Glucose Metabolism and Dynamics of Facilitative Glucose Transporters (GLUTs) under the Influence of Heat Stress in Dairy Cattle
Abstract
1. Introduction
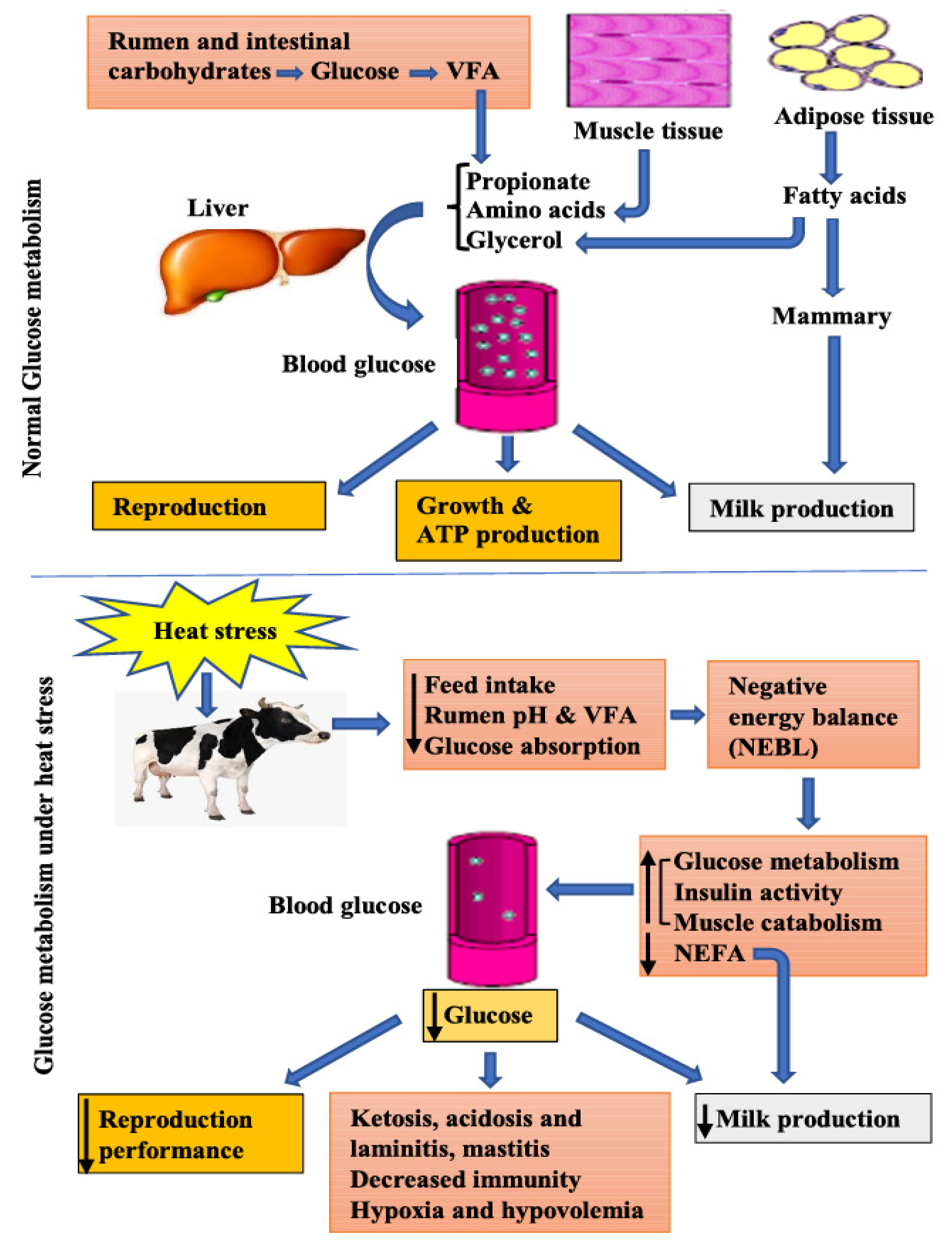
2. Physiology of Glucose Metabolism
3. Regulation of Lactose (Milk Glucose) in Dairy Cattle
4. Heat Stress Effect on Glucose Metabolism
4.1. Decreased Feed Intake and Negative Energy Balance
4.2. Heat Stress Effect on Ruminal and Intestinal Glucose Processing
4.3. Liver Metabolism of Glucose under Heat Stress
4.4. Adipose Tissues Contribution to Glucose Metabolism under Heat Stress
4.5. Nexus of Protein and Glucose Metabolism under Heat Stress
5. Lactose Regulation under Heat Stress
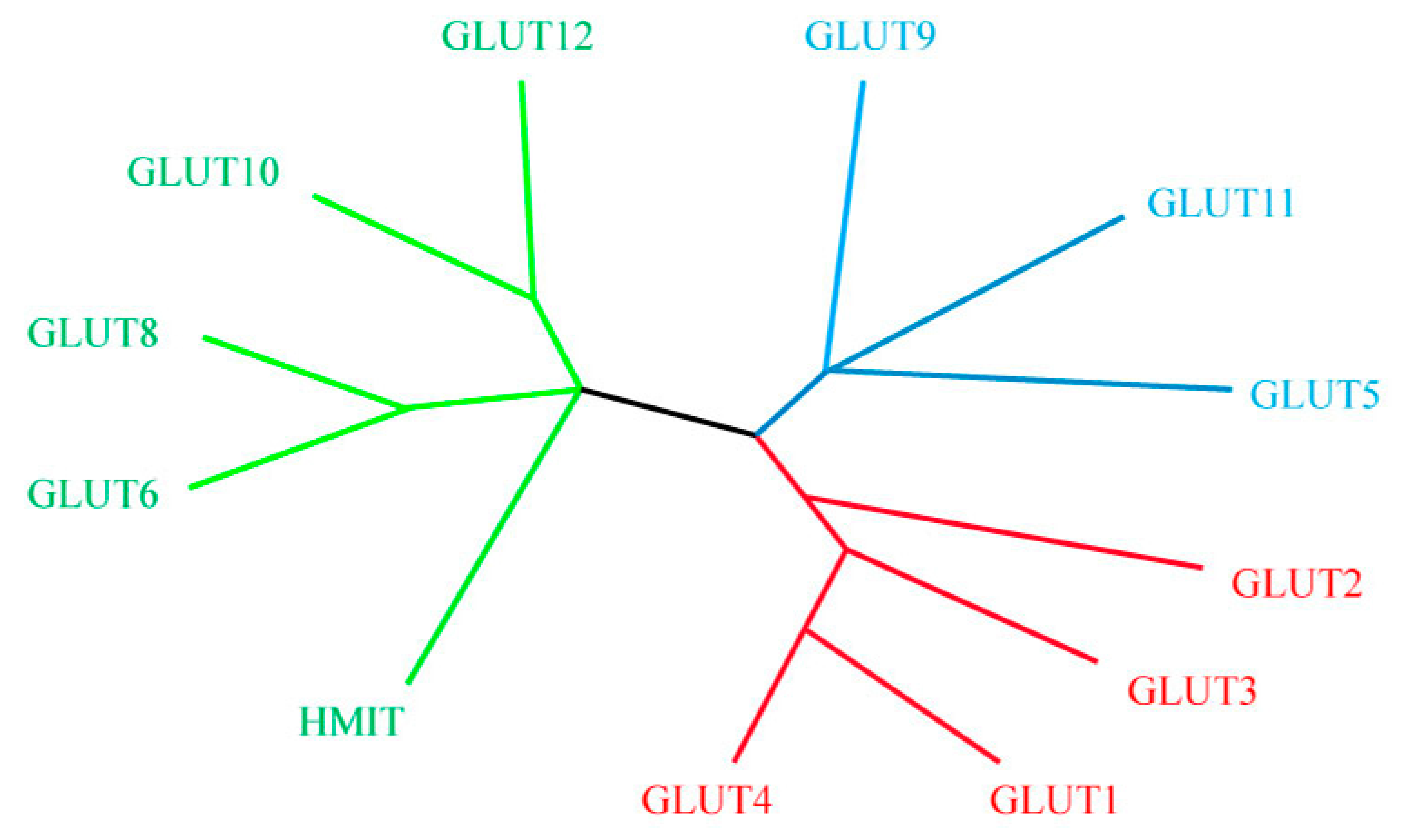
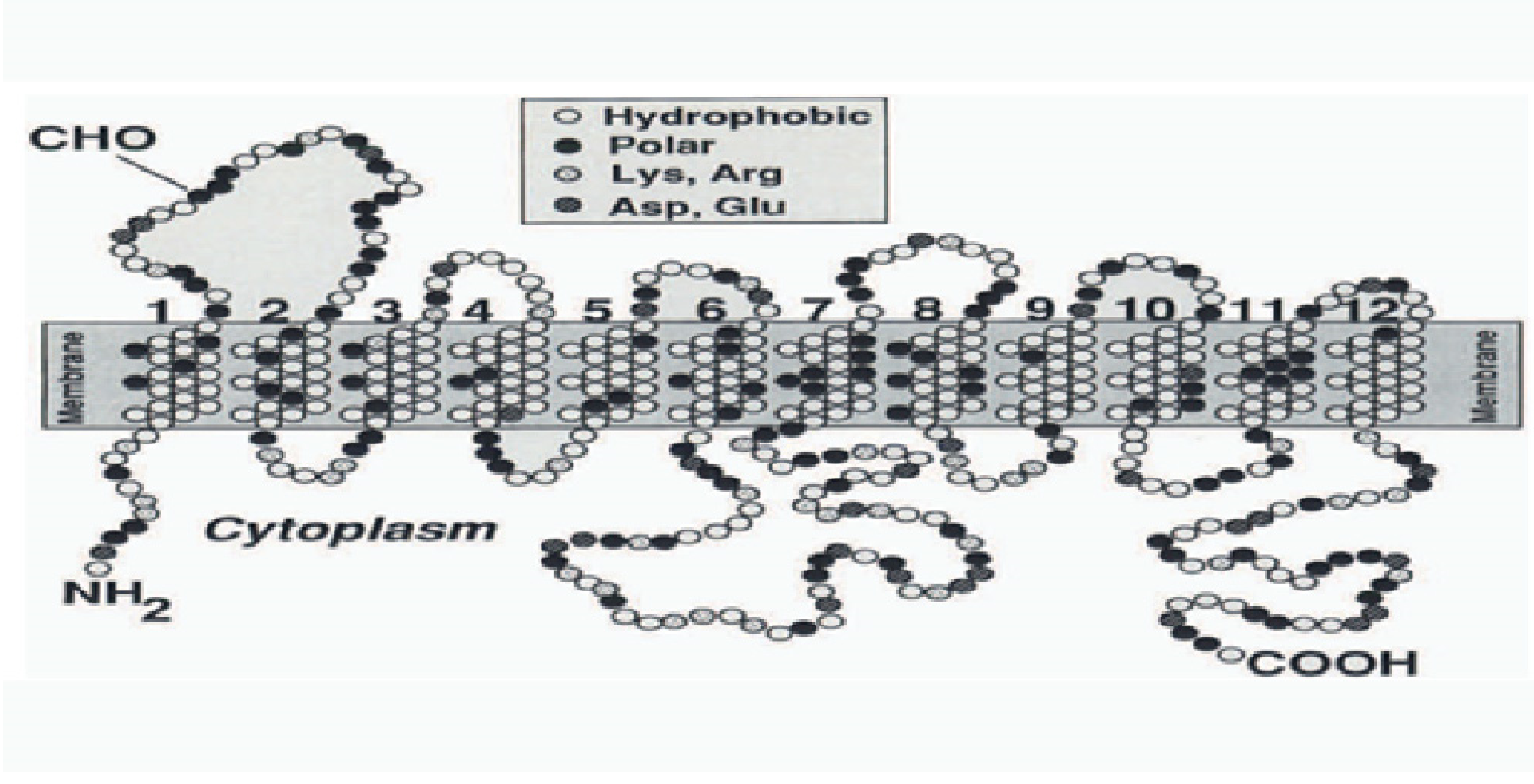
6. Facilitative Glucose Transporters (GLUTs)
7. Heat Stress Effect on Facilitative Glucose Transporters (GLUTs) Family
8. Polymorphism in Facilitative Glucose Transporters (GLUTs) Bringing Sustainable Improvements in Energy Dynamics in Dairy Cattle
9. Mitigation Strategies towards Heat Stress and Its Consequences
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cankaya, M.; Hernandez, A.M.; Ciftci, M.; Beydemir, S.; Ozdemir, H.; Budak, H.; Gulcin, I.; Comakli, V.; Emircupani, T.; Ekinci, D.; et al. An analysis of expression patterns of genes encoding proteins with catalytic activities. BMC Genom. 2007, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Nozière, P.; Ortigues-Marty, I.; Loncke, C.; Sauvant, D. Carbohydrate quantitative digestion and absorption in ruminants: From feed starch and fibre to nutrients available for tissues. Animal 2010, 4, 1057–1074. [Google Scholar] [CrossRef] [PubMed]
- Mayes, P.A. Gluconeogenesis and control of the blood glucose. In Harper’s Biochemistry; McGraw-Hill Education: New York, NY, USA, 1996; pp. 194–204. [Google Scholar]
- Liu, H.; Zhao, K.; Liu, J. Effects of Glucose Availability on Expression of the Key Genes Involved in Synthesis of Milk Fat, Lactose and Glucose Metabolism in Bovine Mammary Epithelial Cells. PLoS ONE 2013, 8, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Scroll, P.; For, D. Potential Function of Its Novel Members. Mol. Membr. Biol. 2007. [Google Scholar] [CrossRef]
- Zhao, F.Q. Biology of glucose transport in the mammary gland. J. Mammary Gland. Biol. Neoplasia 2014, 19, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Braun, E.J.; Sweazea, K.L. Glucose regulation in birds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 1–9. [Google Scholar] [CrossRef]
- Thorens, B. Glucose transporters in the regulation of intestinal, renal, and liver glucose fluxes. Am. J. Physiol. Gastrointest. Liver Physiol. 1996, 270, G541–G553. [Google Scholar] [CrossRef]
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J.; Imtiwati; Kumar, R. Impact of heat stress on health and performance of dairy animals: A review. Vet. World 2016, 9, 260–268. [Google Scholar] [CrossRef]
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat stress: Physiology of acclimation and adaptation. Anim. Front. 2019, 9, 12–19. [Google Scholar] [CrossRef]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional physiology and biochemistry of dairy cattle under the influence of heat stress: Consequences and opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef]
- Koubková, M.; Knížková, I.; Kunc, P.; Härtlová, H.; Flusser, J.; Doležal, O. Influence of high environmental temperatures and evaporative cooling on some physiological, hematological and biochemical parameters in high-yielding dairy cows. Czech J. Anim. Sci. 2002, 47, 309–318. [Google Scholar]
- Moseley, P. Stress proteins and the immune response. Immunopharmacology 2000, 48, 299–302. [Google Scholar] [CrossRef]
- Gupta, M.; Kumar, S.; Dangi, S.; Jangir, B. Physiological, Biochemical and Molecular Responses to Thermal Stress in Goats. Int. J. Livest. Res. 2015, 3, 27. [Google Scholar] [CrossRef]
