Plasma Lipid Profile Reveals Plasmalogens as Potential Biomarkers for Colon Cancer Screening
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinicopathological Characteristics of CC and CTR Subjects
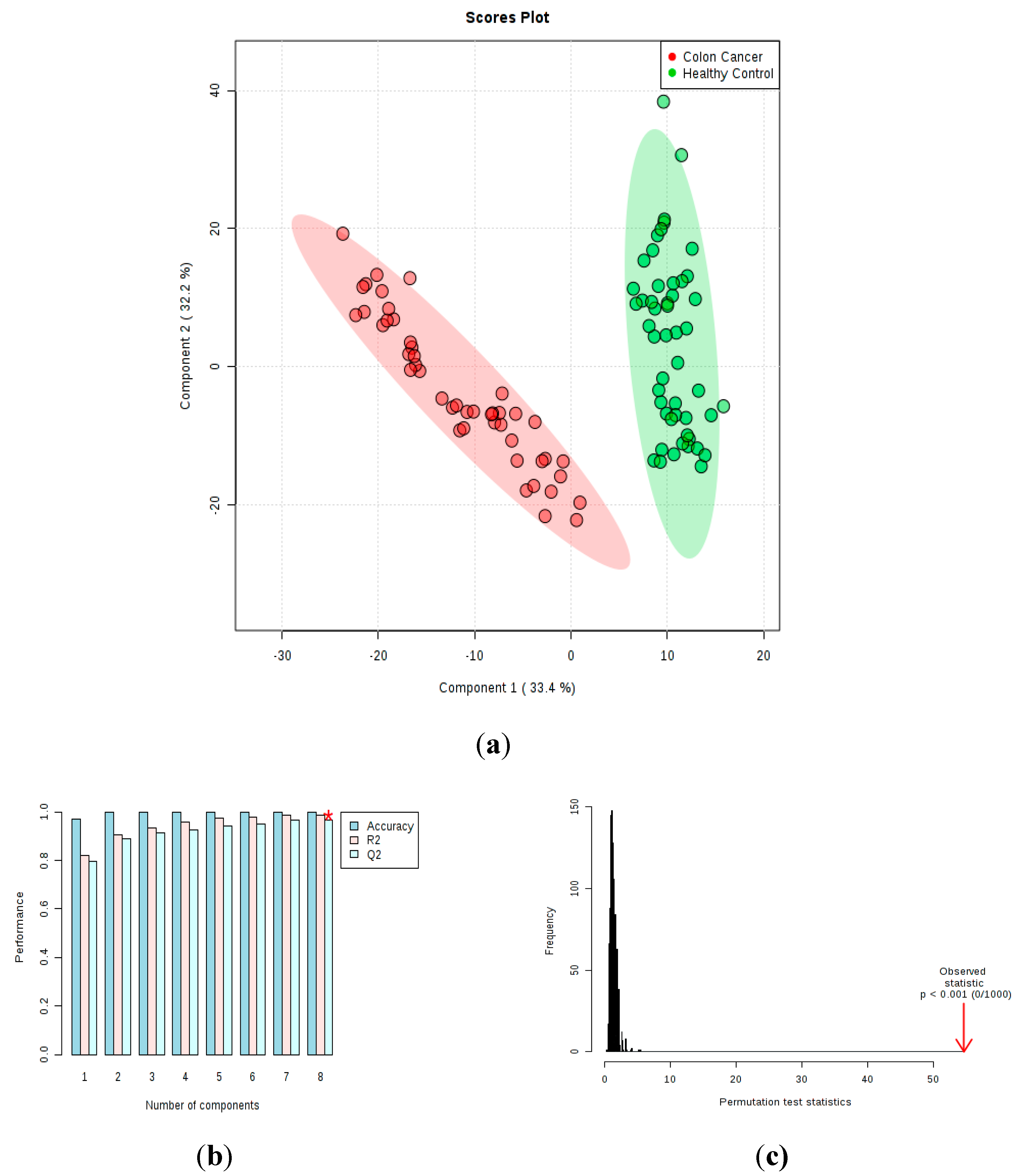
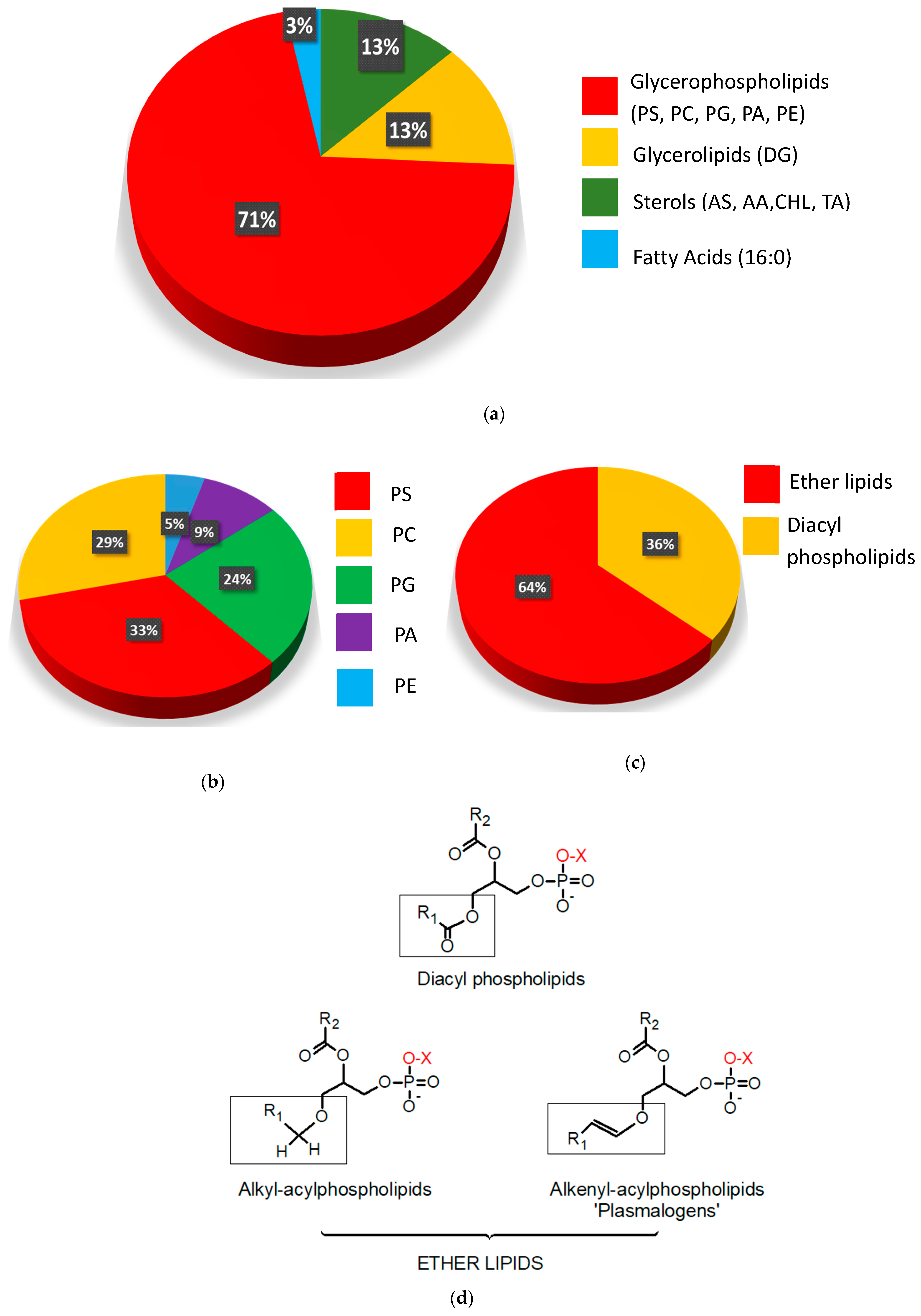
2.2. Untargeted Lipidomic Plasma Analysis and Discrimination Between CC Patients and CTR Volunteers
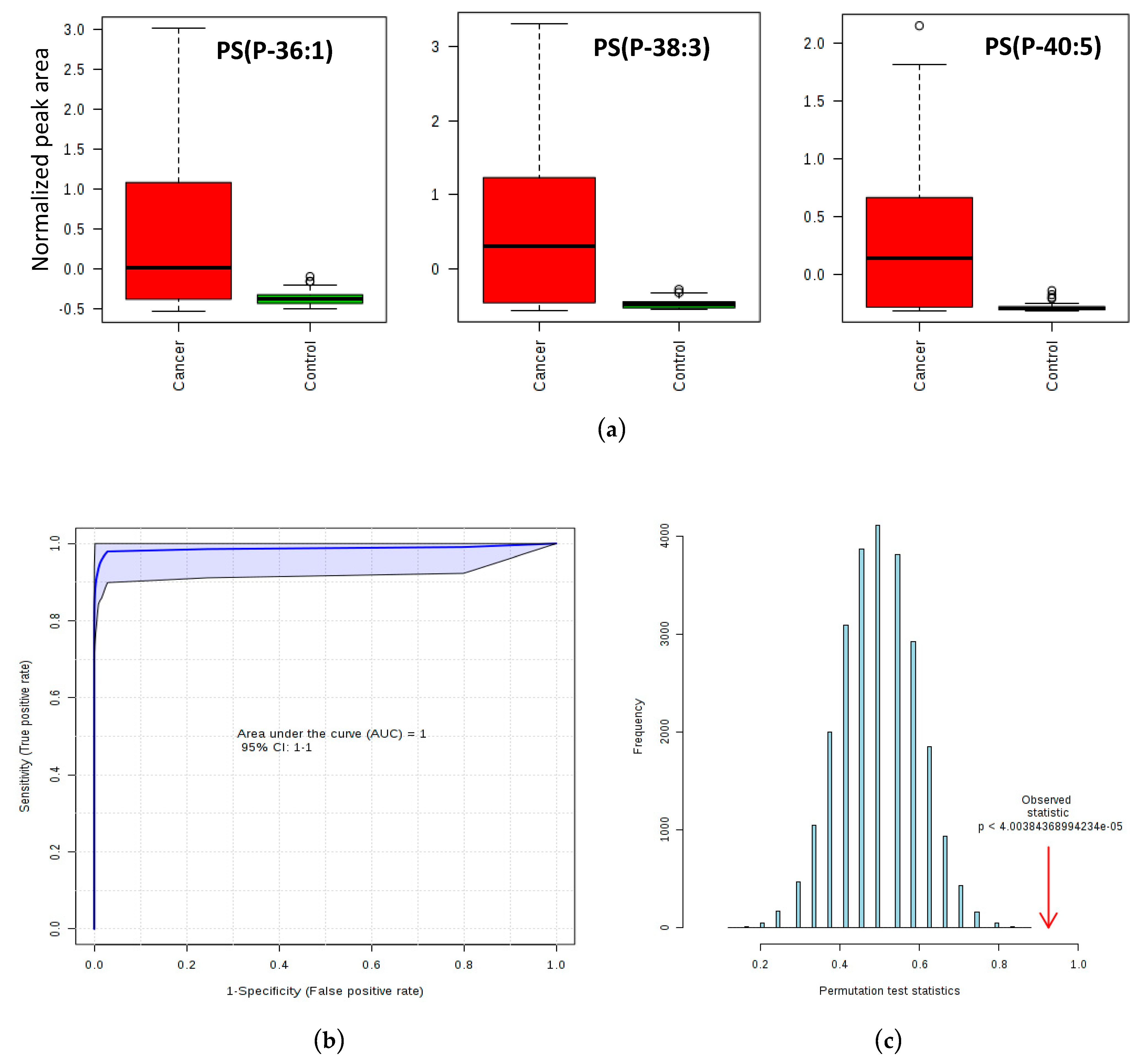
2.3. Phosphatidylserine Plasmalogens as Biomarkers for CC Diagnostics
2.4. Analyses of Fatty Acid Composition by GC
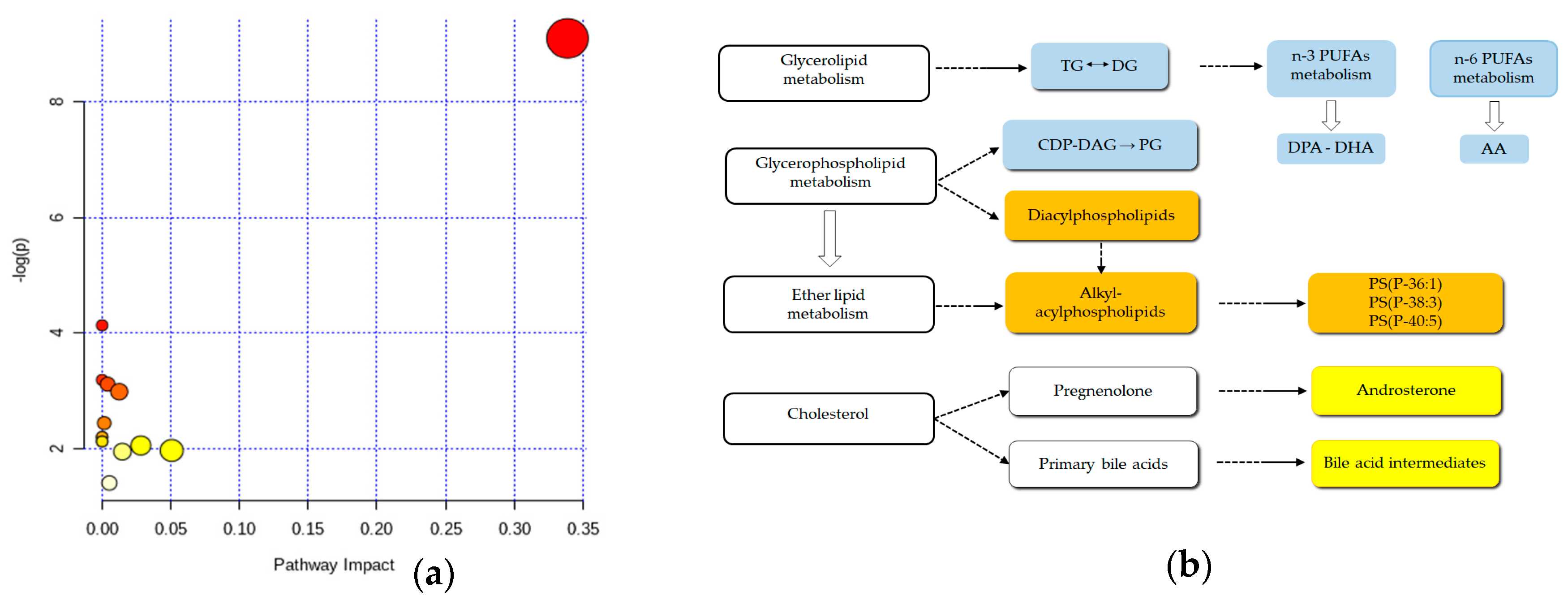
2.5. Metabolic Pathway Analyses Plot
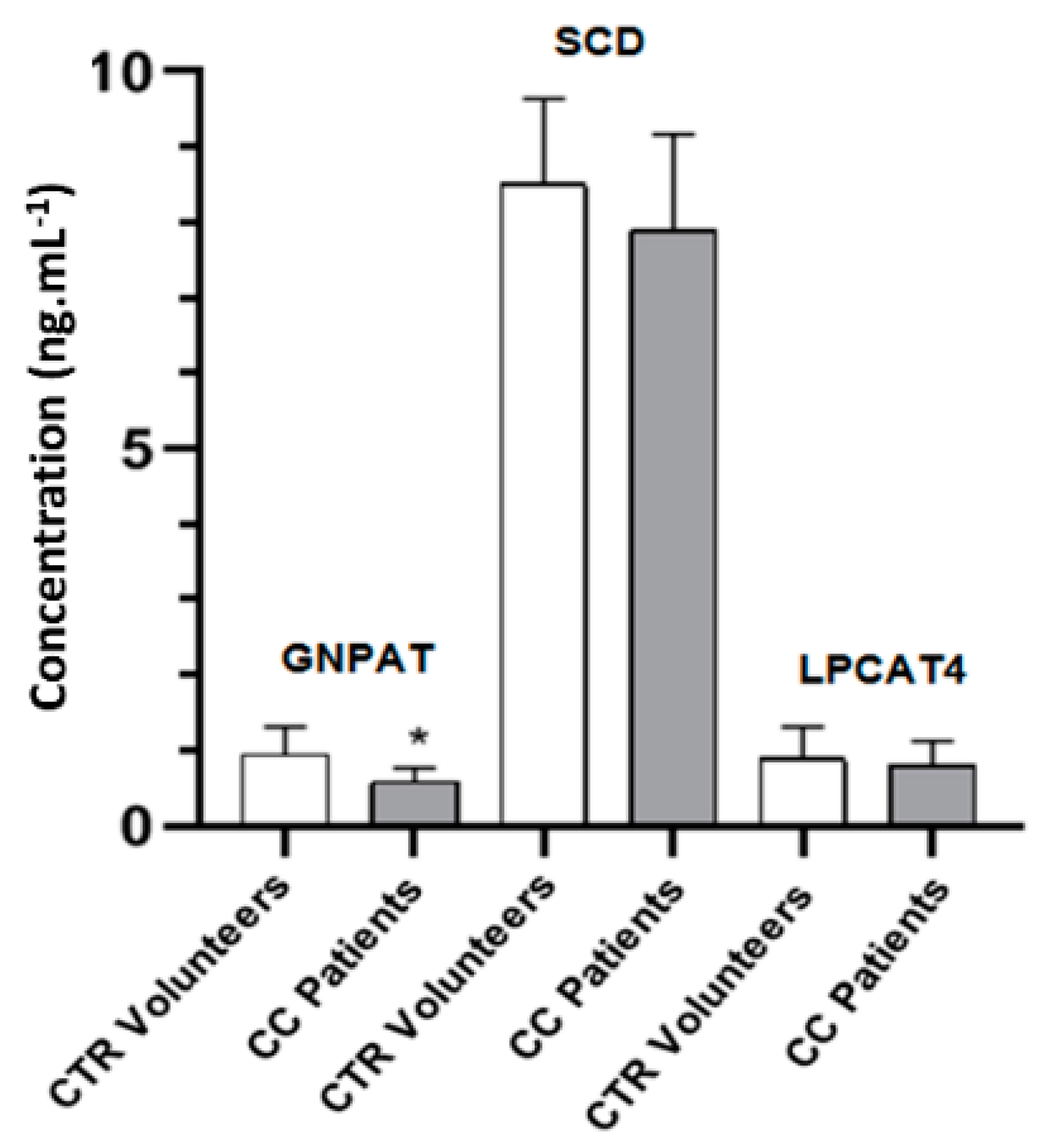
2.6. GNPAT, SCD and LPCAT4 Concentrations in Plasma as Determined by Enzyme-Linked Immunosorbent Assay (ELISA) Assay
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Volunteers, Ethical Consent and Plasma Samples
4.3. Total Lipids Extraction
4.4. Lipid Profile of Plasma Samples by UPLC-QTOF-MSE Analysis
4.5. Fatty Acid Profile by GC-FID Analysis
4.6. Immunodetection of Plasmalogen Synthesis Enzymes LPCAT4, SCD and GNPAT
4.7. Data Processing, Statistical, Biomarker and Pathway Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Orangio, G.R. The Economics of Colon Cancer. Surg. Oncol. Clin. North Am. 2018, 27, 327–347. [Google Scholar] [CrossRef] [PubMed]
- van der Sijp, M.P.; Bastiaannet, E.; Mesker, W.E.; van der Geest, L.G.; Breugom, A.J.; Steup, W.H.; Marinelli, A.W.; Tseng, L.N.; Tollenaar, R.A.; van de Velde, C.J.; et al. Differences between colon and rectal cancer in complications, short-term survival and recurrences. Int. J. Color. Dis. 2016, 31, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Simon, K. Colorectal cancer development and advances in screening. Clin. Interv. Aging 2016, 11, 967–976. [Google Scholar] [CrossRef]
- Ghosh, A.; Nishtala, K. Biofluid lipidome: A source for potential diagnostic biomarkers. Clin. Transl. Med. 2017, 6, 22. [Google Scholar] [CrossRef]
- Hyotylainen, T.; Oresic, M. Bioanalytical techniques in nontargeted clinical lipidomics. Bioanalysis 2016, 8, 351–364. [Google Scholar] [CrossRef]
- Quehenberger, O.; Dennis, E.A. The human plasma lipidome. N. Engl. J. Med. 2011, 365, 1812–1823. [Google Scholar] [CrossRef]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5. [Google Scholar] [CrossRef]
- Knittelfelder, O.L.; Weberhofer, B.P.; Eichmann, T.O.; Kohlwein, S.D.; Rechberger, G.N. A versatile ultra-high performance LC-MS method for lipid profiling. J. Chromatogr. B 2014, 951, 119–128. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Lin, R.C. UPLC-MS(E) application in disease biomarker discovery: The discoveries in proteomics to metabolomics. Chem. Interactions 2014, 215, 7–16. [Google Scholar] [CrossRef]
