Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons
Abstract
1. Introduction
2. Results
2.1. Population Characteristics
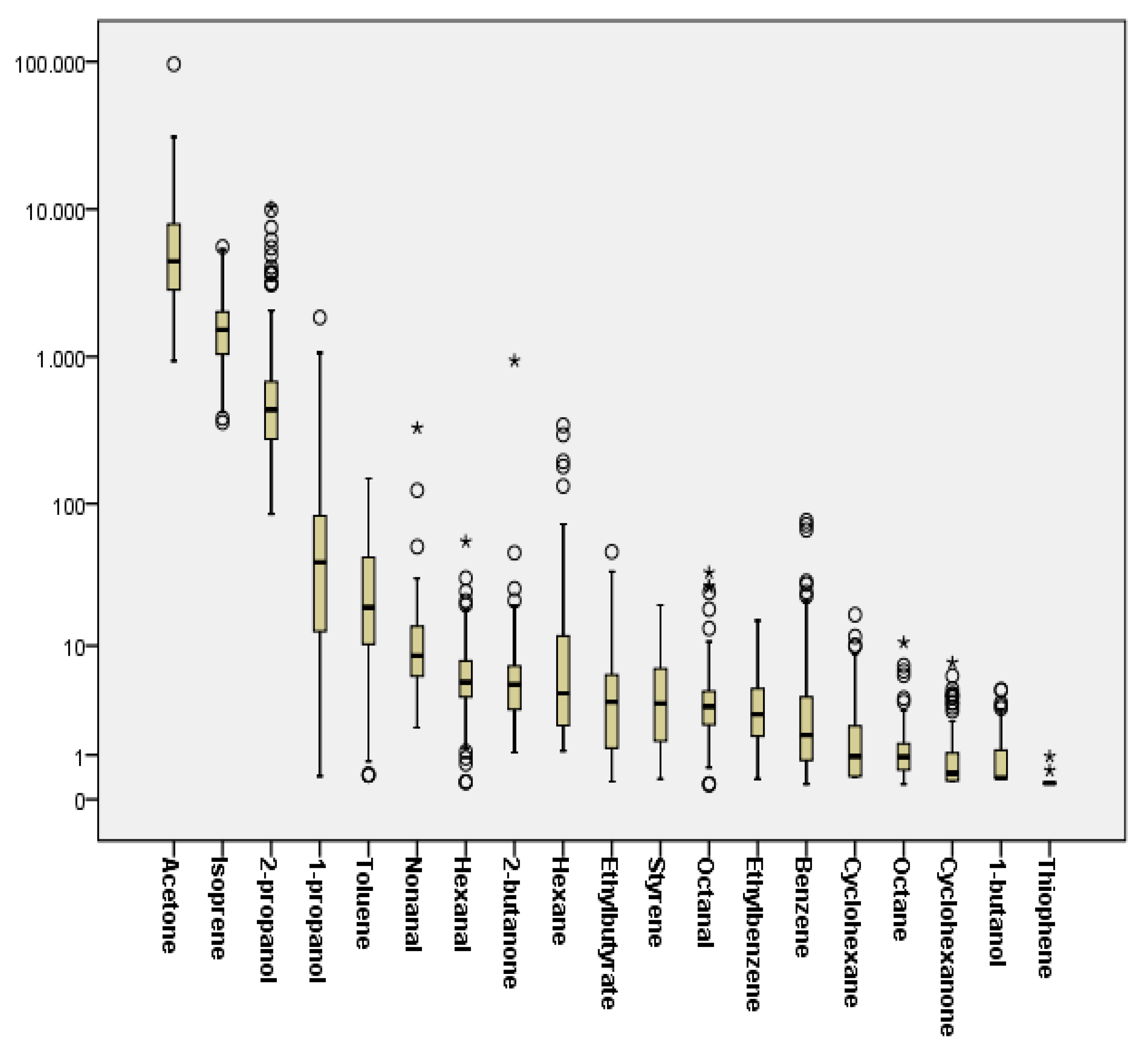
2.2. Breath Concentrations and Determinants of Investigated VOCs
2.3. Association of Exhaled Breath VOCs with Disease Status
2.4. Application of Machine Learning Methods to Determine the Diagnostic Efficiency of the Breath Test
3. Discussion
4. Materials and Methods
4.1. Experimental
4.1.1. Reagents and Materials
4.1.2. Breath Sampling
4.1.3. Solid Phase Microextraction
4.1.4. GC-MS Analysis
4.1.5. Evaluation of Analytical Method Performance
4.2. Study Participants Recruitment
4.3. Statistical Analysis and Machine Learning Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 25 May 2020).
- Sam, D.; Cheung, W.Y. A population-level comparison of cancer-related and non-cancer-related health care costs using publicly available provincial administrative data. Curr. Oncol. 2019, 26, 94–97. [Google Scholar] [CrossRef]
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.-M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018, 124, 2785–2800. [Google Scholar] [CrossRef]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Wender, R.C.; Brawley, O.W.; Fedewa, S.A.; Gansler, T.; Smith, R.A. A blueprint for cancer screening and early detection: Advancing screening’s contribution to cancer control. CA Cancer J. Clin. 2019, 69, 50–79. [Google Scholar] [CrossRef]
- Loud, J.T.; Murphy, J. Cancer Screening and Early Detection in the 21st Century. Semin. Oncol. Nurs. 2017, 33, 121–128. [Google Scholar] [CrossRef]
- Medina-Cleghorn, D.; Nomura, D.K. Chemical approaches to study metabolic networks. Pflug. Arch. Eur. J. Physiol. 2013, 465, 427–440. [Google Scholar] [CrossRef]
- Gaude, E.; Nakhleh, M.K.; Patassini, S.; Boschmans, J.; Allsworth, M.; Boyle, B.; van der Schee, M.P. Targeted breath analysis: Exogenous volatile organic compounds (EVOC) as metabolic pathway-specific probes. J. Breath Res. 2019, 13, 032001. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Antoniou, S.X.; Gaude, E.; Ruparel, M.; van der Schee, M.P.; Janes, S.M.; Rintoul, R.C.; The LuCID Group. The potential of breath analysis to improve outcome for patients with lung cancer. J. Breath Res. 2019, 13. [Google Scholar] [CrossRef]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Câmara, J.S. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef]
- Van der Vliet, A.; Janssen-Heininger, Y.M.W.; Anathy, V. Oxidative stress in chronic lung disease: From mitochondrial dysfunction to dysregulated redox signaling. Mol. Aspects Med. 2018, 63, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Bos, L.D.; van der Schee, M.P.; van Schooten, F.