- Dervisevik, M.; Dinevska-Kjovkarovska, S.; Miova, B.; Mitev, S.; Velkovski, M.; Susleski, D. Heat acclimation-induced changes in heart glycogen/glucose metabolism in rats. J. Physiol. Sci. 2011, 61, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Garriga, C.; Hunter, R.R.; Amat, C.; Planas, J.M.; Mitchell, M.A.; Moretó, M. Heat stress increases apical glucose transport in the chicken jejunum. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 290, R195–R201. [Google Scholar] [CrossRef]
- Horowitz, M. Matching the Heart to Heat-Induced Circulatory Load: Heat-Acclimatory Responses. News Physiol. Sci. 2003, 18, 215–221. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci 2009, 92, 1986–1997. [Google Scholar] [CrossRef]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Wheelock, J.B.; Sanders, S.R.; Moore, C.E.; Green, H.B.; Waldron, M.R.; Rhoads, R.P. Postabsorptive carbohydrate adaptations to heat stress and monensin supplementation in lactating Holstein cows. J. Dairy Sci. 2011, 94, 5620–5633. [Google Scholar] [CrossRef]
- Sano, H.; Ambo, K.; Tsuda, T. Blood Glucose Kinetics in Whole Body and Mammary Gland of Lactating Goats Exposed to Heat. J. Dairy Sci. 1985, 68, 2557–2564. [Google Scholar] [CrossRef]
- Habashy, W.S.; Milfort, M.C.; Fuller, A.L.; Attia, Y.A.; Rekaya, R.; Aggrey, S.E. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int. J. Biometeorol. 2017, 61, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Rajesh, C.; Mishra, S.K.; Gurao, A.; Vohra, V.; Niranjan, S.K.; Kataria, R.S. Comparative Expression Profiling of Heat-stress Tolerance Associated HSP60 and GLUT-1 Genes in Indian Buffaloes. Indian J. Dairy Sci. 2018, 71, 183–186. [Google Scholar]
- Donkin, S.S.; Hammon, H. Chapter 15 Hepatic gluconeogenesis in developing ruminants. In Biology of Growing Animals; Elsevier: Amsterdam, The Netherlands, 2005; Volume 3, pp. 375–390. [Google Scholar]
- Ocquettea, J.H.; Beb, H.A. Facilitative glucose transporters in livestock species. Reprod. Nutr. Dev. 2000, 40, 517–533. [Google Scholar]
- Reynolds, C.K. Glucose Balance in Cattle. In Proceedings of the Florida Ruminant Nutrition Symposium, Gainesvilla, FL, USA, 2 February 2005; pp. 143–154. [Google Scholar]
- Threadgold, L.C.; Kuhn, N.J. Glucose-6-phosphate hydrolysis by lactating rat mammary gland. Int. J. Biochem. 1979, 10, 683–685. [Google Scholar] [CrossRef]
- Kronfeld, D.S. Major Metabolic Determinants of Milk Volume, Mammary Efficiency, and Spontaneous Ketosis in Dairy Cows. J. Dairy Sci. 1982, 65, 2204–2212. [Google Scholar] [CrossRef]
- Bell, A.W. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef]
- Drackley, J.; Overton, T.; Douglas, G. Adaptations of Glucose and Long-Chain Fatty Acid Metabolism in Liver of Dairy Cows during the Periparturient Period. J. Dairy Sci. 2001, 84, E100–E112. [Google Scholar] [CrossRef]
- Nayeri, S.; Stothard, P. Tissues, Metabolic Pathways and Genes of Key Importance in Lactating Dairy Cattle. Springer Sci. Rev. 2016, 4, 49–77. [Google Scholar] [CrossRef]
- Harper, D.; Chandler, B. Splanchnic circulation. BJA Educ. 2016, 16, 66–71. [Google Scholar] [CrossRef]
- Bickerstaffe, R.; Annison, E.F.; Linzell, J.L. The metabolism of glucose, acetate, lipids and amino acids in lactating dairy cows. J. Agric. Sci. 1974, 82, 71–85. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, X.; Hou, X.; Qu, B.; Gao, X.; Li, Q. Effects of glucose on lactose synthesis in mammary epithelial cells from dairy cow. BMC Vet. Res. 2016, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, R.P.; Nardone, A.; Ronchi, B.; Bernabucci, U.; Lacetera, N.; Baumgard, L.H. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef]
- Guo, J.; Gao, S.; Quan, S.; Zhang, Y.; Bu, D.; Wang, J. Blood amino acids profile responding to heat stress in dairy cows. Asian Australas. J. Anim. Sci. 2018, 31, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Du, R.; Gu, X.H.; Li, F.C.; Zhang, Z.Y. A study on the plasma biochemical indices of heat-stressed broilers. Asian Australas. J. Anim. Sci. 2000, 13, 1210–1218. [Google Scholar] [CrossRef]
- Miova, B.; Dinevska-Kjovkarovska, S.; Cvetkovska, F.; Mitev, S.; Dzimrevska, A.; Dimitrovska, M. Liver carbohydrate metabolism in rats in the period of recovery after acute heat stress. Maced. J. Med Sci. 2013, 6, 16–23. [Google Scholar]
