef]
- Pozo, M.J.; López-Ráez, J.A.; Azcón-Aguilar, C.; García-Garrido, J.M. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 2015, 205, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Gonzalez, F.J. Challenges and opportunities of metabolomics. J. Cell. Physiol. 2012, 227, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Raftery, D. Comparing and combining NMR spectroscopy and mass spectrometry in metabolomics. Anal. Bioanal. Chem. 2006, 387, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Lankadurai, B.P.; Nagato, E.G.; Simpson, A. Environmental metabolomics: An emerging approach to study organism responses to environmental stressors. Environ. Rev. 2013, 21, 180–205. [Google Scholar] [CrossRef]
- Allwood, J.W.; Goodacre, R. An introduction to liquid chromatography-mass spectrometry instrumentation applied in plant metabolomic analyses. Phytochem. Anal. 2010, 21, 33–47. [Google Scholar] [CrossRef]
- Hagel, J.M.; Facchini, P.J. Plant metabolomics: Analytical platforms and integration with functional genomics. Phytochem. Rev. 2007, 7, 479–497. [Google Scholar] [CrossRef]
- Dunn, W.B.; Erban, A.; Weber, R.J.M.; Creek, D.J.; Brown, M.; Breitling, R.; Hankemeier, T.; Goodacre, R.; Neumann, S.; Kopka, J.; et al. Mass appeal: Metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 2012, 9, 44–66. [Google Scholar] [CrossRef]
- Miozzi, L.; Vaira, A.M.; Catoni, M.; Fiorilli, V.; Accotto, G.P.; Lanfranco, L. Arbuscular mycorrhizal symbiosis: Plant friend or foe in the fight against viruses? Front. Microbiol. 2019, 10, 1238. [Google Scholar] [CrossRef]

| Secondary Metabolites | Plant Parts | AMF Species | Plant Species | Environmental Condition | Increase/Decrease | Reference |
|---|---|---|---|---|---|---|
| Carotenoid pathway | ||||||
| Blumenols | Roots | Rhizophagus irregularis | Senecio jacobea (Ragwort plant) | - | Increase | [118] |
| Blumenols | Shoot | Rhizophagus irregularis | Nicotiana attenuate | - | Increase | [59] |
| Carotenoids | Leaves | Glomus intraradices and G. mossae | Lettuce | - | Increase | [120] |
| Cyclohexenone conjugates | Roots | Glomus intraradices | Hordeum vulgare | - | Increase | [121] |
| Cyclohexenone derivatives with blumenin | Roots | Glomus intraradices | Hordeum vulgare and Triticum aestivum | - | Increase | [122] |
| Cyclohexenone derivatives | Roots | Glomus intraradices, Glomus mossae and Gigaspora rosea | Hordeum vulgare, Triticum aestivum and Zea mays | - | Increase | [123] |
| Roots | Glomus intraradices | Tobacco and Tomato plants | - | Increase | [87] | |
| Roots | Glomus intraradices | Allium porrum | - | Increase | [124] | |
| Roots | Glomus intraradices | Medicago truncatula | - | Increase | [70] | |
| Mono-, di-, and branched triglycosides of blumenol | Roots | Glomus intraradices | Ornithogalum umbellatum | - | Increase | [125] |
| Mycorradicin | Roots | Glomus intraradices | Ornithogalum umbellatum | - | Increase | [125] |
| Sesquiterpenoid cyclohexenone derivatives | Roots | Glomus intraradices | 61 members of poaceae | - | Increase | [126] |
| Phenylpropanoid pathway | ||||||
| Bound phenolics | Roots | Glomus intraradices | Medicago truncatula | - | No change | [70] |
| Caffeoylshikimate | Leaves | Rhizophagus irregularis | Salix purpurea | - | Increase | [89] |
| Coniferyl alcohol | Roots | Rhizophagus irregularis+ Funneliformis mossae | Solanum lycopersicum | - | Increase | [64] |
| Coumarate | Leaves | Rhizophagus irregularis | Salix purpurea | - | Increase | [89] |
| Coumaryl acetate | Leaves | Rhizophagus irregularis | Salix purpurea | - | Increase | [89] |
| Coumaryl alcohol | Roots | Rhizophagus irregularis+ Funneliformis mossae | Solanum lycopersicum | - | Increase | [64] |
| Daidzein (Isoflavonoid) | Roots | Glomus intraradices | Medicago truncatula | - | Increase | [70] |
| Epicatechin | Leaves | Rhizophagus irregularis | Salix purpurea | - | Decrease | [89] |
| Ferulic acid | Roots | Rhizophagus irregularis+ Funneliformis mossae | Solanum lycopersicum | - | Increase | [64] |
| Flavonoids | Leaves | Funneliformis mossae | Cucumis sativa (Cucumber) | Chilling stress | Increase | [119] |
| Flavonoids | Leaves | Gigaspora albida+ Acaulospora longula | Anadenanthera colubrina | Increasing P concentration | Increase | [69] |
| Hydroxycinnamates | Shoots | Rhizophagus irregularis | Cichorium intybus | Metal toxicity | Increases without stress, no difference under stress | [127] |
| Hydroxycinnamic acid amides | Roots | Glomus intraradices | Hordeum vulgare | - | Increase | [128] |