-J.; Sterk, P.J. Exhaled Molecular Fingerprinting in Diagnosis and Monitoring: Validating Volatile Promises. Trends Mol. Med. 2015, 21, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.B.; Boshier, P.R.; Markar, S.R.; Romano, A. Accuracy and Methodologic Challenges of Volatile Organic Compound-Based Exhaled Breath Tests for Cancer Diagnosis: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Zhu, Y.; Liu, H. Detection of volatile organic compounds in exhaled breath to screen lung cancer: A systematic review. Future Oncol. 2018, 14, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Marzorati, D.; Mainardi, L.; Sedda, G.; Gasparri, R.; Spaggiari, L.; Cerveri, P. A review of exhaled breath: A key role in lung cancer diagnosis. J. Breath Res. 2019, 13, 034001. [Google Scholar] [CrossRef]
- Capuano, R.; Santonico, M.; Pennazza, G.; Ghezzi, S.; Martinelli, E.; Roscioni, C.; Lucantoni, G.; Galluccio, G.; Paolesse, R.; Natale, C.D. The lung cancer breath signature: A comparative analysis of exhaled breath and air sampled from inside the lungs. Sci. Rep. 2015, 5, 16491. [Google Scholar] [CrossRef]
- Filipiak, W.; Filipiak, A.; Sponring, A.; Schmid, T.; Zelger, B.; Ager, C.; Klodzinska, E.; Denz, H.; Pizzini, A.; Lucciarini, P.; et al. Comparative analyses of volatile organic compounds (VOCs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. J. Breath Res. 2014, 8, 027111. [Google Scholar] [CrossRef]
- Knight, S.B.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7. [Google Scholar] [CrossRef]
- Montero-Montoya, R.; López-Vargas, R.; Arellano-Aguilar, O. Volatile organic compounds in air: Sources, distribution, exposure and associated illnesses in children. Ann. Glob. Health 2018, 84, 225–238. [Google Scholar] [CrossRef]
- Capone, S.; Tufariello, M.; Forleo, A.; Longo, V.; Giampetruzzi, L.; Radogna, A.V.; Casino, F.; Siciliano, P. Chromatographic analysis of VOC patterns in exhaled breath from smokers and nonsmokers. Biomed. Chromatogr. 2018, 32. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Kremer, R.; Tisch, U.; Gevorkyan, A.; Shiban, A.; Best, L.A.; Haick, H. A nanomaterial-based breath test for short-term follow-up after lung tumor resection. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 15–21. [Google Scholar] [CrossRef]
- Corradi, M.; Poli, D.; Banda, I.; Bonini, S.; Mozzoni, P.; Pinelli, S.; Alinovi, R.; Andreoli, R.; Ampollini, L.; Casalini, A.; et al. Exhaled breath analysis in suspected cases of non-small-cell lung cancer: A cross-sectional study. J. Breath Res. 2015, 9, 027101. [Google Scholar] [CrossRef]
- Poli, D.; Carbognani, P.; Corradi, M.; Goldoni, M.; Acampa, O.; Balbi, B.; Bianchi, L.; Rusca, M.; Mutti, A. Exhaled volatile organic compounds in patients with non-small cell lung cancer: Cross sectional and nested short-term follow-up study. Respir. Res. 2005, 6, 71. [Google Scholar] [CrossRef]