- O’Brien, M.D.; Rhoads, R.P.; Sanders, S.R.; Duff, G.C.; Baumgard, L.H. Metabolic adaptations to heat stress in growing cattle. Domest. Anim. Endocrinol. 2010, 38, 86–94. [Google Scholar] [CrossRef]
- Settivari, R.; Spain, J.; Ellersieck, M.; Byatt, J.; Collier, R.; Spiers, D. Relationship of Thermal Status to Productivity in Heat-Stressed Dairy Cows Given Recombinant Bovine Somatotropin. J. Dairy Sci. 2007, 90, 1265–1280. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Drackley, J.K. ADSA foundation scholar award: Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Koch, F.; Lamp, O.; Eslamizad, M.; Weitzel, J.; Kuhla, B. Metabolic Response to heat stress in late-pregnant and early lactation dairy cows: Implications to liver-muscle crosstalk. PLoS ONE 2016, 11, 1–19. [Google Scholar] [CrossRef]
- Kvidera, S.K.; Horst, E.A.; Abuajamieh, M.; Mayorga, E.J.; Fernandez, M.V.S.; Baumgard, L.H. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 2017, 100, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Alamer, M. The role of prolactin in thermoregulation and water balance during heat stress in domestic ruminants. Asian J. Anim. Vet. Adv. 2011, 6, 1153–1169. [Google Scholar] [CrossRef]
- Ahmed, N.; Berridge, M.V. Transforming oncogenes regulate glucose transport by increasing transporter affinity for glucose: Contrasting effects of oncogenes and heat stress in a murine marrow-derived cell line. Life Sci. 1998, 63, 1887–1903. [Google Scholar] [CrossRef]
- Knapp, D.M.; Grummer, R.R. Response of Lactating Dairy Cows to Fat Supplementation During Heat Stress. J. Dairy Sci. 1991, 74, 2573–2579. [Google Scholar] [CrossRef]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Nafikov, R.A.; Beitz, D.C. Carbohydrate and Lipid Metabolism in Farm Animals. J. Nutr. 2007, 137, 702–705. [Google Scholar] [CrossRef]
- James, A.; DeShazer James, A. DeShazer Livestock Energetics and Thermal Environmental Management. Livest. Energetics Therm. Environ. Manag. 2013. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Wheelock, J.B.; Shwartz, G.; O’Brien, M.; VanBaale, M.J.; Collier, R.J.; Rhoads, M.L.; Rhoads, R.P. Effects of Heat Stress on Nutritional Requirements of Lactating Dairy Cattle. In Proceedings of the 5th Annual Arizona Dairy Production Conference, Tempe, AZ, USA, 10 October 2006; pp. 8–17. [Google Scholar]
- Cai, L.; Yu, J.; Hartanto, R.; Zhang, J.; Yang, A.; Qi, D. Effects of heat challenge on growth performance, ruminal, blood and physiological parameters of Chinese crossbred goats. Small Rumin. Res. 2019, 174, 125–130. [Google Scholar] [CrossRef]
- Fan, C.; Su, D.; Tian, H.; Li, X.; Li, Y.; Ran, L.; Hu, R.; Cheng, J. Liver metabolic perturbations of heat-stressed lactating dairy cows. Asian Australas. J. Anim. Sci. 2018, 31, 1244–1251. [Google Scholar] [CrossRef]
- McGuire, M.A.; Beede, D.K.; Collier, R.J.; Buonomo, F.C.; De Lorenzo, M.A.; Wilcox, C.J.; Huntington, G.B.; Reynolds, C.K. Effects of acute thermal stress and amount of feed intake on concentrations of somatotropin, insulin-like growth factor (IGF)-I and IGF-II, and thyroid hormones in plasma of lactating Holstein cows. J. Anim. Sci. 1991, 69, 2050–2056. [Google Scholar] [CrossRef]
- Yousef, M.K.; Johnson, H.D. Calorigenesis of cattle as influenced by growth hormone and environmental temperature. J. Anim. Sci. 1966, 25, 1076–1082. [Google Scholar] [CrossRef]
- Marinković, M.D.; Belić, B.; Cincović, M.R.; Đoković, R.; Lakić, I.; Stojanac, N.; Stevančević, O.; Devečerski, G. Relationship between insulin, glucose, non-esterified fatty acid and indices of insuliresistance in obese cows during the dry period and early lactation. Acta Vet. Brno 2019, 88, 143–155. [Google Scholar] [CrossRef]
- Bauman, D. Effects of Exogenous Bovine Somatotropin on Lactation. Annu. Rev. Nutr. 1993, 13, 437–461. [Google Scholar] [CrossRef] [PubMed]
- Galster, A.D.; Clutter, W.E.; Cryer, P.E.; Collins, J.A.; Bier, D.M. Epinephrine plasma thresholds for lipolytic effects in man. Measurements of fatty acid transport with [1-13C]palmitic acid. J. Clin. Investig. 1981, 67, 1729–1738. [Google Scholar] [CrossRef]