- Perrotti, F.; Rosa, C.; Cicalini, I.; Sacchetta, P.; Del Boccio, P.; Genovesi, D.; Pieragostino, D. Advances in Lipidomics for Cancer Biomarkers Discovery. Int. J. Mol. Sci. 2016, 17, 1992. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Han, X. Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends Biochem. Sci. 2016, 41, 954–969. [Google Scholar] [CrossRef] [PubMed]
- Bandu, R.; Mok, H.J.; Kim, K.P. Phospholipids as cancer biomarkers: Mass spectrometry-based analysis. Mass Spectrom. Rev. 2018, 37, 107–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, Z.; Shen, X.; Li, L.; Zhong, J.; Min, L.S.; Xu, L.; Li, H.; Zhang, J.; Dai, L. Serum Lipidomics Profiling to Identify Biomarkers for Non-Small Cell Lung Cancer. BioMed Res. Int. 2018, 2018, 5276240. [Google Scholar] [CrossRef]
- Cala, M.P.; Aldana, J.; Medina, J.; Sanchez, J.; Guio, J.; Wist, J.; Meesters, R.J.W. Multiplatform plasma metabolic and lipid fingerprinting of breast cancer: A pilot control-case study in Colombian Hispanic women. PLoS ONE 2018, 13, e0190958. [Google Scholar] [CrossRef]
- Duscharla, D.; Bhumireddy, S.R.; Lakshetti, S.; Pospisil, H.; Murthy, P.V.; Walther, R.; Sripadi, P.; Ummanni, R. Prostate Cancer Associated Lipid Signatures in Serum Studied by ESI-Tandem Mass Spectrometryas Potential New Biomarkers. PLoS ONE 2016, 11, e0150253. [Google Scholar] [CrossRef]
- Fernandes Messias, M.C.; Mecatti, G.C.; Figueiredo Angolini, C.F.; Eberlin, M.N.; Credidio, L.; Real Martinez, C.A.; Rodrigues Coy, C.S.; de Oliveira Carvalho, P. Plasma Lipidomic Signature of Rectal Adenocarcinoma Reveals Potential Biomarkers. Front. Oncol. 2017, 7, 325. [Google Scholar] [CrossRef]
- Pakiet, A.; Kobiela, J.; Stepnowski, P.; Sledzinski, T.; Mika, A. Changes in lipids composition and metabolism in colorectal cancer: A review. Lipids Heal. Dis. 2019, 18, 29. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, X.; Wang, Y.; Xu, G.; Zhang, H.; Dai, W.; He, B.; Zhang, Q.; Ji, J.; Wang, X. A sensitive liquid chromatography/electrospray tandem mass spectroscopy method for simultaneous quantification of a disulfide bond doxorubicin conjugation prodrug and activated doxorubicin: Application to cellular pharmacokinetic and catabolism studies. J. Chromatogr. B 2017, 1065-1066, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Perttula, K.; Schiffman, C.; Edmands, W.M.B.; Petrick, L.; Grigoryan, H.; Cai, X.; Gunter, M.J.; Naccarati, A.; Polidoro, S.; Dudoit, S.; et al. Untargeted lipidomic features associated with colorectal cancer in a prospective cohort. BMC Cancer 2018, 18, 996. [Google Scholar] [CrossRef]