- Schallschmidt, K.; Becker, R.; Jung, C.; Bremser, W.; Walles, T.; Neudecker, J.; Leschber, G.; Frese, S.; Nehls, I. Comparison of volatile organic compounds from lung cancer patients and healthy controls-challenges and limitations of an observational study. J. Breath Res. 2016, 10, 046007. [Google Scholar] [CrossRef]
- Marchand, A.; Aranda-Rodriguez, R.; Tardif, R.; Nong, A.; Haddad, S. Human inhalation exposures to toluene, ethylbenzene, and m-xylene and physiologically based pharmacokinetic modeling of exposure biomarkers in exhaled air, blood, and urine. Toxicol. Sci. 2015, 144, 414–424. [Google Scholar] [CrossRef]
- Ager, C.; Unterkofler, K.; Mochalski, P.; Teschl, S.; Teschl, G.; Mayhew, C.A.; King, J. Modeling-based determination of physiological parameters of systemic VOCs by breath gas analysis, part 2. J. Breath Res. 2018, 12, 036011. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Sun, X.; Yang, X. Human respiratory system as sink for volatile organic compounds: Evidence from field measurements. Indoor Air 2019, 29, 968–978. [Google Scholar] [CrossRef]
- Cheng, S.; Bois, F.Y. A mechanistic modeling framework for predicting metabolic interactions in complex mixtures. Environ. Health Perspect. 2011, 119, 1712–1718. [Google Scholar] [CrossRef]
- Ramsey, J.C.; Andersen, M.E. A physiologically based description of the inhalation pharmacokinetics of styrene in rats and humans. Toxicol. Appl. Pharmacol. 1984, 73, 159–175. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshimura, K.; Enomoto, Y.; Yasui, H.; Hozumi, H.; Karayama, M.; Furuhashi, K.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci. Rep. 2018, 8, 14074. [Google Scholar] [CrossRef]
- Koureas, M.; Karagkouni, F.; Rakitskii, V.; Hadjichristodoulou, C.; Tsatsakis, A.; Tsakalof, A. Serum levels of organochlorine pesticides in the general population of Thessaly, Greece, determined by HS-SPME GC-MS method. Environ. Res. 2016, 148, 318–321. [Google Scholar] [CrossRef]
- Jansen, A.; Lyche, J.L.; Polder, A.; Aaseth, J.; Skaug, M.A. Increased blood levels of persistent organic pollutants (POP) in obese individuals after weight loss-A review. J. Toxicol. Environ. Health Part B Crit. Rev. 2017, 20, 22–37. [Google Scholar] [CrossRef]
- Di Gilio, A.; Catino, A.; Lombardi, A.; Palmisani, J.; Facchini, L.; Mongelli, T.; Varesano, N.; Bellotti, R.; Galetta, D.; de Gennaro, G.; et al. Breath Analysis for Early Detection of Malignant Pleural Mesothelioma: Volatile Organic Compounds (VOCs) Determination and Possible Biochemical Pathways. Cancers 2020, 12, 1262. [Google Scholar] [CrossRef]
- Castellanos, M.; Xifra, G.; Fernández-Real, J.M.; Sánchez, J.M. Breath gas concentrations mirror exposure to sevoflurane and isopropyl alcohol in hospital environments in non-occupational conditions. J. Breath Res. 2016, 10, 016001. [Google Scholar] [CrossRef]