- Streffer, C. Aspects of Metabolic Change After Hyperthermia. In Application of Hyperthermia in the Treatment of Cancer; Springer: Berlin/Heidelberg, Germany, 1988; pp. 7–16. [Google Scholar] [CrossRef]
- Patel, M.S.; Korotchkina, L.G. Regulation of the pyruvate dehydrogenase complex. Biochem. Soc. Trans. 2006, 34, 217–222. [Google Scholar] [CrossRef]
- Rhoads, R.; Baumgard, L.H.; Suagee, J.K. 2011 AND 2012 EARLY CAREERS ACHIEVEMENT AWARDS: Metabolic priorities during heat stress with an emphasis on skeletal muscle1,2. J. Anim. Sci. 2013, 91, 2492–2503. [Google Scholar] [CrossRef]
- Rhoads, R.P.; La Noce, A.J.; Wheelock, J.B.; Baumgard, L.H. Short communication: Alterations in expression of gluconeogenic genes during heat stress and exogenous bovine somatotropin administration. J. Dairy Sci. 2011, 94, 1917–1921. [Google Scholar] [CrossRef]
- Tian, H.; Wang, W.; Zheng, N.; Cheng, J.; Li, S.; Zhang, Y.; Wang, J. Identification of diagnostic biomarkers and metabolic pathway shifts of heat-stressed lactating dairy cows. J. Proteom. 2015, 125, 17–28. [Google Scholar] [CrossRef]
- Bell, A.W.; Burhans, W.S.; Overton, T.R. Protein nutrition in late pregnancy, maternal protein reserves and lactation performance in dairy cows. Proc. Nutr. Soc. 2000, 59, 119–126. [Google Scholar] [CrossRef]
- Sejian, V.; Indu, S.; Naqvi, S.M.K. Impact of short term exposure to different environmental temperature on the blood biochemical and endocrine responses of Malpura ewes under semi-arid tropical environment. Indian J. Anim. Sci. 2013, 83, 1155–1159. [Google Scholar]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.L.; Beede, D.K.; Wilcox, C.J. Nycterohemeral patterns of acid-base status, mineral concentrations and digestive function of lactating cows in natural or chamber heat stress environments. J. Anim. Sci. 1988, 66, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Joksimovic-Todorovic, M.; Davidovic, V.; Hristov, S.; Stankovic, B. Effect of heat stress on milk production in dairy cows. Biotechnol. Anim. Husb. 2011, 27, 1017–1023. [Google Scholar] [CrossRef]
- Ikari, A.; Nakano, M.; Suketa, Y.; Harada, H.; Takagi, K. Reorganization of ZO-1 by sodium-dependent glucose transporter activation after heat stress in LLC-PK1 cells. J. Cell. Physiol. 2005, 203, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A. Alterations in energy metabolism during exercise and heat stress. Sports Med. 2001, 31, 47–59. [Google Scholar] [CrossRef]
- Mehaba, N.; Salama, A.A.; Such, X.; Albanell, E.; Caja, G. Lactational Responses of Heat-Stressed Dairy Goats to Dietary L-Carnitine Supplementation. Animals 2019, 9, 567. [Google Scholar] [CrossRef]
- Mueckler, M. Facilitative glucose transporters. Eur. J. Biochem. 1994, 219, 713–725. [Google Scholar] [CrossRef]
- Ostrowska, M.; Jarczak, J.; Zwierzchowski, L. Glucose transporters in cattle—A review. Anim. Sci. Pap. Rep. 2015, 33, 191–212. [Google Scholar]
- Wood, I.S.; Trayhurn, P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br. J. Nutr. 2003, 89, 3–9. [Google Scholar] [CrossRef]
- Uldry, M.; Thorens, B. The SLC2 family of facilitated hexose and polyol transporters. Pflug. Arch. Eur. J. Physiol. 2004, 447, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Q.; Dixon, W.T.; Kennelly, J.J. Localization and gene expression of glucose transporters in bovine mammary gland. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 115, 127–134. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Okine, E.K.; Kennelly, J.J. Glucose transporter gene expression in bovine mammary gland. J. Anim. Sci. 1999, 77, 2517–2522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Q.; Glimm, D.R.; Kennelly, J.J. Distribution of mammalian facilitative glucose transporter messenger rna in bovine tissues. Int. J. Biochem. 1993, 25, 1897–1903. [Google Scholar] [CrossRef]