- Li, F.; Qin, X.; Chen, H.; Qiu, L.; Guo, Y.; Liu, H.; Chen, G.; Song, G.; Wang, X.; Li, F.; et al. Lipid profiling for early diagnosis and progression of colorectal cancer using direct-infusion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Yuan, J.M.; Huang, J.Y.; Su, J.; Wang, R.; Koh, W.P.; Ong, C.N. Plasma fatty acids and risk of colon and rectal cancers in the Singapore Chinese Health Study. NPJ Precis. Oncol. 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Geijsen, A.; Brezina, S.; Keski-Rahkonen, P.; Baierl, A.; Bachleitner-Hofmann, T.; Bergmann, M.M.; Boehm, J.; Brenner, H.; Chang-Claude, J.; van Duijnhoven, F.J.B.; et al. Plasma metabolites associated with colorectal cancer: A discovery-replication strategy. Int. J. Cancer 2019, 145, 1221–1231. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, Y.; Elson, P.; Tan, H.; Plummer, S.J.; Berk, M.; Aung, P.P.; Lavery, I.C.; Achkar, J.P.; Li, L.; et al. Plasma lysophosphatidylcholine levels: Potential biomarkers for colorectal cancer. J. Clin. Oncol. 2007, 25, 2696–2701. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Rudge, S.A.; Zhang, Q.; Wakelam, M.J. Using lipidomics analysis to determine signalling and metabolic changes in cells. Curr. Opin. Biotechnol. 2017, 43, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.H.; Bestard-Escalas, J.; Garate, J.; Maimo-Barcelo, A.; Fernandez, R.; Reigada, R.; Khorrami, S.; Ginard, D.; Okazaki, T.; Fernandez, J.A.; et al. Tissue-selective alteration of ethanolamine plasmalogen metabolism in dedifferentiated colon mucosa. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.M.; Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef]
- Lv, J.; Lv, C.Q.; Xu, L.; Yang, H. Plasma Content Variation and Correlation of Plasmalogen and GIS, TC, and TPL in Gastric Carcinoma Patients: A Comparative Study. Med. Sci. Monit. Basic Res. 2015, 21, 157–160. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual, 8th ed.; American College of Surgeons: Chicago, IL, USA, 2018. [Google Scholar]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- The LipidWeb. Available online: https://www.lipidhome.co.uk/ (accessed on 11 April 2019).
- Plumb, R.S.; Johnson, K.A.; Rainville, P.; Smith, B.W.; Wilson, I.D.; Castro-Perez, J.M.; Nicholson, J.K. UPLC/MS(E); a new approach for generating molecular fragment information for biomarker structure elucidation. Rapid Commun. Mass Spectrom. 2006, 20, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Vallabhapurapu, S.D.; Blanco, V.M.; Sulaiman, M.K.; Vallabhapurapu, S.L.; Chu, Z.; Franco, R.S.; Qi, X. Variation in human cancer cell external phosphatidylserine is regulated by flippase activity and intracellular calcium. Oncotarget 2015, 6, 34375–34388. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Ghosh, S.; Sen, T.; Shadab, M.; Banerjee, I.; Basu, S.; Ali, N. A Novel Therapeutic Strategy for Cancer Using Phosphatidylserine Targeting Stearylamine-Bearing Cationic Liposomes. Mol. Ther. Nucleic Acids 2018, 10, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Kanwar, S.S. Phosphatidylserine: A cancer cell targeting biomarker. Semin. Cancer Biol. 2018, 52, 17–25. [Google Scholar] [CrossRef]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 2012, 1822, 1442–1452. [Google Scholar] [CrossRef]
- Snyder, F.; Wood, R. Alkyl and Alk-1-enyl Ethers of Glycerol in Lipids from Normal and Neoplastic Human Tissues. Cancer Res. 1969, 29, 251–257. [Google Scholar]
- Snyder, F.; Cress, E.A.; Stephens, N. An unidentified lipid prevalent in tumors. Lipids 1966, 1, 381–386. [Google Scholar] [CrossRef]
- Jaffres, P.A.; Gajate, C.; Bouchet, A.M.; Couthon-Gourves, H.; Chantome, A.; Potier-Cartereau, M.; Besson, P.; Bougnoux, P.; Mollinedo, F.; Vandier, C. Alkyl ether lipids, ion channels and lipid raft reorganization in cancer therapy. Pharmacol. Ther. 2016, 165, 114–131. [Google Scholar] [CrossRef]
- Glade, M.J.; Smith, K. Phosphatidylserine and the human brain. Nutrition 2015, 31, 781–786. [Google Scholar] [CrossRef]
- Messias, M.C.F.; Mecatti, G.C.; Priolli, D.G.; de Oliveira Carvalho, P. Plasmalogen lipids: Functional mechanism and their involvement in gastrointestinal cancer. Lipids Health Dis. 2018, 17, 41. [Google Scholar] [CrossRef]
- Inadomi, J.M. Screening for Colorectal Neoplasia. N. Engl. J. Med. 2017, 376, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Voorneveld, P.W.; Reimers, M.S.; Bastiaannet, E.; Jacobs, R.J.; van Eijk, R.; Zanders, M.M.J.; Herings, R.M.C.; van Herk-Sukel, M.P.P.; Kodach, L.L.; van Wezel, T.; et al. Statin Use After Diagnosis of Colon Cancer and Patient Survival. Gastroenterology 2017, 153, 470–479.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Zheng, S.H.; Yang, W.G.; Yang, C.; Yuan, W.T. Targeting colon cancer stem cells with novel blood cholesterol drug pitavastatin. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 8. [Google Scholar]