- Ruzsanyi, V.; Peter Kalapos, M. Breath acetone as a potential marker in clinical practice. J. Breath Res. 2017, 11, 024002. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Liu, Y.; Cheng, S.; Duan, Y. Exhaled isopropanol: New potential biomarker in diabetic breathomics and its metabolic correlations with acetone. RSC Adv. 2017, 7, 17480–17488. [Google Scholar] [CrossRef]
- Orywal, K.; Szmitkowski, M. Alcohol dehydrogenase and aldehyde dehydrogenase in malignant neoplasms. Clin. Exp. Med. 2017, 17, 131–139. [Google Scholar] [CrossRef]
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009, 9, 348. [Google Scholar] [CrossRef]
- Karl, T.; Prazeller, P.; Mayr, D.; Jordan, A.; Rieder, J.; Fall, R.; Lindinger, W. Human breath isoprene and its relation to blood cholesterol levels: New measurements and modeling. J. Appl. Physiol. (Bethesda, Md: 1985) 2001, 91, 762–770. [Google Scholar] [CrossRef]
- Chen, X.; Xu, F.; Wang, Y.; Pan, Y.; Lu, D.; Wang, P.; Ying, K.; Chen, E.; Zhang, W. A study of the volatile organic compounds exhaled by lung cancer cells in vitro for breath diagnosis. Cancer 2007, 110, 835–844. [Google Scholar] [CrossRef]
- Gordon, S.M.; Szidon, J.P.; Krotoszynski, B.K.; Gibbons, R.D.; O’Neill, H.J. Volatile organic compounds in exhaled air from patients with lung cancer. Clin. Chem. 1985, 31, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Gleeson, K.; Hughes, J.M.B.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Sakumura, Y.; Koyama, Y.; Tokutake, H.; Hida, T.; Sato, K.; Itoh, T.; Akamatsu, T.; Shin, W. Diagnosis by Volatile Organic Compounds in Exhaled Breath from Lung Cancer Patients Using Support Vector Machine Algorithm. Sensors 2017, 17, 287. [Google Scholar] [CrossRef]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2643–2651. [Google Scholar] [CrossRef]
- Van de Kant, K.D.; van der Sande, L.J.; Jöbsis, Q.; van Schayck, O.C.P.; Dompeling, E. Clinical use of exhaled volatile organic compounds in pulmonary diseases: A systematic review. Respir. Res. 2012, 13, 117. [Google Scholar] [CrossRef]
- Wang, M.; Sheng, J.; Wu, Q.; Zou, Y.; Hu, Y.; Ying, K.; Wan, H.; Wang, P. Confounding effect of benign pulmonary diseases in selecting volatile organic compounds as markers of lung cancer. J. Breath Res. 2018, 12, 046013. [Google Scholar] [CrossRef]
- Li, M.; Yang, D.; Brock, G.; Knipp, R.J.; Bousamra, M.; Nantz, M.H.; Fu, X.A. Breath carbonyl compounds as biomarkers of lung cancer. Lung Cancer 2015, 90, 92–97. [Google Scholar] [CrossRef]
- Levitt, M.D.; Ellis, C.; Furne, J. Influence of method of alveolar air collection on results of breath tests. Dig. Dis. Sci. 1998, 43, 1938–1945. [Google Scholar] [CrossRef]
- Phillips, C.; Mac Parthaláin, N.; Syed, Y.; Deganello, D.; Claypole, T.; Lewis, K. Short-Term Intra-Subject Variation in Exhaled Volatile Organic Compounds (VOCs) in COPD Patients and Healthy Controls and Its Effect on Disease Classification. Metabolites 2014, 4, 300–318. [Google Scholar] [CrossRef]