- Liao, S.F.; Harmon, D.L.; Vanzant, E.S.; McLeod, K.R.; Boling, J.A.; Matthews, J.C. The small intestinal epithelia of beef steers differentially express sugar transporter messenger ribonucleic acid in response to abomasal versus ruminal infusion of starch hydrolysate. J. Anim. Sci. 2010, 88, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, H.; Matsutani, R.; Yamamoto, S.; Takahashi, T.; Hayashi, K.G.; Miyamoto, A.; Hamano, S.; Tetsuka, M. Gene expression of glucose transporter (GLUT) 1, 3 and 4 in bovine follicle and corpus luteum. J. Endocrinol. 2006, 188, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Augustin, R.; Pocar, P.; Navarrete-Santos, A.; Wrenzycki, C.; Gandolfi, F.; Niemann, H.; Fischer, B. Glucose transporter expression is developmentally regulated in in vitro derived bovine preimplantation embryos. Mol. Reprod. Dev. 2001, 60, 370–376. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Miller, P.J.; Wall, E.H.; Zheng, Y.C.; Dong, B.; Neville, M.C.; McFadden, T.B. Bovine glucose transporter GLUT8: Cloning, expression, and developmental regulation in mammary gland. Biochim. Biophys. Acta Gene Struct. Expr. 2004, 1680, 103–113. [Google Scholar] [CrossRef]
- McVie-Wylie, A.J.; Lamson, D.R.; Chen, Y.T. Molecular cloning of a novel member of the GLUT family of transporters, SLC2A10 (GLUT10), localized on chromosome 20q13.1: A candidate gene for NIDDM susceptibility. Genomics 2001, 72, 113–117. [Google Scholar] [CrossRef]
- Wu, X.; Li, W.; Sharma, V.; Godzik, A.; Freeze, H.H. Cloning and characterization of glucose transporter 11, a novel sugar transporter that is alternatively spliced in various tissues. Mol. Genet. Metab. 2002, 76, 37–45. [Google Scholar] [CrossRef]
- Komatsu, T.; Itoh, F.; Sakumoto, R.; Hodate, K.; Obara, Y.; Kushibiki, S. Changes in the gene expression of adiponectin and glucose transporter 12 (GLUT12) in lactating and non-lactating cows. Anim. Sci. J. 2007, 78, 98–102. [Google Scholar] [CrossRef]
- Uldry, M.; Ibberson, M.; Horisberger, J.D.; Chatton, J.Y.; Riederer, B.M.; Thorens, B. Identification of a mammalian H+-myo-inositol symporter expressed predominantly in the brain. EMBO J. 2001, 20, 4467–4477. [Google Scholar] [CrossRef]
- Malago, J.J.; Koninkx, J.F.J.G.; Van Dijk, J.E. The heat shock response and cytoprotection of the intestinal epithelium. Cell Stress Chaperones 2002, 7, 191–199. [Google Scholar] [CrossRef]
- Simpson, I.A.; Appel, N.M.; Hokari, M.; Oki, J.; Holman, G.D.; Maher, F.; Koehler-Stec, E.M.; Vannucci, S.J.; Smith, Q.R. Blood-brain barrier glucose transporter: Effects of hypo and hyperglycemia revisited. J. Neurochem. 1999, 72, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, H.; Sheikhahmadi, A.; Wang, Y.; Jiao, H.; Lin, H.; Song, Z. Effects of heat stress on the gene expression of nutrient transporters in the jejunum of broiler chickens (Gallus gallus domesticus). Int. J. Biometeorol. 2014, 59, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bánhegyi, G.; Benedetti, A.; Margittai, É.; Marcolongo, P.; Fulceri, R.; Németh, C.E.; Szarka, A. Subcellular compartmentation of ascorbate and its variation in disease states. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 1909–1916. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat Stress Reduces Intestinal Barrier Integrity and Favors Intestinal Glucose Transport in Growing Pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, M.; Cota, M.; Arce, N.; Castillo, G.; Avelar, E.; Espinoza, S.; Morales, A. Effect of heat stress on performance and expression of selected amino acid and glucose transporters, HSP90, leptin and ghrelin in growing pigs. J. Therm. Biol. 2016, 59, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.Q.; Liao, T.T.; Yang, W.R.; Wang, Y.; Luo, H.Y.; Wang, X.Z. Heat stress–induced autophagy promotes lactate secretion in cultured immature boar Sertoli cells by inhibiting apoptosis and driving SLC2A3, LDHA, and SLC16A1 expression. Theriogenology 2017, 87, 339–348. [Google Scholar] [CrossRef]
- Gaughan, J.; Lacetera, N.; Valtorta, S.E.; Khalifa, H.H.; Hahn, L.; Mader, T. Response of Domestic Animals to Climate Challenges. In Biometeorology for Adaptation to Climate Variability and Change. Biometeorology; Springer: Dordrecht, The Netherlands, 2009; Volume 1, pp. 131–170. ISBN 9781402089213. [Google Scholar]
- Horowitz, M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 131, 475–483. [Google Scholar] [CrossRef]