- Berendse, K.; Klouwer, F.C.; Koot, B.G.; Kemper, E.M.; Ferdinandusse, S.; Koelfat, K.V.; Lenicek, M.; Schaap, F.G.; Waterham, H.R.; Vaz, F.M.; et al. Cholic acid therapy in Zellweger spectrum disorders. J. Inherit. Metab. Dis. 2016, 39, 859–868. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Schutgens, R.B.H.; Heymans, H.S.A. Deficient cholesterol side chain oxidation in patients without peroxisomes (Zellweger syndrome): Evidence for the involvement of peroxisomes in bile acid synthesis in man. Clin. Chim. Acta 1987, 162, 295–301. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; Garcia-Irigoyen, O.; Moschetta, A. Bile acids and colon cancer: Is FXR the solution of the conundrum? Mol. Asp. Med. 2017, 56, 66–74. [Google Scholar] [CrossRef]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary bile acids: An underrecognized cause of colon cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef]
- Thomas, A.M.; Manghi, P.; Asnicar, F.; Pasolli, E.; Armanini, F.; Zolfo, M.; Beghini, F.; Manara, S.; Karcher, N.; Pozzi, C.; et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 2019, 25, 667–678. [Google Scholar] [CrossRef]
- Dury, A.Y.; Ke, Y.; Gonthier, R.; Isabelle, M.; Simard, J.N.; Labrie, F. Validated LC-MS/MS simultaneous assay of five sex steroid/neurosteroid-related sulfates in human serum. J. Steroid Biochem. Mol. Biol. 2015, 149. [Google Scholar] [CrossRef]
- Mitamura, K.; Setaka, M.; Shimada, K.; Honma, S.; Namiki, M.; Koh, E.; Mizokami, A. Determination of sulfates of androsterone and epiandrosterone in human serum using isotope diluted liquid chromatography-electrospray ionization-mass spectrometry. Biomed. Chromatogr. 2005, 19, 796–801. [Google Scholar] [CrossRef]
- Steers, W.D. 5α-reductase activity in the prostate. Urology 2001, 58, 17–24. [Google Scholar] [CrossRef]
- Kondo, Y.; Nishiumi, S.; Shinohara, M.; Hatano, N.; Ikeda, A.; Yoshie, T.; Kobayashi, T.; Shiomi, Y.; Irino, Y.; Takenawa, T.; et al. fatty acid profiling of colorectal cancer by gas chromatography/mass spectrometry. Biomark. Med. 2011, 5, 451–460. [Google Scholar] [CrossRef]
- Rohrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Dix, T.A.; Aikens, J. Mechanisms and biological relevance of lipid peroxidation initiation. Chem. Res. Toxicol. 1993, 6, 2–18. [Google Scholar] [CrossRef]
- Carracedo, A.; Cantley, L.C.; Pandolfi, P.P. Cancer metabolism: Fatty acid oxidation in the limelight. Nat. Rev. Cancer 2013, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, Y.Y.; Harris, J.W.; Mitov, M.I.; Kim, J.T.; Butterfield, D.A.; Lee, E.Y.; Weiss, H.L.; Gao, T.; Evers, B.M. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget 2015, 6, 18891–18904. [Google Scholar] [CrossRef] [PubMed]
- Okuno, M.; Hamazaki, K.; Ogura, T.; Kitade, H.; Matsuura, T.; Yoshida, R.; Hijikawa, T.; Kwon, S.; Arita, S.; Itomura, I.; et al. Abnormalities in Fatty Acids in Plasma, Erythrocytes and Adipose Tissue in Japanese Patients with Colorectal Cancer. In Vivo 2013, 27, 203–210. [Google Scholar]