| Patients Ca+ | Patients Ca− | HC | p-Value * | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||||||
| Age | 70.9 (8.1) | 65.2 (13.2) | 66.8 (10.7) | 0.055 | ||||
| BMI | 26.48 (4.37) | 27.41 (4.72) | 27.97(5.43) | 0.365 | ||||
| n | % | n | % | n | % | |||
| Smoking status | Active smoker (daily) | 7 | 13.7% | 8 | 21.1% | 20 | 37.7% | |
| Active smoker (occasionally) | 1 | 2.0% | 2 | 5.3% | 0 | 0.0% | ||
| Former smoker | 40 | 78.4% | 21 | 55.3% | 18 | 34.0% | ||
| Never | 3 | 5.9% | 7 | 18.4% | 15 | 28.3% | ||
| Gender | Male | 44 | 86.3% | 31 | 81.6% | 36 | 67.9% | |
| Female | 7 | 13.7% | 7 | 18.4% | 17 | 32.1% | ||
| Total | 51 | 100% | 38 | 100% | 53 | 100% | ||
| Gender | Smoking Status * | Ambient Air Concentrations | Body Mass Index | Age | |
|---|---|---|---|---|---|
| p-Value (Mann–Whitney Test) | Trend/p-Value (Mann–Whitney Test) | Correlation Coefficient **/p-Value | Correlation Coefficient **/p-Value | Correlation Coefficient **/p-Value | |
| isoprene | 0.575 | ↑/0.646 | 0.117/0.058 | −0.021/0.800 | −0.092/0.260 |
| acetone | 0.272 | ↓/0.246 | 0.044/0.585 | −0.129/0.111 | 0.138/0.088 |
| 2-propanol | 0.994 | ↓/0.332 | 0.383/<0.001 | 0.009/0.914 | −0.067/0.408 |
| hexane | 0.676 | ↑/0.640 | 0.689/<0.001 | 0.007/0.993 | −0.095/0.241 |
| 1-propanol | 0.803 | ↓/0.827 | 0.545/<0.001 | −0.061/0.453 | −0.024/0.768 |
| 2-butanone | 0.134 | ↑/0.080 | 0.176/0.029 | −0.086/0.289 | −0.068/0.406 |
| cyclohexane | 0.653 | ↑/0.702 | 0.730/<0.001 | −0.001/0.987 | 0.049/0.549 |
| benzene | 0.834 | ↑/<0.001 | 0.416/<0.001 | −0.076/0.350 | −0.131/0.107 |
| 1-butanol | 0.241 | ↑/0.752 | 0.347/<0.001 | −0.050/0.537 | −0.198/0.018 |
| toluene | 0.178 | ↑/0.007 | 0.131/0.106 | −0.056/0.494 | −0.135/0.095 |
| octane | 0.280 | ↑/0.005 | 0.200/0.013 | 0.069/0.394 | −0.166/0.040 |
| ethyl butyrate | 0.238 | ↓/0.643 | 0.401/<0.001 | −0.019/0.813 | −0.125/0.125 |
| hexanal | 0.739 | ↑/< 0.001 | 0.332/<0.001 | −0.039/0.613 | −0.004/0.964 |
| ethylbenzene | 0.639 | ↑/0.104 | 0.253/0.002 | −0.095/0.241 | −0.177/0.029 |
| styrene | 0.334 | ↑/0.148 | −0.160/0.048 | −0.027/0.927 | −0.240/0.003 |
| cyclohexanone | 0.997 | ↑/0.500 | 0.273/<0.001 | −0.078/0.340 | −0.033/0.690 |
| n-octanal | 0.931 | ↓/0.82 | 0.468/<0.001 | −0.007/0.927 | −0.063/0.441 |
| nonanal | 0.990 | ↑/0.187 | 0.731/<0.001 | −0.034/0.674 | 0.006/0.940 |
| Patients Ca+ | Patients Ca− | Healthy Contrοls | Ca+ vs. HC | Ca+ vsCa− | All Groups | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Substance | %† | Median(IQR) | %† | Median(IQR) | %† | Median(IQR) | p * | p * | p ** | |
| Isoprene | Br | 100% | 1486(1037–1986) | 100% | 1661(1123–2578) | 100% | 1493(1029–1952) | 0.909 | 0.328 | 0.501 |
| Sbtr | 1471(1020–1962) | 1643(1099–2560) | 1459(1011–1936) | 0.843 | 0.332 | 0.481 | ||||
| Acetone | Br | 100% | 4565(3157–7921) | 100% | 3944(2839–5877) | 100% | 4303(2761–9580) | 0.997 | 0.236 | 0.442 |