- Hall, D.M.; Buettner, G.R.; Oberley, L.W.; Xu, L.; Matthes, R.D.; Gisolfi, C.V. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.M.; Baumgardner, K.R.; Oberley, T.D.; Gisolfi, C.V. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 276. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, R.; Liu, Y.; Zhu, W.; Mao, S. Intravenous lipopolysaccharide challenge alters ruminal bacterial microbiota and disrupts ruminal metabolism in dairy cattle. Br. J. Nutr. 2014, 112, 170–182. [Google Scholar] [CrossRef]
- Lambert, G.P.; Gisolfi, C.V.; Berg, D.J.; Moseley, P.L.; Oberley, L.W.; Kregel, K.C. Selected contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J. Appl. Physiol. 2002, 92, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.R. Heat stroke and cytokines. Prog. Brain Res. 2007, 162, 481–524. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef]
- Carabaño, M.J.; Ramón, M.; Menéndez-Buxadera, A.; Molina, A.; Díaz, C. Selecting for heat tolerance. Anim. Front. 2019, 9, 62–68. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.C.; Hu, L.R.; Kang, L. The effect of temperature stress on milk production traits and blood biochemical parameters of Chinese Holstein cows. In Proceedings of the World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand, 11 February 2018. [Google Scholar]
- West, J.W.; Mullinix, B.G.; Bernard, J.K. Effects of hot, humid weather on milk temperature, dry matter intake, and milk yield of lactating dairy cows. J. Dairy Sci. 2003, 86, 232–242. [Google Scholar] [CrossRef]
- Amamou, H.; Beckers, Y.; Mahouachi, M.; Hammami, H. Thermotolerance indicators related to production and physiological responses to heat stress of holstein cows. J. Therm. Biol. 2019, 82, 90–98. [Google Scholar] [CrossRef]
- Ng, D.P.K.; Canani, L.; Araki, S.I.; Smiles, A.; Moczulski, D.; Warram, J.H.; Krolewski, A.S. Minor effect of GLUT1 polymorphisms on susceptibility to diabetic nephropathy in type 1 diabetes. Diabetes 2002, 51, 2264–2269. [Google Scholar] [CrossRef]
- Grabellus, F.; Sheu, S.Y.; Bachmann, H.S.; Lehmann, N.; Otterbach, F.; Heusner, T.A.; Antoch, G.; Bockisch, A.; Kimmig, R.; Schmid, K.W.; et al. The XbaI G>T polymorphism of the glucose transporter 1 gene modulates 18F-FDG uptake and tumor aggressiveness in breast cancer. J. Nucl. Med. 2010, 51, 1191–1197. [Google Scholar] [CrossRef]
- Seefried, F.R. Genomic Characterisation and Polymorphism Analysis of Candidate Genes for Milk Production Traits and Association Studies in Three Cattle Breeds. Ph.D. Thesis, The Technical University of Munich, München, Germany, 2008. [Google Scholar]
- Herbut, P.; Angrecka, S.; Godyń, D. Effect of the duration of high air temperature on cow’s milking performance in moderate climate conditions. Ann. Anim. Sci. 2018, 18, 195–207. [Google Scholar] [CrossRef]
- Berman, A. An overview of heat stress relief with global warming in perspective. Int. J. Biometeorol. 2019, 63, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.W.; Chastain, J.P.; Hemken, R.W.; Gates, R.S.; Crist, W.L. Reducing Heat Stress in Dairy Cows Through Sprinkler and Fan Cooling. Appl. Eng. Agric. 1992, 8, 251–256. [Google Scholar] [CrossRef]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Lees, A.M.; Sejian, V.; Wallage, A.L.; Steel, C.C.; Mader, T.L.; Lees, J.C.; Gaughan, J.B. The impact of heat load on cattle. Animals 2019, 9, 322. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Chen, X.; Lu, Y.; Wang, D. Effects of heat stress on body temperature, milk production, and reproduction in dairy cows: A novel idea for monitoring and evaluation of heat stress—A review. Asian Australas. J. Anim. Sci. 2019, 32, 1332–1339. [Google Scholar] [CrossRef]
- Tang, Y.; Li, J.; Liao, S.; Qi, M.; Kong, X.; Tan, B.; Yin, Y.; Wang, J. The effect of dietary protein intake on immune status in pigs of different genotypes. Food Agric. Immunol. 2018, 29, 776–784. [Google Scholar] [CrossRef]
- Caroprese, M.; Albenzio, M.; Marino, R.; Santillo, A.; Sevi, A. Dietary glutamine enhances immune responses of dairy cows under high ambient temperature. J. Dairy Sci. 2013, 96, 3002–3011. [Google Scholar] [CrossRef]