- Wood, P.L.; Donohue, M.M.; Cebak, J.E.; Beckmann, T.G.; Messias, M.C.F.; Credidio, L.; Coy, C.S.R.; Carvalho, P.O.; Crotti, S.; D’Aronco, S.; et al. Reduced Plasma Levels of Very-Long-Chain Dicarboxylic Acid 28:4 in Italian and Brazilian Colorectal Cancer Patient Cohorts. Metabolites 2018, 8, 91. [Google Scholar] [CrossRef]
- Alexander, J.; Gildea, L.; Balog, J.; Speller, A.; McKenzie, J.; Muirhead, L.; Scott, A.; Kontovounisios, C.; Rasheed, S.; Teare, J.; et al. A novel methodology for in vivo endoscopic phenotyping of colorectal cancer based on real-time analysis of the mucosal lipidome: A prospective observational study of the iKnife. Surg. Endosc. 2017, 31, 1361–1370. [Google Scholar] [CrossRef]
- Liu, T.; Tan, Z.; Yu, J.; Peng, F.; Guo, J.; Meng, W.; Chen, Y.; Rao, T.; Liu, Z.; Peng, J. A conjunctive lipidomic approach reveals plasma ethanolamine plasmalogens and fatty acids as early diagnostic biomarkers for colorectal cancer patients. Expert Rev. Proteom. 2020, 17, 233–242. [Google Scholar] [CrossRef]
- Honsho, M.; Asaoku, S.; Fujiki, Y. Posttranslational regulation of fatty acyl-CoA reductase 1, Far1, controls ether glycerophospholipid synthesis. J. Biol. Chem. 2010, 285, 8537–8542. [Google Scholar] [CrossRef]
- Honsho, M.; Abe, Y.; Fujiki, Y. Plasmalogen biosynthesis is spatiotemporally regulated by sensing plasmalogens in the inner leaflet of plasma membranes. Sci. Rep. 2017, 7, 43936. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; LEES, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Metherel, A.H.; Stark, K.D. The stability of blood fatty acids during storage and potential mechanisms of degradation: A review. Prostaglandins Leukot. Essent. Fat. Acids 2016, 104, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Burla, B.; Arita, M.; Arita, M.; Bendt, A.K.; Cazenave-Gassiot, A.; Dennis, E.A.; Ekroos, K.; Han, X.; Ikeda, K.; Liebisch, G.; et al. MS-based lipidomics of human blood plasma: A community-initiated position paper to develop accepted guidelines. J. Lipid Res. 2018, 59, 2001–2017. [Google Scholar] [CrossRef] [PubMed]
- Lipid Maps. Available online: http://www.lipidmaps.org/ (accessed on 10 April 2019).
- Human Metabolome Database (HMDB). Available online: http://www.hmdb.ca/ (accessed on 10 April 2019).
- Metaboanalyst. Available online: www.metaboanalyst.ca (accessed on 11 April 2019).
| CC Patients | CTR Volunteers | |
|---|---|---|
| Number of patients (N) | 50 | 50 |
| Sex (Male/Female) | 24/26 | 25/25 |
| Age (years) | 62.4 ± 9.4 | 57.2 ± 12.9 † |
| BMI (kg.m−2) | 23.9 ± 6.1 | 29.2 ± 6.7 † |
| Stages (%) | ||