| Sbtr | 4463(3090–7820) | 3844(2763–5859) | 4239(2697–9530) | 0.982 | 0.229 | 0.412 | ||||
| 2-propanol | Br | 21.6% | 528(324–804) | 15.8% | 490(382–702) | 43.4% | 315(218–497) | 0.002 | 0.636 | 0.002 |
| Sbtr | neg.(neg.-neg.) | neg.(neg.-neg.) | neg.(neg.−130.97) | 0.041 | 0.491 | 0.015 | ||||
| Hexane | Br | 25.5% | 4.43(1.13–24.48) | 28.9% | 9.63(3.25–36.05) | 52.8% | 3.42(2.25–5.19) | 0.239 | 0.121 | 0.007 |
| Sbtr | neg.(neg.−0.05) | neg.(neg.−0.73) | 0.11(neg.−1.45) | 0.006 | 0.504 | 0.022 | ||||
| 1-propanol | Br | 11.8% | 30.78(7.14–57.81) | 21.0% | 24.13(8.14–60.85) | 35.8% | 63.84(38.46–103.63) | <0.001 | 0.684 | <0.001 |
| Sbtr | neg.(neg.-neg.) | neg.(neg.-neg.) | neg.(neg.−14.09) | 0.005 | 0.255 | 0.014 | ||||
| 2-butanone | Br | 82.3% | 4.39(3.03–6.9) | 92.1% | 4.71(3.41–6.78) | 92.4% | 5.25(3.27–7) | 0.358 | 0.507 | 0.626 |
| Sbtr | 2.68(1.08–5.31) | 2.98(1.39–5.31) | 3.28(1.77–5.04) | 0.321 | 0.531 | 0.576 | ||||
| Cyclohexane | Br | 29.4% | 0.69(0.43–2) | 36.8% | 0.92(0.61–1.99) | 39.6% | 1.46(0.43–2.48) | 0.050 | 0.165 | 0.115 |
| Sbtr | neg.(neg.−0.22) | neg.(neg.−0.44) | neg.(neg.−0.49) | 0.415 | 0.492 | 0.681 | ||||
| Benzene | Br | 49.0% | 1.33(0.66–3.17) | 60.5% | 1.63(0.88–3.23) | 45.3% | 2.42(1.21–5.15) | 0.028 | 0.156 | 0.072 |
| Sbtr | neg.(neg.−0.79) | 0.19(neg.−1.54) | neg.(neg.−3.68) | 0.615 | 0.254 | 0.54 | ||||
| Thiophene *** | Br | 0.0% | Nd | 2.6% | nd | 1.9% | nd | - | - | - |
| Sbtr | Nd | nd | nd | - | - | - | ||||
| 1-butanol | Br | 19.6% | nd(nd−1.05) | 28.9% | nd(nd−1.07) | 18.9% | nd(nd−1.41) | 0.42 | 0.575 | 0.707 |
| Sbtr | neg.(neg.-neg.) | neg.(neg.−0.1) | neg.(neg.-neg.) | 0.865 | 0.282 | 0.51 | ||||
| Toluene | Br | 86.2% | 27.36(15.35–66.04) | 76.3% | 24.39(18.14–51.17) | 58.5% | 12.33(6.27–21.37) | <0.001 | 0.816 | <0.001 |
| Sbtr | 18.16(2.32–56.58) | 18.34(0.95–48.08) | 0.87(neg.−4.21) | <0.001 | 0.592 | <0.001 | ||||
| Octane | Br | 74.5% | 0.81(0.5–1.38) | 73.7% | 0.88(0.55–1.5) | 77.3% | 0.99(0.75–1.33) | 0.09 | 0.871 | 0.186 |
| Sbtr | 0.42(neg.−0.86) | 0.32(neg.−0.71) | 0.36(0.06–0.76) | 0.883 | 0.907 | 0.965 | ||||
| Ethyl butyrate | Br | 58.8% | 3.11(0.84–6.03) | 52.6% | 2.08(0.52–4.95) | 79.2% | 4.28(2.49–6.24) | 0.085 | 0.221 | 0.015 |
| Sbtr | 0.9(neg.−3.11) | 0.26(neg−3.45) | 2.49(0.53–3.9) | 0.021 | 0.461 | 0.010 | ||||
| Hexanal | Br | 29.4% | 5.13(3.43–6.95) | 28.9% | 5.24(4.35–7.2) | 22.6% | 5.36(4.04–10.76) | 0.172 | 0.275 | 0.313 |
| Sbtr | neg.(neg.−0.33) | Neg.(neg.−1.17) | neg.(neg.-neg.) | 0.634 | 0.918 | 0.856 | ||||
| Ethyl_benzene | Br | 76.4% | 3.85(2.44–6.26) | 76.3% | 3.13(2.02–5.36) | 49.0% | 2.01(1.30–2.89) | <0.001 | 0.476 | <0.001 |
| Sbtr | 1.99(0.18–4.15) | 2.05(0.06–3.56) | neg.(neg.−1.78) | <0.001 | 0.442 | <0.001 | ||||