| Hydroxycinnamic acid amides | Roots | Glomus intraradices | Hordeum vulgare and Triticum aestivum | - | Increase | [122] |
| Lignans: Secoisolariciresinol Yatein | Root | Rhizophagus irregularis(Ri), Funneliformis mossae(Fm) and Claroideoglomu etunicatum(Ce) | Solanum lycopersicum | Salt stress | Increase | [86] |
| Lignin | Leaves | Funneliformis mossae | Cucumis sativa (Cucumber) | Chilling stress | Increase | [119] |
| Luteolin-7-O-glucoside | Leaves | Rhizophagus irregularis | Salix purpurea | - | Increase | [89] |
| Malonylononin (Isoflavonoid) | Roots | Glomus intraradices | Medicago truncatula | - | Increase | [70] |
| Monolignans | Roots | Rhizophagus irregularis+ Funneliformis mossae | Solanum lycopersicum | - | Increase | [64] |
| Ononin (Isoflavonoid) | Roots | Glomus intraradices | Medicago truncatula | - | Increase | [70] |
| Phenolic acids | Roots+ shoots | Glomus mossae | Groundnut | - | Increase | [128] |
| Phenolic compounds: Cinnamic acid p-coumaric acid caffeic acid ferulic acid | Leaves | Funneliformis mossae | Cucumis sativa (Cucumber) | Chilling stress | Increase | [119] |
| Phenols | Leaves | Gigaspora albida+ Acaulospora longula | Anadenanthera colubrina | Increasing P concentration | Increase | [69] |
| Pinostrobin | Leaves | Rhizophagus irregularis | Salix purpurea | - | Increase | [89] |
| Quercetin (flavonoid) | Stems and leaves | Mixture of Glomus fasciculatum, G. mossae, and G. intraradices | Vitis vinifera | - | Increase | [129] |
| Rutin | Leaves | Rhizophagus irregularis | Salix purpurea | - | Increase | [89] |
| Scopoletin and its glucoside scopolin | Roots | Glomus intraradices | Tobacco and Tomato plants | - | Decrease | [87] |
| Scopolin | Leaves | Rhizophagus irregularis | Salix purpurea | - | Decrease | [89] |
| Tannins | Leaves | Gigaspora albida+ Acaulospora longula | Anadenanthera colubrina | Increasing P concentration | Increase | [69] |
| Alkaloids | ||||||
| Benzylisoquinoline alkaloids | Roots | Rhizophagus irregularis+ Funneliformis mossae | Solanum lycopersicum | - | Increase | [64] |
| Pyrrolizidine alkaloids | Roots | Rhizophagus irregularis | Senecio jacobea (Ragwort plant) | - | Increase | [118] |
| Trigonelline (pyridine alkaloid) | Roots | Gigaspora rosea | Prosopis laevigata | - | Increase | [130] |
| Phytoalexins | ||||||
| Rishitin | Roots | Glomus etunicatum | Potato plantlets | Rhizoctonia solani pathogen | Increase | [131] |
| Solavetivone | Roots | Glomus etunicatum | Potato plantlets | Rhizoctonia solani pathogen | Increase | [131] |
| Antioxidants | ||||||
| Bioactive compounds: Picrocrocin Crocin II Quercitrin | saffron | Rhizophagus intraradices | Crocus sativus (saffron) | - | Increase | [132] |
| Caffeic acids | Shoot | Glomus caledonium and Glomus mossae | Sweet basil | - | Increase | [133] |
| Caffeic acids | Shoot | Glomus intraradices | Sweet basil | - | Same as non-mycorrhizal | [133] |
| Rosmarinic acid | Shoot | Glomus caledonium and Glomus mossae | Sweet basil | - | Increase | [133] |
| Rosmarinic acid | Shoot | Glomus intraradices | Sweet basil | - | Same as non-mycorrhizal | [133] |
| Plant defense and hormones | ||||||
| Abscisic acid | Leaf | Rhizophagus irregularis | Medicago truncatula | - | Increase | [134] |
| Catalpol | Leaf | Rhizophagus irregularis | ** Monocot and dicots | - | Increased in Plantago lanceolate, slightly decreased in Veronica chamaedrys | [76] |
| Glucosinolates | Root | Funneliformis mossae | Triticum aestivum and Triticum durum | Water stress | Mostly decreased | [92] |
| Jasmonic acid Methyl jasmonate | Root | Rhizophagus irregularis(Ri), Funneliformis mossae(Fm) and Claroideoglomu etunicatum(Ce) | Solanum lycopersicum | Salt stress | Increase | [86] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, S.; Suseela, V. Unraveling Arbuscular Mycorrhiza-Induced Changes in Plant Primary and Secondary Metabolome. Metabolites 2020, 10, 335. https://doi.org/10.3390/metabo10080335
Kaur S, Suseela V. Unraveling Arbuscular Mycorrhiza-Induced Changes in Plant Primary and Secondary Metabolome. Metabolites. 2020; 10(8):335. https://doi.org/10.3390/metabo10080335
Chicago/Turabian StyleKaur, Sukhmanpreet, and Vidya Suseela. 2020. "Unraveling Arbuscular Mycorrhiza-Induced Changes in Plant Primary and Secondary Metabolome" Metabolites 10, no. 8: 335. https://doi.org/10.3390/metabo10080335
APA StyleKaur, S., & Suseela, V. (2020). Unraveling Arbuscular Mycorrhiza-Induced Changes in Plant Primary and Secondary Metabolome. Metabolites, 10(8), 335. https://doi.org/10.3390/metabo10080335