| I | 11 (22) | - |
| II | 18 (36) | - |
| III/IV | 12 (24) | - |
| No information | 9 (18) | - |
| Number | Compound a,b | Molecular Formula | FDR c | Mass Error (ppm) | log2(FC) d | Trend |
|---|---|---|---|---|---|---|
| 1 | Androsterone sulfate | C19H30O5S | 1.26 × 10−04 | −2.42 | 2.25 | High |
| 2 | Apocholic acid | C24H38O4 | 5.18 × 10−17 | −0.76 | 1.14 | High |
| 3 | Cholesterol | C27H46O | 7.82 × 10−11 | −0.12 | 5.00 | High |
| 4 | TA | C13H24O2 | 1.64 × 10−20 | −0.28 | 1.45 | High |
| 5 | PA(32:0) | C35H69O8P | 4.36 × 10−23 | 3.79 | 4.25 | High |
| 6 | PA(O-34:0) | C37H75O7P | 1.26 × 10−04 | −3.61 | 2.23 | High |
| 7 | PE(32:2) | C37H70NO8P | 5.18 × 10−17 | −3.61 | 1.61 | High |
| 8 | PG(P-40:5) or | C46H81O9P | 7.82 × 10−11 | −0.72 | 3.94 | High |
| PG(O-38:6) | ||||||
| 9 | PS(O-38:1) | C46H84NO9P | 1.33 × 10−10 | −2.01 | 4.06 | High |
| 10 | PS(40:4) | C46H82NO10P | 9.54 × 10−16 | −2.92 | 2.46 | High |
| 11 | PS(O-40:3) | C50H85NO7 | 4.15 × 10−11 | −2.52 | 5.76 | High |
| 12N | PS(P-36:1) | C42H80NO9P | 2.31 × 10−07 | −1.42 | 2.49 | High |
| 12P | PS(P-36:1) | C42H80NO9P | 2.69 × 10−10 | −1.42 | 4.46 | High |
| 13 | PS(P-38:1) | C46H84NO10P | 1.74 × 10−11 | 1.81 | 3.91 | High |
| 14N | PS(P-38:3) | C44H80NO9P | 7.34 × 10−09 | −1.72 | 3.56 | High |
| 14P | PS(P-38:3) | C44H80NO9P | 7.48 × 10−10 | −1.22 | 4.61 | High |
| 15 | PS(P-40:5) | C46H80NO9P | 1.80 × 10−08 | −2.14 | 4.30 | High |
| 16 | DG(38:1) | C31H58O5 | 2.91 × 10−27 | 4.46 | 3.68 | High |
| 17 | DG(42:5) | C45H78O5 | 1.78 × 10−28 | −2.90 | 2.23 | High |
| 18 | DG(32:1) | C35H66O5 | 2.01 × 10−21 | −2.89 | −1.80 | Low |
| 19 | DG(34:2) | C37H68O5 | 1.01 × 10−16 | −0.91 | −1.09 | Low |
| 20 | PA(20:3) | C23H30O6 | 5.37 × 10−14 | −2.22 | −1.40 | Low |
| 21 | PA(20:4) | C23H39O7P | 2.27 × 10−14 | −0.97 | −2.15 | Low |
| 22 | Palmitic acid | C16H32O2 | 1.00 × 10−15 | 2.73 | −1.27 | Low |
| 23 | PC(36:4) | C44H80NO8P | 5.71 × 10−07 | 0.41 | −1.54 | Low |
| 24 | PC(O-34:3) | C42H80NO7P | 4.16 × 10−10 | −1.35 | −1.26 | Low |
| 25 | PC(P-36:4) or | C44H80NO7P | 2.52 × 10−11 | −0.81 | −1.31 | Low |
| PC(O-36:5) | ||||||
| 26 | PC(P-36:3) or | C44H82NO7P | 1.63 × 10−14 | −1.06 | −1.38 | Low |
| PC(O36:4) | ||||||
| 27 | PC(P-38:3) or | C46H86NO7P | 6.20 × 10−11 | −4.35 | −1.39 | Low |
| PC(O-38:4) | ||||||
| 28 | PC(P-38:4) or | C46H84NO7P | 5.13 × 10−13 | −1.74 | −1.26 | Low |
| PC(O-38:5) | ||||||
| 29 | PG(38:5) | C44H77O10P | 7.38 × 10−13 | −0.76 | −2.78 | Low |
| 30 | PG(38:6) | C44H75O10P | 1.09 × 10−19 | 3.96 | −2.65 | Low |
| 31 | PG(38:7) | C44H73O10P | 4.18 × 10−18 | 3.81 | −2.85 | Low |
| Fatty Acids | CTR Volunteers | Stage I | Stage II | Stage III/IV |