| Styrene | Br | 86.2% | 4.83(2.36–7.87) | 89.5% | 4.85(1.71–8.26) | 62.3% | 2.11(1.25–3.53) | <0.001 | 0.914 | <0.001 |
| Sbtr | 4.02(0.53–6.93) | 3.97(0.62–7.48) | 0.28(neg.−1.57) | <0.001 | 0.888 | <0.001 | ||||
| Cyclohexanone | Br | 39.2% | 0.58(nd−1.04) | 42.1% | nd(nd−1.53) | 39.6% | 0.49(0.34–0.92) | 0.466 | 0.875 | 0.799 |
| Sbtr | neg.(neg.−0.36) | neg.(neg.−0.81) | neg.(neg.−0.28) | 0.687 | 0.513 | 0.579 | ||||
| Octanal | Br | 19.6% | 3.1(1.65–4.99) | 26.3% | 2.97(2.06–3.90) | 13.2% | 3.46(2.66–4.44) | 0.354 | 0.947 | 0.416 |
| Sbtr | neg.(neg.-neg.) | neg.(neg.−0.11) | neg.(neg.-neg.) | 0.464 | 0.323 | 0.287 | ||||
| Nonanal | Br | 7.8% | 9.3(5.72–16.03) | 7.9% | 7.71(6.02–11.71) | 0.0% | 8.61(6.15–13.38) | 0.407 | 0.131 | 0.317 |
| Sbtr | neg.(neg.-neg.) | neg.(neg.-neg.) | neg.(neg.-neg.) | 0.117 | 0.993 | 0.114 | ||||
| Ca+ vs. HC (p-Value) * | Ca+ vs. Ca− (p-Value) * | ||||
|---|---|---|---|---|---|
| Substance | Variable | Active Smokers | Former/Never Smokers | Active Smokers | Former/Never smokers |
| Isoprene | Br | 0.601 | 0.882 | 0.068 | 0.787 |
| Sbtr | 0.636 | 0.746 | 0.068 | 0.805 | |
| Acetone | Br | 0.862 | 0.674 | 0.965 | 0.300 |
| Sbtr | 0.862 | 0.694 | 0.965 | 0.290 | |
| 2-propanol | Br | 0.199 | 0.021 | 0.672 | 0.751 |
| Sbtr | 0.746 | 0.011 | 0.829 | 0.635 | |
| Hexane | Br | 0.182 | 0.336 | 0.762 | 0.170 |
| Sbtr | 0.123 | 0.039 | 0.122 | 0.520 | |
| 1-propanol | Br | 0.003 | 0.002 | 0.460 | 0.621 |
| Sbtr | 0.409 | 0.008 | 0.460 | 0.534 | |
| 2-butanone | Br | 1.000 | 0.486 | 0.762 | 0.502 |
| Sbtr | 0.940 | 0.495 | 0.897 | 0.945 | |
| Cyclohexane | Br | 0.469 | 0.007 | 0.696 | 0.088 |
| Sbtr | 0.553 | 0.226 | 0.829 | 0.635 | |
| Benzene | Br | 0.746 | 0.404 | 1.000 | 0.225 |
| Sbtr | 0.746 | 0.079 | 0.629 | 0.918 | |
| 1-butanol | Br | 0.901 | 0.294 | 0.897 | 0.844 |
| Sbtr | 0.636 | 0.672 | 0.897 | 0.352 | |
| Toluene | Br | 0.043 | <0.001 | 0.515 | 0.724 |
| Sbtr | 0.033 | <0.001 | 0.633 | 0.435 | |
| Octane | Br | 1.000 | 0.253 | 0.829 | 0.911 |
| Sbtr | 0.566 | 0.664 | 0.762 | 0.725 | |
| Ethyl butyrate | Br | 0.237 | 0.255 | 0.630 | 0.540 |
| Sbtr | 0.033 | 0.252 | 0.897 | 0.526 | |
| Hexanal | Br | 0.940 | 0.408 | 0.696 | 0.685 |
| Sbtr | 0.438 | 0.580 | 0.897 | 0.510 | |
| Ethyl_benzene | Br | 0.011 | <0.001 | 1.000 | 0.638 |
| Sbtr | 0.018 | <0.001 | 0.762 | 0.538 | |
| Styrene | Br | 0.079 | <0.001 | 0.274 | 0.742 |
| Sbtr | 0.028 | <0.001 | 0.315 | 0.671 | |
| Cyclohexanone | Br | 0.258 | 0.734 | 0.696 | 0.955 |
| Sbtr | 0.601 | 0.785 | 1.000 | 0.642 | |
| Octanal | Br | 0.150 | 0.904 | 0.762 | 0.944 |
| Sbtr | 0.940 | 0.284 | 0.315 | 0.882 | |
| Nonanal | Br | 0.381 | 0.175 | 0.696 | 0.066 |
| Sbtr | 0.636 | 0.124 | 0.762 | 0.536 | |
| % Correctly Classified | |||||
|---|---|---|---|---|---|
| Comparison Groups | VOCs Included in the Analysis | Naïve Bayes | Logistic | RF | AUC for RF |
| Breath concentrations (Cexhaled) | |||||