- Wu, G.; Meier, S.A.; Knabe, D.A. Dietary Glutamine Supplementation Prevents Jejunal Atrophy in Weaned Pigs. J. Nutr. 1996, 126, 2578–2584. [Google Scholar] [CrossRef]
- Sciences, D. Effect of trace mineral supplementation on selected minerals, energy metabolites, oxidative stress and immune parameters and its association with uterine diseases in dairy cattle. J. Dairy Sci. 2014, 97, 4281–4295. [Google Scholar] [CrossRef]
- Yadav, B.; Singh, G.; Wankar, A.; Dutta, N.; Chaturvedi, V.B.; Verma, M.R. Effect of simulated heat stress on digestibility, methane emission and metabolic adaptability in crossbred cattle. Asian Australas. J. Anim. Sci. 2016, 29, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- West, J.W. Nutritional strategies for managing the heat-stressed dairy cow. J. Anim. Sci. 1999, 77, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; La, S.K.; Zhang, G.W.; Du, H.S.; Wu, Z.Z.; Wang, C.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; et al. Diet supplementation of palm fat powder and coated folic acid on performance, energy balance, nutrient digestion, ruminal fermentation and blood metabolites of early lactation dairy cows. Anim. Feed Sci. Technol. 2020, 265. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Jenkins, T.C. Fat in Lactation Rations: Review. J. Dairy Sci. 1980, 63, 1–14. [Google Scholar] [CrossRef]
- Arieli, A.; Adin, G.; Bruckental, I. The effect of protein intake on performance of cows in hot environmental temperatures. J. Dairy Sci. 2004, 87, 620–629. [Google Scholar] [CrossRef]
- Bruno, R.G.S.; Rutigliano, H.M.; Cerri, R.L.; Robinson, P.H.; Santos, J.E.P. Effect of feeding Saccharomyces Cerevisiae on performance of dairy cows during summer heat stress. Anim. Feed Sci. Technol. 2009, 150, 175–186. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Guan, R.; Shi, K.; Wei, Z.; Liu, J.; Liu, H. Effects of dietary rumen-protected betaine supplementation on performance of postpartum dairy cows and immunity of newborn calves. Animals 2019, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Erdman, R.A. Dietary Buffering Requirements of the Lactating Dairy Cow: A Review. J. Dairy Sci. 1988, 71, 3246–3266. [Google Scholar] [CrossRef]
- Melendez, P.; Donovan, G.A.; Risco, C.A.; Littell, R.; Goff, J.P. Effect of calcium-energy supplements on calving-related disorders, fertility and milk yield during the transition period in cows fed anionic diets. Theriogenology 2003, 60, 843–854. [Google Scholar] [CrossRef]
- Costanzo, A.D.I.; Spiers, D.E. Supplementation of Nicotinic Acid for Lactating Holstein Cows Under Heat Stress Conditions. J. Dairy Sci. 1997, 80, 1200–1206. [Google Scholar] [CrossRef]
- Barreras, A.; Castro-Pérez, B.I.; López-Soto, M.A.; Torrentera, N.G.; Montaño, M.F.; Estrada-Angulo, A.; Ríos, F.G.; Dávila-Ramos, H.; Plascencia, A.; Zinn, R.A. Influence of ionophore supplementation on growth performance, dietary energetics and carcass characteristics in finishing cattle during period of heat stress. Asian Australas. J. Anim. Sci. 2013, 26, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, S.; Chen, J.; Peng, D.; Gu, X. Predicting rectal temperature and respiration rate responses in lactating dairy cows exposed to heat stress. J. Dairy Sci. 2020, 103, 5466–5484. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Shimadzu, H.; Endo, T. Modelling temperature effects on milk production: A study on Holstein cows at a Japanese farm. Springer Plus 2014, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Bowman, P.J.; Haile-Mariam, M.; Nieuwhof, G.J.; Hayes, B.J.; Pryce, J.E. Short communication: Implementation of a breeding value for heat tolerance in Australian dairy cattle. J. Dairy Sci. 2017, 100, 7362–7367. [Google Scholar] [CrossRef]
- Berman, A. Invited review: Are adaptations present to support dairy cattle productivity in warm climates? J. Dairy Sci. 2011, 94, 2147–2158. [Google Scholar] [CrossRef]
| Specie | Glucose Level HS/TNZ | Milk Lactose HS/TNZ | Milk Yield HS/TNZ | p-Value | Reference |
|---|---|---|---|---|---|
| Cow | 6.3 mg/L ↓ | 0.12% ↓ | 6.65 kg ↓ | p < 0.05 | [19] |
| Cow | 3.15 mg/L ↓ | 0.06% ↓ | 5.25 kg ↓ | p < 0.05 | [20] |