|---|---|---|---|---|
| 14:0 | 3.06 ± 1.63 | 2.08 ± 0.25 | 1.64 ± 0.88 * | 4.51 ± 2.43 |
| 16:0 | 27.33 ± 4.37 | 30.23 ± 2.00 | 30.63 ± 5.79 | 33.25 ± 3.31 |
| 18:0 | 7.68 ± 1.44 | 7.39 ± 0.88 | 7.62 ± 1.70 | 7.38 ± 0.92 |
| ∑ SFA | 38.07 ± 7.44 | 39.70 ± 3.13 | 39.89 ± 8.37 | 45.14 ± 6.66 |
| 16:1 n-7 (palmitoleic acid) | 5.08 ± 0.79 | 4.40 ± 0.81 | 5.35 ± 2.99 | 5.50 ± 1.22 |
| 18:1 n-9 (oleic acid) | 20.06 ± 3.36 | 23.04 ± 1.02 | 21.25 ± 5.26 | 19.81 ± 0.88 |
| ∑ MUFA | 25.14 ± 4.15 | 27.44 ± 1.83 | 26.60 ± 8.25 | 25.31 ± 2.10 |
| 18:2 n-6 (linoleic acid) | 28.35 ± 5.66 | 27.96 ± 3.41 | 27.77 ± 5.75 | 25.46 ± 1.44 |
| 20:4 n-6 (ARA) | 5.12 ± 1.01 | 3.87 ± 2.69 | 4.42 ± 2.21 | 3.33 ± 0.62 * |
| ∑ n-6 PUFA | 33.47 ± 6.67 | 31.83 ± 6.10 | 32.19 ± 7.96 | 28.79 ± 2.06 |
| 18:3 n-3 (linolenic acid) | 1.35 ± 1.38 | 0.30 ± 0.23 | 0.63 ± 0.32 | 0.42 ± 0.31 |
| 20:5 n-3 (EPA) | 0.54 ± 0.42 | 0.35 ± 0.08 | 0.18 ± 0.09 | 0.21 ± 0.12 |
| 22:5 n-3 (DPA) | 0.33 ± 0.19 | 0.09 ± 0.04 * | 0.19 ± 0.13 | 0.18 ± 0.01 * |
| 22:6 n-3 (DHA) | 0.63 ± 0.28 | 0.22 ± 0.15 * | 0.28 ± 0.07 * | 0.18 ± 0.04 * |
| ∑ n-3 PUFA | 2.85 ± 2.27 | 0.96 ± 0.32 | 1.30 ± 0.54 | 0.98 ± 0.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, A.M.A.P.; Messias, M.C.F.; Duarte, G.H.B.; de Santis, G.K.D.; Mecatti, G.C.; Porcari, A.M.; Murgu, M.; Simionato, A.V.C.; Rocha, T.; Martinez, C.A.R.; et al. Plasma Lipid Profile Reveals Plasmalogens as Potential Biomarkers for Colon Cancer Screening. Metabolites 2020, 10, 262. https://doi.org/10.3390/metabo10060262
Fernandes AMAP, Messias MCF, Duarte GHB, de Santis GKD, Mecatti GC, Porcari AM, Murgu M, Simionato AVC, Rocha T, Martinez CAR, et al. Plasma Lipid Profile Reveals Plasmalogens as Potential Biomarkers for Colon Cancer Screening. Metabolites. 2020; 10(6):262. https://doi.org/10.3390/metabo10060262
Chicago/Turabian StyleFernandes, Anna Maria A.P., Marcia C.F. Messias, Gustavo H.B. Duarte, Gabrielle K.D. de Santis, Giovana C. Mecatti, Andreia M. Porcari, Michael Murgu, Ana Valéria C. Simionato, Thalita Rocha, Carlos A.R. Martinez, and et al. 2020. "Plasma Lipid Profile Reveals Plasmalogens as Potential Biomarkers for Colon Cancer Screening" Metabolites 10, no. 6: 262. https://doi.org/10.3390/metabo10060262
APA StyleFernandes, A. M. A. P., Messias, M. C. F., Duarte, G. H. B., de Santis, G. K. D., Mecatti, G. C., Porcari, A. M., Murgu, M., Simionato, A. V. C., Rocha, T., Martinez, C. A. R., & Carvalho, P. O. (2020). Plasma Lipid Profile Reveals Plasmalogens as Potential Biomarkers for Colon Cancer Screening. Metabolites, 10(6), 262. https://doi.org/10.3390/metabo10060262