| Ca+ vs. HC | All 19 VOCs | 72.1 | 80.7 | 88.5 | 0.940 |
| * Ethylbenzene, Toluene, Styrene, Benzene, Cyclohexane, 1-propanol, 2-propanol | 70.2 | 78.8 | 83.7 | 0.908 | |
| ** Ethylbenzene, Toluene, styrene, 1-propanol, 2-propanol, hexane | 73.1 | 74.3 | 76.3 | 0.839 | |
| Smokers Ca+ vs. HC | All 19 VOCs | 71.4 | 71.4 | 85.7 | 0.934 |
| * Ethylbenzene, Toluene, Styrene, Benzene, Cyclohexane, 1-propanol, 2-propanol | 85.7 | 71.4 | 89.2 | 0.769 | |
| ** Ethylbenzene, Toluene, styrene, 1-propanol, 2-propanol, hexane | 85.7 | 82.1 | 82.1 | 0.822 | |
| Non-smokers Ca+ vs. HC | All 19 VOCs | 73.7 | 82.9 | 895 | 0.970 |
| * Ethylbenzene, Toluene, Styrene, Benzene, Cyclohexane, 1-propanol, 2-propanol | 69.7 | 80.2 | 86.8 | 0.910 | |
| ** Ethylbenzene, Toluene, styrene, 1-propanol, 2-propanol, hexane | 67.1 | 76.3 | 77.6 | 0.898 | |
| Ca+ vs. Ca− | All 19 VOCs | 40.4 | 41.6 | 35.9 | 0.342 |
| Breath Subtracts ΔC = Cexhaled − Cambient | |||||
| Ca+ vs. HC | All 19 VOCs | 55.7 | 80.8 | 78.8 | 0.888 |
| * Ethylbenzene, Toluene, Styrene,1-propanol, 2-propanol, hexane, ethylbutyrate | 67.3 | 76.9 | 78.8 | 0.86 | |
| ** Ethylbenzene, Toluene, Styrene, 1-propanol, ethylbutyrate | 65.4 | 78.8 | 78.8 | 0.853 | |
| Smokers Ca+ vs. HC | All 19 VOCs | 71.4 | 71.4 | 85.7 | 0.831 |
| * Ethylbenzene, Toluene, Styrene,1-propanol, 2-propanol, hexane, ethylbutyrate | 85.7 | 85.7 | 89.3 | 0.947 | |
| ** Ethylbenzene, Toluene, Styrene,1-propanol, ethylbutyrate | 89.3 | 82.1 | 85.7 | 0.95 | |
| Non-smokers Ca+ vs. HC | All 19 VOCs | 64.5 | 82.9 | 78.9 | 0.892 |
| * Ethylbenzene, Toluene, Styrene,1-propanol, 2-propanol, hexane, ethylbutyrate | 77.6 | 77.6 | 81.6 | 0.901 | |
| ** Ethylbenzene, Toluene, Styrene, 1-propanol, ethylbutyrate | 76.3 | 76.3 | 84.2 | 0.881 | |
| Ca+ vs. Ca− | All 19 VOCs | 43.8 | 52.8 | 41.6 | 0.266 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koureas, M.; Kirgou, P.; Amoutzias, G.; Hadjichristodoulou, C.; Gourgoulianis, K.; Tsakalof, A. Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons. Metabolites 2020, 10, 317. https://doi.org/10.3390/metabo10080317
Koureas M, Kirgou P, Amoutzias G, Hadjichristodoulou C, Gourgoulianis K, Tsakalof A. Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons. Metabolites. 2020; 10(8):317. https://doi.org/10.3390/metabo10080317
Chicago/Turabian StyleKoureas, Michalis, Paraskevi Kirgou, Grigoris Amoutzias, Christos Hadjichristodoulou, Konstantinos Gourgoulianis, and Andreas Tsakalof. 2020. "Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons" Metabolites 10, no. 8: 317. https://doi.org/10.3390/metabo10080317
APA StyleKoureas, M., Kirgou, P., Amoutzias, G., Hadjichristodoulou, C., Gourgoulianis, K., & Tsakalof, A. (2020). Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons. Metabolites, 10(8), 317. https://doi.org/10.3390/metabo10080317