| Cow | 6.5 mg/dL ↓ | 0.14% ↓ | 7.5 kg ↓ | p < 0.05 | [18] |
| Cow | ------ | 0.42% ↓ | 3.14 kg ↓ | p < 0.05 | [68] |
| Goat | 202 µmol ↓ | 11% ↓ | 11–13% ↓ (0.21 kg) | p < 0.05 | [71] |
| Protein | Gene | Chr. Location | Exon No. | Accession No | Protein Size | Main Tissue Localization | Functional Characteristics | References | |
|---|---|---|---|---|---|---|---|---|---|
| Gene | Protein | ||||||||
| GLUT1 | SLC2A1 | Chr.3 | 10 | NC_037330.1 | NP_777027.1 | 492 aa | Mammary gland, kidney, brain, omental fat, skeletal muscle, bovine follicle, bovine ovary, and corpus luteum | Basal glucose transport across blood tissue barriers | [76,77] |
| GLUT2 | SLC2A2 | Chr.1 | 11 | NC_037328.1 | NP_001096692 | 510 aa | Small intestine, liver, Islets, kidney, and jejunal region | Glucose (low affinity) | [78,79] |
| GLUT3 | SLC2A3 | Chr.5 | 11 | NC_037332.1 | NP_777028 | 494 aa | Bovine ovary, follicles, corpus luteum, and brain. | Glucose (high affinity | [80] |
| GLUT4 | SLC2A4 | Chr.19 | 11 | NC_037346.1 | NP_777029 | 509 aa | Heart, muscle, brain and adipose tissue | Transport of glucose in all insulin-responsive tissues | [78] |
| GLUT5 | SLC2A5 | Chr.14 | 13 | NC_037341.1 | NP_001094512 | 501 aa | Small intestine, testes, kidney, muscle, brain and adipose tissue | Fructose (high affinity), glucose (low affinity) | [79,80,81] |
| GLUT6 | SLC2A6 | Chr.11 | 10 | NC_037338.1 | NP_001073725 | 507 aa | Brain, spleen, and peripheral leukocytes. | not determined | |
| GLUT8 | SLC2A8 | Chr.11 | 10 | NC_037338.1 | NP_963286 | 478 aa | Mammary gland, testis, kidney, intestinal epithelia, skeletal muscle, blastocyst and liver | Insulin-responsive transport in blastocyst | [82] |
| GLUT9 | SLC2A9 | Chr.6 | 18 | NC_037333.1 | XP_002688502 | 506 aa | Kidney and liver | not determined | [5] |
| GLUT 10 | SLC2A 10 | Chr.13 | 5 | NC_037340.1 | NP_001179368 | 536 aa | Liver and pancreas | not determined | [83] |
| GLUT 11 | SLC2A 11 | Chr.17 | 12 | NC_037344.1 | NP_001180026 | 496 aa | Heart, muscle (short form) liver, lung, trachea, and brain (long form). | Glucose (low affinity), transport of fructose (long form) | [84] |
| GLUT 12 | SLC2A 12 | Chr.9 | 7 | NC_037336.1 | NP_001011683 | 621 aa | Skeletal muscle, spleen, kidney, testes, mammary gland, liver, lung, and intestine | Insulin-dependent glucose uptake in mammary gland | [85] |
| HMIT | SLC2A 13 | Chr.5 | 10 | NC_037332.1 | NP_001179892 | 648 aa | Brain | H+/myo-inositol transporter | [86] |
| Protein | Gene | Animals | Tissue | mRNA Expression | Reference |
|---|---|---|---|---|---|
| Buffalos | Blood | Up-regulated | [23] | ||
| GLUT1 | SLC2A1 | Chicken | Intestine | Down-regulated | [22] |
| Chicken | Intestine | Down-regulated | [89] | ||
| GLUT2 | SLC2A2 | Pigs | Intestine | Up-regulated | [91] |
| GLUT3 | SLC2A3 | Boar | Sertoli cells | Down-regulated | [93] |
| GLUT4 | SLC2A4 | Pigs | Liver, Muscle | Up-regulated | [92] |
| GLUT5 | SLC2A5 | Chicken | Intestine | Up-regulated | [22] |
| GLUT10 | SLC2A10 | Chicken | Intestine | Up-regulated | [22] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, Z.; Sammad, A.; Hu, L.; Fang, H.; Xu, Q.; Wang, Y. Glucose Metabolism and Dynamics of Facilitative Glucose Transporters (GLUTs) under the Influence of Heat Stress in Dairy Cattle. Metabolites 2020, 10, 312. https://doi.org/10.3390/metabo10080312
Abbas Z, Sammad A, Hu L, Fang H, Xu Q, Wang Y. Glucose Metabolism and Dynamics of Facilitative Glucose Transporters (GLUTs) under the Influence of Heat Stress in Dairy Cattle. Metabolites. 2020; 10(8):312. https://doi.org/10.3390/metabo10080312
Chicago/Turabian StyleAbbas, Zaheer, Abdul Sammad, Lirong Hu, Hao Fang, Qing Xu, and Yachun Wang. 2020. "Glucose Metabolism and Dynamics of Facilitative Glucose Transporters (GLUTs) under the Influence of Heat Stress in Dairy Cattle" Metabolites 10, no. 8: 312. https://doi.org/10.3390/metabo10080312
APA StyleAbbas, Z., Sammad, A., Hu, L., Fang, H., Xu, Q., & Wang, Y. (2020). Glucose Metabolism and Dynamics of Facilitative Glucose Transporters (GLUTs) under the Influence of Heat Stress in Dairy Cattle. Metabolites, 10(8), 312. https://doi.org/10.3390/metabo10080312







